ABSTRACT
Pancreatic cancer is one of the most malignant tumors that are difficult to diagnose at its early stage and there is no effective therapy. Recent studies uncovered that many non-protein-coding RNAs including the class of long noncoding RNAs (lncRNAs) are differentially expressed in various types of tumors and they are potent regulators of tumor progression and metastasis. LncRNA can mediate tumor initiation, proliferation, migration and metastasis through modulating epigenetic modification, alternative splicing, transcription, and protein translation. In this review, we discuss the molecular mechanism of lncRNAs in the involvement of tumor growth, survival, epithelial-mesenchymal transition, tumor microenvironment, cancer stem cells and chemoresistance in pancreatic ductal adenocarcinoma (PDAC).
Introduction
Pancreatic cancer is one of the most devastating human tumors. According to the most recent statistical data, the 5-y survival of pancreatic cancer is 8%, which is very low in contrast to the steady increase in survival in other types of cancers.Citation1 The mortality rate of pancreatic cancer continued to increase (by 0.3% per year) in men. Recent advances revealed that only <2% transcripts of human genome code for proteins and the remaining 98% transcripts encode different classes of non-coding RNAs.Citation2,3 Understanding the potential functions of the noncoding transcriptome is of great importance. An important feature of lncRNAs is that their genomic location is transcribed in relation to protein-coding genes. On this basis, lncRNAs can be broadly classified as 1) intronic, where they originate from within coding gene loci; 2) cis-antisense, where they are transcribed from the opposite stand to other transcripts, or 3) intergenic, where they are transcribed outside of, or between, coding genes.Citation4 LncRNAs can also be transcribed from other genomic loci including bi-directional regions (either head-to-head or tail-to-tail) with other transcripts,Citation5 3′ from coding genes (uaRNAs)Citation6 and from promoter regions (promoter-associated).Citation7
Though lncRNAs are generally defined as RNA transcripts longer than 200 nucleotides (nt) with no protein-coding potential, they are involved in diverse biological processes, including cell growth, differentiation and proliferation.Citation8–10 Accumulating evidence indicates that lncRNAs are frequently aberrantly expressed in cancers.Citation11,12 Some of them play significant roles in oncogenic or tumor-suppressive pathways and have diagnostic and prognostic values.Citation13–15
Notably, increasing data suggests that lncRNAs play essential roles in the progression of pancreatic ductal adenocarcinoma (PDAC). For instance, lncRNA HULC serves as a promoting factor in pancreatic cancer development and may become a candidate prognostic biomarker for pancreatic cancer.Citation16 Expression of gas5 is significantly decreased in pancreatic cancer tissues and its overexpression in pancreatic cancer cells inhibits cell proliferation.Citation17 Although genomic analyses have discovered a growing list of lncRNAs,Citation18 the mechanisms of lncRNAs in pancreatic cancer development remain largely unknown. In this review, we address the recent advances in our understanding of the contribution of lncRNAs related to microRNA, EMT, pancreatic cancer stem cells (PCSCs), epigenetics, hypoxia, chemoresistance and tumor microenvironment during pancreatic cancer progression. The function of several important lncRNAs in pancreatic cancer is summarized in .
Table 1. The biologic functions of lncRNAs in pancreatic cancer (PC).
LncRNAs and microRNA
MicroRNAs (miRNAs) are a small non-coding RNAs shorter than 22 nucleotides that differentially expressed in various types of tumors including pancreatic cancer.Citation19 It exerts either oncogenic or tumor suppressive roles depending on its downstream targets. Increasing evidence indicates that lncRNAs can function as a miRNA sponge to regulate the expression of specific genes targeted by miRNA which involved in progression of cancer.Citation20 For instance, Qu L et al. identified lncRNA ARSR as a mediator of sunitinib resistance in renal cell carcinoma by acting as a competing endogenous RNA(ceRNA) for miR-34 and miR-449.Citation21 Moreover, lncRNA UCA1 contributes to the progression of hepatocellular carcinoma through inhibiting miR-216b and activating FGFR1/ERK signaling pathways.Citation22 Thus, lncRNAs exhibited their regulatory function with different miRNAs.
LincROR which was originally identified in the induction of pluripotent stem cells (iPS), functions to maintain stem cell pluripotency.Citation23 In the study of Song G et al.,Citation24 they observed that ROR was substantially overexpressed in pancreatic cancer tissues and acted as an endogenous sponge of miR-145, thereby activating the core transcription factor Nanog. Consequently, the up-regulated Nanog significantly increased the tumourigenicity of pancreatic cancer stem cells (PCSCs).
Another example, H19, as an imprinted gene, is highly expressed from the early stages of embryogenesis to fetal life in many organs. However, H19 barely expressed after birth.Citation25 Emerging evidences have shown that H19 was overexpressed in many types of cancers including bladder and breast cancer.Citation26,27 It was reportedCitation28 that up-regulated expression of H19 in pancreatic cancer promoted cell invasion and metastasis partially by increasing HMGA2-mediated epithelial-mesenchymal transition (EMT) through sponging let-7(a miRNA). This work provided a positive correlation between H19 and HMGA2 and shed a light on the potential therapeutic target in pancreatic cancer.
NEAT1(nuclear enriched abundant transcript 1), a newly identified nuclear-restricted lncRNA, acts as a transcriptional regulator for numerous genes.Citation29 Previous study showed that NEAT1 dysregulation promoted tumorigenesis in a variety of human cancers.Citation30 Huang B et al.Citation31 observed that NEAT1 could promote pancreatic cancer progression through binding to miR-506–3p, a tumor suppressor reported in hepatocellular carcinoma.Citation32
Moreover, in the study of Yang H et al.[33], they proved that the lncRNA, MIR31HG, was upregulated in PDAC through microarray gene-expression profile and qRT–PCR assay. Subsequently, they showed that the tumor suppressor miR-193bCitation34,35 negatively regulated MIR31HG through binding to target sequence, a mechanism similar to that of miRNA-mediated silencing of target mRNAs. In addition, MIR31HG also functioned as endogenous decoy for miR-193b to affect its distribution on specific targets without inducing miR-193b destabilization, as the level of miR-193b was not affected following MIR31HG knockdown or overexpression. Therefore, effect of MIR31HG on PDAC cell proliferation and invasion could be explained, in part by its function as a molecular sponge of miR-193b.
KRAS gene mutation was found at high rates in pancreatic carcinoma, and KRAS was commonly considered as an oncogene in pancreatic cancer tumorigenesis.Citation36 In addition to the mutation of KRAS, some studies also demonstrated that KRAS overexpression was related to pancreatic tumorigenesis.Citation37 Xiang L and colleaguesCitation38 found that lncRNA NUTF2P3–001 was significantly increased in pancreatic cancer and it up-regulated KRAS expression by depriving the inhibition of miR-3923 on KRAS. Similarly, another studyCitation39 found that the HOTAIR-miR-613-notch3 axis may be a promising therapeutic target for pancreatic cancer. Taken together, lncRNAs could affect development and progression of pancreatic cancer through lncRNA-miRNA interaction.
LncRNAs and epithelial to mesenchymal transition
Epithelial to mesenchymal transition (EMT) is a developmental process during which polarized epithelial cells loss tight intercellular adhesions and acquire a mesenchymal cell phenotype with the capacity to spread to distant locations.Citation40 In recent years, EMT was considered playing a critical role in the progression of cancers. EMT documented by the loss of E-cadherin expression and the acquisition of mesenchymal markers, such as N-cadherin or vimentin, led to increased cell migratory and invasive properties.Citation41,42 Recent studies have indicated that lncRNAs can regulate cancer epithelial to mesenchymal transition. It was shown that inhibiting the expression of linc-ROR could not only increase the sensitivity of cancer cells to docetaxel and reduce the capacity of cancer cell proliferation, but also reverse EMT process in docetaxel resistant lung adenocarcinoma (LAD) cells.Citation43 Another study showed that LncRNA T-Cell Factor-7 (LncTCF7) was highly expressed in HCC and mediated IL6-induced EMT causing cancer cell invasion.Citation44
The HOTTIP lncRNA which located at the 5′ end of the HOXA cluster, was recently functionally characterized. HOTTIP significantly expressed in human fibroblasts.Citation45 In 2015, Zhihua L et al. identified that HOTTIP contributed to EMT in pancreatic cancer cell, and HOTTIP achieved its the regulatory function in PDAC, by controlling HOXA13.Citation46
Metastasis-associated lung adenocarcinoma transcript-1 (MALAT-1), an evolutionarily highly conserved lncRNA with a length of 8,700 nucleotides, has been linked to several human tumor types since its discovery in non-small cell lung cancer.Citation47 Jiao F et al. demonstrated that the expression of MALAT-1 was higher in pancreatic cancer cells compared to human pancreatic ductal epithelial (HPDE) cells. Suppressing MALAT-1 decreased expression of genes MMP-2 and MMP-9. Suppressing MALAT-1 also down-regulated EMT biomarkers including Snail, Slug, N-cadherin, and vimentin, and significantly upregulated the expression of E-cadherin. Furthermore, morphological change was observed in CFPAC-1 after MALAT-1 down-regulation.Citation48 Another work indicated that EZH2 could be recruited by MALAT-1 and increased H3K27me3 at the E-cadherin promoter, thereby suppressing E-cadherin expression and leading to a malignant phenotype with enhanced tumor migration and invasion.Citation49 EZH2, which functions predominately as a transcriptional repressor that silences tumor suppressor gene, has been found to mediate E-cadherin repression in tongue squamous cell carcinoma.Citation50
Shangyou Z et al. utilized microarrays to explore the expression profiles of mRNAs and lncRNAs in PDAC(GSE61166). Numerous lncRNAs differentially expressed in PDAC and non-tumorous tissues. Among them a novel lncRNA LOC389641 was remarkably increased in PDAC tissues. Additionally, LOC389641 suppressed the expression of EMT-related epithelial marker E-cadherin and induce the mesenchymal markers Vimentin and Snail. The loss of E-cadherin expression directly triggered EMT. The underlying mechanism of LOC389641-suppressed E-cadherin might be related to TNFRSF10A.Citation51
Despite linc-ROR functions as a microRNA sponge to influence progression of PDAC, Han-xiang Z and colleagues proposed that linc-ROR-induced EMT in pancreatic cancer cells may be partly through activation of ZEB1 and inhibition of p53 expression.Citation24,52
Based upon, the LncRNAs have shown significant function in epithelial to mesenchymal transition in PDAC. As most of the pancreatic cancer have metastasized to other organs or locally progressed when diagnosed, the LncRNAs may predict the early metastasis and help making therapeutic decision in the future.
LncRNAs and pancreatic cancer stem cells (PCSCs)
Recently it was demonstrated that the presence of a minor population of cells, termed cancer stem cells (CSCs), are capable of self-renewing, differentiating into other cell types that associated with tumor initiation, metastasis, chemoresistance and recurrence.Citation53 Studies have shown that lncRNAs play pivotal roles in mediating the pluripotency of the embryonic stem cells and differentiation of cancer stem cells.Citation54,55 Peng F et al.Citation56 found that ectopic overexpression of lncRNA H19 significantly promote breast cancer cell colony formation, migration and sphere-forming ability. Furthermore, it was reported that lncRNA RBM5-AS1 contributed to the stem-like characteristics of colon cancer cells in the Wnt pathway via physical interactions with β-catenin.Citation57
Pancreatic cancer stem cells (PCSCs) have been identified not only based on the expression of CD24, CD44, and epithelial-specific antigen (ESA), but also several other cell-surface markers, such as ALDH, CD133.Citation58,59,60 Li et al.Citation61 observed that CD44+CD24+ESA+ cells had a higher tumorigenic ability in immunodeficient mice than PDAC cells that had those markers negative.
In a previous study of Jiao F48, it was found that induced expression of MALAT-1 served as an oncogenic lncRNA in pancreatic cancer by upregulating expression of CSCs markers. However, the detailed molecular mechanisms of MALAT-1 regulating pancreatic CSCs and tumor progression remained unknown. MALAT-1 upregulated the expression of a self-renewal factor Sox2 to give rise to the stem cell-like phenotypes of pancreatic cancer in PDAC cells.Citation62 It was revealed that high level of MALAL-1 was expressed in CD133+ cell and CD133 expression was reduced after MALAT-1 downregulation. However, the detailed molecular mechanisms of MALAT-1 upregulating Sox2 still need to be clarified. On the contrary, other researchers documented that Linc-ROR can function as a competing endogenous RNA to sponge miR-145, leading to an increase in expression of a miR-145 target, the transcription factor Nanog, which effectively induce the malignant tumor characteristics of PCSCs.Citation24 Collectively, the above data suggested that dysregulating lncRNAs in pancreatic cancer is related to its stem cell-like properties.
LncRNAs and Hypoxia
The presence of intratumoral hypoxia is a negative prognostic indicator for many patients as it has been associated with aggressive tumor cell phenotypes and distant metastasis.Citation63 High level of hypoxia often occurred in pancreatic cancers.Citation64 Hypoxia-inducible factor- 1α (HIF-1α) is the best characterized biomarker among the transcriptional regulators under hypoxia.Citation65 In addition, the overexpression of HIF-1α was reported to play a critical role in pancreatic cancer under hypoxia and promoted invasion and metastasis.Citation66 LncRNAs have been reported to function in diverse regulatory mechanisms in the hypoxia process. Sun YW et al. found that hyaluronan synthase 2 antisense 1 (HAS2-AS1) increased in oral squamous cell carcinoma and in cells cultured under hypoxia. Moreover, the expression of hypoxia-induced HAS2-AS1 is dependent on HIF-1α which directly binds to and activates the transcription of HAS2-AS1.Citation67
Recent research showedCitation68 that ENST00000480739 functioned as an important anti-metastatic lncRNA, which downregulated HIF-1α by upregulating OS-9 and served as an independent risk factor for the overall survival of pancreatic cancer patients () after surgery. Meanwhile, HIF-1α could transcriptionally upregulated lncRNA-NUTF2P3–001 by binding to hypoxia-response elements (HRE) of promoter area, which significantly promoted proliferation and invasive ability of pancreatic cancer.Citation38 However, more explorations are needed to demonstrate how lncRNAs participate in this process in pancreatic cancer.
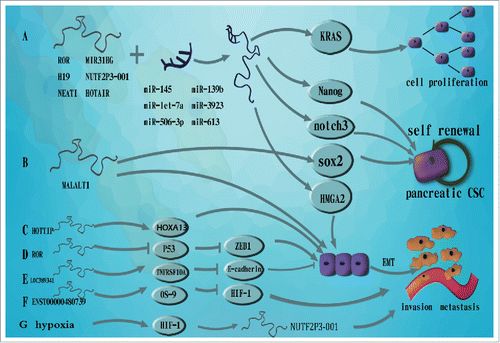
Figure 1. The molecular mechanism of lncRNAs in the progression of pancreatic cancer. A. lncRNAs bind to specific microRNAs to activate the expression of targeted genes which involve in carcinoma progression, B. MALAT-1 promotes stem cell-like phenotypes of pancreatic cancer through upregulating self-renewal factor Sox2 and induces EMT. C. HOTTIP contributes to EMT through upregulating HOXA13 in pancreatic cancer cells, D. ROR induces EMT through inhibiting p53 and ZEB1 expression, E. LOC389641 triggers EMT through suppressing E-cadherin expression, F. ENST00000480739 downregulates HIF-1 by upregulating OS-9 resulted in facilitating pancreatic cancer invasion and metastasis, G. HIF-1α transcriptionally upregulates lncRNA-NUTF2P3–001 under hypoxia which promotes invasive ability of pancreatic cancer.
lncRNA-mediated modulation of epigenetic modifications
lncRNAs participated in genomic transcriptional control through association with epigenetic modifier complexes.Citation69 Polycomb repressive complex 2 (PRC2) is one of the main histone modification complexes tethered by lncRNAs since a substantial fraction of lncRNAs was found to interact with this complex.Citation70,71 Enhancer of zeste homolog 2 (EZH2) acts as a catalytic subunit of PRC2, which is overexpressed in a wide range of tumors and functions predominately as a transcriptional repressor that silences tumor suppressor gene targets via methylation of lysine 27 of histone 3 (H3K27).Citation72–74 Accumulating evidence indicated that long noncoding RNA contributed to the epigenetic regulation of tumor initiation. Terashima M and his coworkers revealed that MEG3 regulated the recruitment of JARID2 and EZH2 and histone H3 methylation on the regulatory regions of CDH1 and microRNA-200 family genes for transcriptional repression in lung cancer cells.Citation75 Another study showed that lncRNA HOXA11-AS can also function as a scaffold to recruit EZH2 along with the histone demethylase LSD1 or DNMT1 to serve as critical effectors in tumorigenesis and progression of gastric cancer.
Similarity, EZH2 was overexpressed in pancreatic cancer promoted tumor cell proliferation, migration and invasion via epigenetic repression of target genes. Intriguingly, it was reported that EZH2 recruitment by MALAT-1 played a key role in the pathogenesis of pancreatic cancer through subsequent involvement of the complex in repressing E-cadherin. In addition, RNA immunoprecipitation (RIP) assay confirmed that EZH2 directly bound to MALAT-1.Citation49
Another example, the first HOX-associated lncRNA, HOTAIR, was initially identified as a scaffold RNA associated with histone modification complexes, namely polycomb repressive complex (PRC2) and the LSD1/CoREST/REST complex.Citation76 Chi-Han L and colleaguesCitation77 demonstrated that HOTAIR could guide EZH2 to affect the level of the tumor suppressor miR-34a in PDAC cells, thereby promoting cancer cell proliferation. Apart from the conventional repressing mechanism of H3K27 trimethylation, EZH2 could also induce heterochromatin formation at the promoter of miR-34a. Similarity, evidences showed that HOTAIR also suppressed another tumor suppressor miR-663b in pancreatic cancer cells by modifying histone methylation on miR-663b promoter.Citation78 However, another studyCitation79 showed that silencing HOTAIR decreased interaction of EZH2 with the growth inhibitory/pro-apoptotic gene GDF15 promoter region in Panc1 and L3.6pL cells, thus activating derepression of GDF15 and promoting apoptosis of tumor cells.
Since there was evidence that HOTTIP interacted with both chromatin-modifying complexes, PRC2 and MLL1/WDR5Citation45, Yating C et al.Citation80 downregulated HOTTIP and the other two complexes. They performed microarray analysis and found that <40% of all HOTTIP-regulated genes were coregulated by HOTTIP and the two complexes. Though the regulation of gene expression by HOTTIP in pancreatic cancer cells involved interaction with complexes in addition to PRC2 and MLL1/WDR5, the interactions need more explorations.
Meanwhile, studies have found lncRNA IRAIN, which is 5359 nt in length and located in chromosome 15q26.3, may contribute to the progression of non-small cell lung cancer (NSCLC).Citation81 Also, its overexpression was significant and correlated with advanced pathological stage, larger tumor size and lymph node metastasis in patients with pancreatic cancer.Citation82 Yifan L and coworkers found IRAIN can directly bind to EZH2 and LSD1(another important role in the epigenetic regulation of gene transcription) complexes and repress two key tumor suppressors, KLF2 and P15, responsible for cell apoptosis and cell cycle control ().Citation83,84
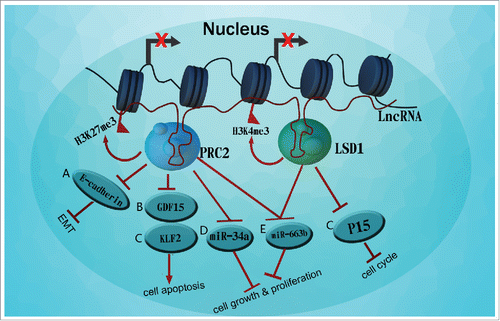
Figure 2. LncRNAs contribute to the epigenetic regulation of pancreatic cancer tumorigenesis, A. EZH2(one unit of PRC2 complex) recruited by MALAT-1 and suppresses E-cadherin expression through increasing H3K27me3 at the E-cadherin promoter, B. HOTAIR increases interaction of EZH2 with GDF15 promoter region, C. IRAIN binds to EZH2 and LSD1 complexes and repress two key tumor suppressors, KLF2 and P15. D. HOTAIR guides EZH2 to target the tumor suppressor miR-34a, E. HOTAIR suppresses tumor suppressor miR-663b in pancreatic cancer cells by LSD1 and PRC2 modification on H3K4me3 or H3K27me3 of miR-663b promoter.
Although lncRNAs showed a critical role in epigenetic modulation of pancreatic cancer, more complex cases of epigenetic regulation need to be clarified. For instance, the radiation-induced PARTICLE represses tumor suppressive gene MAT2A in breast cancer cell via forming a DNA-lncRNA triplex within the 456 bp upstream of the CpG island in the MAT2A promoter. Besides, it could also induce methylation of the MAT2A promoter through interaction with transcription-repressive complex proteins G9a and SUZ12 (subunit of PRC2).Citation85
LncRNAs and Chemoresistance
Recent evidence indicated a close link between lncRNAs and the acquisition of chemoresistance in various carcinoma cells. It was found that decreased expression of linc-ROR effectively reversed EMT in docetaxel-resistant lung adenocarcinoma cells and sensitized them to chemotherapy.[43] However, lncRNA RP11–838N2.4 could enhances the cytotoxic effects of temozolomide to glioblastomas cells both in vivo and in vitro.Citation86
PDAC is an intractable disease as a result of its rapid dissemination and innate or acquired resistance to chemotherapy. Recent findings provide novel information on lncRNAs expression profiles that might be related to chemoresistance in PDAC. Considering the effect of HOTTIP on tumor cell proliferation, cell cycle, and EMT identified above, Zhihua L et al.Citation46 further investigated whether down-regulation of HOTTIP affected the resistance of PDAC cells to gemcitabine. Interestingly, SW1990-shHOTTIP knockdown cells exhibited much slower growth and a lower IC50 for gemcitabine. Moreover, combined treatment with gemcitabine and HOTTIP knockdown led to an even further reduction in tumor volume in vivo.
In a previous study, Feng J et al.Citation48 shown that MALAT-1 increased the proportion of pancreatic CSCs. They investigated the anti-proliferative effects of gemcitabine in MALAT-1-nc and MALAT-1-si cells, and found that the resistance index(RI) of gemcitabine in AsPC-1/M-nc and CFPAC-1/M-nc was 2.96 and 4.29 times higher than that of M-si group, respectively.Citation62 Nevertheless, the increased chemosensitivity may be a reflection of the slower growth rate of the MALAT-1 knockdown. Resistance to chemotherapy is a property that distinguished pancreatic CSCs from other cancer cells.Citation87
Meanwhile, through a piggyBac transposon-based genome-wide mutagenesis strategy, Lei Y et al.Citation88 identified that functional inactivation of gene PVT1 led to increased sensitivity of gemcitabine in human pancreatic ASPC-1 cells. PVT1, a long non-coding RNA (lncRNA), was observed to play a key role in tumorigenesis through DNA rearrangements or amplifications in non-small cell lung cancer.Citation89 Furthermore, PVT1 was expressed significantly higher in gastric cancer patients and might be a novel biomarker for predicting gastric cancer.Citation90 Xian-wen Z et al.Citation91 concluded that up-regulation of PVT1 promoted the development of multidrug resistance in gastric cancer via increasing the expression of multidrug resistant related gene (MDR1, MRP, mTOR and HIF-1a). However, the exact mechanism of PVT1 on the pancreatic cancer chemoresistance needs further study.
Although comprehensive therapies have been used for the treatment of pancreatic cancer, it is urgent to identify reliable biomarkers for predicting therapeutic efficacy and for choosing individualized therapeutic strategies in patients with pancreatic cancer.
LncRNAs and tumor microenvironment
The complex microenvironment surrounding tumor cells includes inflammatory cells, fibroblasts, extracellular matrix, and small blood and lymphatic vessels. Emerging evidence supported that tumor stroma or tumor microenvironment influences the aggressiveness and drug resistance of cancer cells, thus accelerating the progression of carcinomas.Citation92–94
The desmoplastic stroma of pancreatic cancer results from the proliferation and differentiation of fibroblasts. The increase in fibrosis within the stroma associates with invasiveness and poor prognosis.Citation95–97 Cancer-associated fibroblast (CAF), which is one of the major components in the tumor stroma, plays a critical role in tumor growth and proliferation.Citation98 A new data in bladder cancer showed that lncRNA ZEB2NAT was upregulated by CAF-CM(Cancer-associated fibroblast condition medium) through secreted TGFβ1, and the knockdown of ZEB2NAT reversed CAF-CM induced ZEB2 protein level, which was a major transcription factor involved in EMT.Citation99,100 In another important study, CAF-secreted CXCL14 caused lncRNA LINC00092 expression alteration in ovarian cancer cells to promote cancer progression.Citation101 LINC00092 modulated glycolysis to promote ovarian cancer metastasis through directly interaction with PFKFB2, which was a key enzyme of glycolysis in cancer.Citation102 More interestingly, PFKFB2-induced glycolytic phenotype of ovarian cancer cells sustained the CAFs-like features of fibroblasts in turn, indicating a reciprocal feedback loop between CXCL14-positive CAFs and ovarian cancer cells. As for pancreatic cancer, evidence provided that expression of SHH(Sonic Hedgehog) or TGFβ which derived from cancer cells may contribute to the formation of desmoplasia that impeded proper drug delivery.Citation103,104
Tumor neovascularization is a another critical tumor-supportive element for the stroma, where tumor cells release proangiogenic signals to recruit endothelial cells to form the neovasculature, thereby obtaining nutrients and oxygen or evacuating metabolic wastes during tumor growth and metastasis.Citation105,106 lncRNAs have been evidenced to regulate angiogenic process recently. For example, Jia P and coworkers uncovered that H19 was up-regulated in microvessels from glioma tissues and over-expression of H19 promoted glioma-associated endothelial cell proliferation, migration and tube formation.Citation107 Other research found that the upregulated H19 CD90+ liver cancer cells could modulate endothelial cell phenotype through the release of exosomes, resulting in angiogenic phenotype.Citation108 Remarkably, a xenograft study revealedCitation109 that nutrient starvation-responsive lncRNA JHDM1D-AS1 overexpression increased pancreatic cancer growth in vivo, accompanied by elevated blood vessel formation and macrophage infiltration. Through investigating the expression of major pro- and anti-angiogenic factors by quantitative real-time PCR, they found that several pro-angiogenic factors such as HGF and FGF1 were significantly up-regulated in tumor xenografts comprising hJHDM1D-AS1-expressing PC cells compared to xenografts comprising of control cells. Although overexpression of JHDM1D-AS1 had minor effects on cell growth in vitro, the significant increase of tumor growth in vivo demonstrated that JHDM1D-AS1 affects the tumor microenvironment rather than the cancer cells themselves. These results provided us a new insight into the therapeutic approach for pancreatic cancer in the future.
It is proposed that the tumor microenvironment with selective inflammatory immune cells play important roles in promoting the malignant progression of many types of carcinomas.Citation110,111 In addition, tumor immune evasion is a hallmark of cancer progression.Citation112 There are many immunosuppressive mechanisms in the tumor microenvironment, programmed death receptor(PD-1) is found to be a major factor related to tumor immunity.Citation113,114 PD-1 is mainly expressed in tumor infiltrating lymphocytes (TILs) which inactivates tumor-reactive CTLs that directly target tumor cells for apoptosis.Citation115,116 A study demonstrated that the expression of lncRNA AFAP1-AS1 was positively correlated with PD-1 in nasopharyngeal carcinoma(NPC), and the co-expression of AFAP1-AS1 and PD-1 predicted high incidence of recurrence or metastasis among patients. It was reported that AFAP1-AS1 may indirectly regulate PD-1 expression and the AFAP1-AS1-PD-1 might be ideal candidates for future clinical trials of anti-PD-1 therapy.Citation117 In addition, long non-coding RNA AFAP1-AS1 could predict pancreatic cancer progression within 6 months and 1 y when the areas under receiver operating characteristic (ROC) curves of AFAP1-AS1 were 0.8669 and 0.9370 respectively.Citation118 Although researchers have reported that PD-1 was expressed in 51.2% to 52.1% of pancreatic tumor–infiltrating cytotoxic T lymphocytes,Citation119 the correlations between the PD-1 and AFAP1-AS1 or other tumor associated-lncRNAs in PDAC warranted in-depth study in the future.
Conclusion
Pancreatic cancer is known to have a poor prognosis, mostly due to lack of effective diagnosis at the early stage of tumor development and effective therapy. Although surgical resection of pancreatic cancers diagnosed at early stage remains the potentially curative option for PDAC, it is only possible in a small subset of patients.
More and more lncRNAs with differential expression in PDAC are discovered and most of them are major regulators in the multi-step process of cancer development from tumor initiation to metastasis. Some prominent functions of lncRNAs (such as HOTAIR, HOTTIP, MALAT1, and ROR) are associated with clinicopathologic characteristics of pancreatic cancer and may be potential tumor biomarkers of pancreatic cancer in the future. In-depth investigation on the molecular mechanisms of lncRNA activity is required to better understand the diverse function of lncRNAs. Tumor microenvironment is a driving force in pancreatic cancer progression. It is critical to elucidate the emerging pathophysiologic roles of lncRNA in the crosstalk between pancreatic cancer cells and tumor stroma. We believe that understanding the roles of lncRNAs would provide novel perspectives for developing new therapeutic agents and diagnostic biomarkers.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA CANCER J CLIN. 2017;67(1):7–30. doi:10.3322/caac.21387.
- Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447(7146):799–816. doi:10.1038/nature05874.
- Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F et al. Landscape of transcription in human cells. Nature. 2012;489(7414):101–108. doi:10.1038/nature11233.
- Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs. insights into functions. Nat Rev Genet. 2009;10(3):155–159. doi:10.1038/nrg2521.
- Engstrom PG, Suzuki H, Ninomiya N, Akalin A, Sessa L, Lavorgna G, Brozzi A, Luzi L, Tan SL, Yang L et al. Complex Loci in human and mouse genomes. PLoS Genet. 2006;2(4):e47. doi:10.1371/journal.pgen.0020047.
- Mercer TR, Wilhelm D, Dinger ME, Solda G, Korbie DJ, Glazov EA, Truong V, Schwenke M, Simons C, Matthaei KI et al. Expression of distinct RNAs from 3′ untranslated regions. Nucleic Acids Res. 2011;39(6):2393–2403. doi:10.1093/nar/gkq1158.
- Preker P, Nielsen J, Kammler S, Lykke-Andersen S, Christensen MS, Mapendano CK, Schierup MH, Jensen TH. RNA exosome depletion reveals transcription upstream of active human promoters. Science (New York, NY). 2008;322(5909):1851–1854. doi:10.1126/science.1164096.
- Hirano T, Yoshikawa R, Harada H, Harada Y, Ishida A, Yamazaki T. Long noncoding RNA, CCDC26, controls myeloid leukemia cell growth through regulation of KIT expression. Mol Cancer. 2015;14:90. doi:10.1186/s12943-015-0364-7.
- Yin YF, Yan PX, Lu JL, Song G, Zhu YY, Li ZH, Zhao Y, Shen B, Huang XX, Zhu H et al. Opposing Roles for the lncRNA Haunt and Its Genomic Locus in Regulating HOXA Gene Activation during Embryonic Stem Cell Differentiation. Cell Stem Cell. 2015;16(5):504–516. doi:10.1016/j.stem.2015.03.007.
- Kong R, Zhang EB, Yin DD, You LH, Xu TP, Chen WM, Xia R, Wan L, Sun M, Wang ZX et al. Long noncoding RNA PVT1 indicates a poor prognosis of gastric cancer and promotes cell proliferation through epigenetically regulating p15 and p16. Mol Cancer. 2015;14:82. doi:10.1186/s12943-015-0355-8.
- Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi:10.1186/1476-4598-10-38.
- Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer discovery. 2011;1(5):391–407. doi:10.1158/2159-8290.CD-11-0209.
- Qi P, Du X. The long non-coding RNAs, a new cancer diagnostic and therapeutic gold mine. Mod Pathol: an official journal of the United States and Canadian Academy of Pathology, Inc. 2013;26(2):155–165. doi:10.1038/modpathol.2012.160.
- Li J, Chen Z, Tian L, Zhou C, He MY, Gao Y, Wang S, Zhou F, Shi S, Feng X et al. LncRNA profile study reveals a three-lncRNA signature associated with the survival of patients with oesophageal squamous cell carcinoma. Gut. 2014;63(11):1700–1710. doi:10.1136/gutjnl-2013-305806.
- Zeng S, Xiao YF, Tang B, Hu CJ, Xie R, Yang SM, Li BS. Long Noncoding RNA in Digestive Tract Cancers: Function, Mechanism, and Potential Biomarker. Oncologist. 2015;20(8):898–906. doi:10.1634/theoncologist.2014-0475.
- Peng W, Gao W, Feng JF. Long noncoding RNA HULC is a novel biomarker of poor prognosis in patients with pancreatic cancer. Medical Oncology. 2014;31(12). doi:10.1007/s12032-014-0346-4.
- Lu XX, Fang Y, Wang ZT, Xie JJ, Zhan Q, Deng XX, Chen H, Jin JB, Peng CH, Li HW et al. Downregulation of gas5 increases pancreatic cancer cell proliferation by regulating CDK6. Cell Tissue Res. 2013;354(3):891–896. doi:10.1007/s00441-013-1711-x.
- Fu XL, Liu DJ, Yan TT, Yang JY, Yang MW, Li J, Huo YM, Liu W, Zhang JF, Hong J et al. Analysis of long non-coding RNA expression profiles in pancreatic ductal adenocarcinoma. Scientific Reports. 2016;6:15. doi:10.1038/srep33535.
- Yu J, Li A, Hong SM, Hruban RH, Goggins M. MicroRNA alterations of pancreatic intraepithelial neoplasias. Clin Cancer Res: an official journal of the American Association for Cancer Research. 2012;18(4):981–992. doi:10.1158/1078-0432.CCR-11-2347.
- Xing CY, Hu XQ, Xie FY, Yu ZJ, Li HY, Bin Z, Wu JB, Tang LY, Gao SM: Long non-coding RNA HOTAIR modulates c-KIT expression through sponging miR-193a in acute myeloid leukemia. Febs Letters. 2015;589(15):1981–1987. doi:10.1016/j.febslet.2015.04.061.
- Qu L, Ding J, Chen C, Wu ZJ, Liu B, Gao Y, Chen W, Liu F, Sun W, Li XF et al. Exosome-Transmitted lncARSR Promotes Sunitinib Resistance in Renal Cancer by Acting as a Competing Endogenous RNA. Cancer Cell. 2016;29(5):653–668. doi:10.1016/j.ccell.2016.03.004.
- Wang F, Ying HQ, He BS, Pan YQ, Deng QW, Sun HL, Chen J, Liu X, Wang SK. Upregulated lncRNA-UCA1 contributes to progression of hepatocellular carcinoma through inhibition of miR-216b and activation of FGFR1/ERK signaling pathway. Oncotarget. 2015;6(10):7899–7917. doi:10.18632/oncotarget.3219.
- Loewer S, Cabili MN, Guttman M, Loh YH, Thomas K, Park IH, Garber M, Curran M, Onder T, Agarwal S et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nature genetics. 2010;42(12):1113–1117. doi:10.1038/ng.710.
- Gao S, Wang P, Hua YQ, Xi H, Meng ZQ, Liu T, Chen Z, Liu LM. ROR functions as a ceRNA to regulate Nanog expression by sponging miR-145 and predicts poor prognosis in pancreatic cancer. Oncotarget. 2016;7(2):1608–1618. doi:10.18632/oncotarget.6450.
- Gabory A, Jammes H, Dandolo L. The H19 locus: role of an imprinted non-coding RNA in growth and development. BioEssays: news and reviews in molecular, cellular and developmental biology. 2010, 32(6):473–480. doi:10.1002/bies.200900170.
- Byun HM, Wong HL, Birnstein EA, Wolff EM, Liang G, Yang AS. Examination of IGF2 and H19 loss of imprinting in bladder cancer. Cancer Res. 2007;67(22):10753–10758. doi:10.1158/0008-5472.CAN-07-0329.
- Lottin S, Adriaenssens E, Dupressoir T, Berteaux N, Montpellier C, Coll J, Dugimont T, Curgy JJ: Overexpression of an ectopic H19 gene enhances the tumorigenic properties of breast cancer cells. Carcinogenesis. 2002;23(11):1885–1895. doi:10.1093/carcin/23.11.1885.
- Ma CC, Nong KT, Zhu HD, Wang WW, Huang XY, Yuan Z, Ai KX. H19 promotes pancreatic cancer metastasis by derepressing let-7′s suppression on its target HMGA2-mediated EMT. Tumor Biology. 2014;35(9):9163–9169. doi:10.1007/s13277-014-2185-5.
- Clemson CM, Hutchinson JN, Sara SA, Ensminger AW, Fox AH, Chess A, Lawrence JB. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Molecular cell. 2009;33(6):717–726. doi:10.1016/j.molcel.2009.01.026.
- Chakravarty D, Sboner A, Nair SS, Giannopoulou E, Li R, Hennig S, Mosquera JM, Pauwels J, Park K, Kossai M et al. The oestrogen receptor alpha-regulated lncRNA NEAT1 is a critical modulator of prostate cancer. Nat Commun. 2014;5:5383. doi:10.1038/ncomms6383.
- Huang B, Liu C, Wu Q, Zhang J, Min QH, Sheng TL, Wang XZ, Zou YQ. Long non-coding RNA NEAT1 facilitates pancreatic cancer progression through negative modulation of miR-506-3p. Biochem Biophys Res Commun. 2017;482(4):828–834. doi:10.1016/j.bbrc.2016.11.120.
- Deng Q, Xie L, Li H. MiR-506 suppresses cell proliferation and tumor growth by targeting Rho-associated protein kinase 1 in hepatocellular carcinoma. Biochem Biophys Res Commun. 2015;467(4):921–927. doi:10.1016/j.bbrc.2015.10.043.
- Yang H, Liu P, Zhang J, Peng X, Lu Z, Yu S, Meng Y, Tong WM, Chen J. Long noncoding RNA MIR31HG exhibits oncogenic property in pancreatic ductal adenocarcinoma and is negatively regulated by miR-193b. Oncogene. 2016;35(28):3647–3657. doi:10.1038/onc.2015.430.
- Gastaldi C, Bertero T, Xu N, Bourget-Ponzio I, Lebrigand K, Fourre S, Popa A, Cardot-Leccia N, Meneguzzi G, Sonkoly E et al. miR-193b/365a cluster controls progression of epidermal squamous cell carcinoma. Carcinogenesis. 2014;35(5):1110–1120. doi:10.1093/carcin/bgt490.
- Chen J, Zhang X, Lentz C, Abi-Daoud M, Pare GC, Yang X, Feilotter HE, Tron VA. miR-193b Regulates Mcl-1 in Melanoma. Am J Pathol. 2011, 179(5):2162–2168. doi:10.1016/j.ajpath.2011.07.010.
- Kranenburg O. The KRAS oncogene: past, present, and future. Biochim Bio Acta. 2005;1756(2):81–82.
- Zorde Khvalevsky E, Gabai R, Rachmut IH, Horwitz E, Brunschwig Z, Orbach A, Shemi A, Golan T, Domb AJ, Yavin E et al. Mutant KRAS is a druggable target for pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110(51):20723–20728. doi:10.1073/pnas.1314307110.
- Li X, Deng SJ, Zhu S, Jin Y, Cui SP, Chen JY, Xiang C, Li QY, He C, Zhao SF et al. Hypoxia-induced lncRNA-NUTF2P3–001 contributes to tumorigenesis of pancreatic cancer by derepressing the miR-3923/KRAS pathway. Oncotarget. 2016;7(5):6000–6014. doi:10.18632/oncotarget.6830.
- Cai H, Yao J, An Y, Chen X, Chen W, Wu D, Luo B, Yang Y, Jiang Y, Sun D et al. LncRNA HOTAIR acts a competing endogenous RNA to control the expression of notch3 via sponging miR-613 in pancreatic cancer. Oncotarget. 2017.
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi:10.1172/JCI39104.
- Grunert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat Rev Mol Cell Biol. 2003;4(8):657–665. doi:10.1038/nrm1175.
- Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17(5):548–558. doi:10.1016/j.ceb.2005.08.001.
- Pan Y, Chen J, Tao L, Zhang K, Wang R, Chu X, Chen L. Long noncoding RNA ROR regulates chemoresistance in docetaxel-resistant lung adenocarcinoma cells via epithelial mesenchymal transition pathway. Oncotarget. 2017;8(20):33144–33158.
- Wu J, Zhang J, Shen B, Yin K, Xu J, Gao W, Zhang L. Long noncoding RNA lncTCF7, induced by IL-6/STAT3 transactivation, promotes hepatocellular carcinoma aggressiveness through epithelial-mesenchymal transition. J Exp Clin Cancer Res: CR. 2015;34:116. doi:10.1186/s13046-015-0229-3.
- Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y, Lajoie BR, Protacio A, Flynn RA, Gupta RA et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472(7341):120–124. doi:10.1038/nature09819.
- Li ZH, Zhao XH, Zhou Y, Liu YM, Zhou QB, Ye HL, Wang YX, Zeng JL, Song YD, Gao WC et al. The long non-coding RNA HOTTIP promotes progression and gemcitabine resistance by regulating HOXA13 in pancreatic cancer. J of Transl Med. 2015;13:16. doi:10.1186/s12967-015-0442-z.
- Ji P, Diederichs S, Wang W, Boing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22(39):8031–8041. doi:10.1038/sj.onc.1206928.
- Jiao F, Hu H, Yuan CC, Wang L, Jiang WH, Jin ZL, Guo Z, Wang LW. Elevated expression level of long noncoding RNA MALAT-1 facilitates cell growth, migration and invasion in pancreatic cancer. Oncology Reports. 2014;32(6):2485–2492. doi:10.3892/or.2014.3518.
- Han T, Jiao F, Hu H, Yuan C, Wang L, Jin ZL, Song WF, Wang LW. EZH2 promotes cell migration and invasion but not alters cell proliferation by suppressing E-cadherin, partly through association with MALAT-1 in pancreatic cancer. Oncotarget. 2016;7(10):11194–11207. doi:10.18632/oncotarget.7156.
- Wang C, Liu X, Chen Z, Huang H, Jin Y, Kolokythas A, Wang A, Dai Y, Wong DT, Zhou X. Polycomb group protein EZH2-mediated E-cadherin repression promotes metastasis of oral tongue squamous cell carcinoma. Mol Carcinog. 2013;52(3):229–236. doi:10.1002/mc.21848.
- Zheng S, Chen H, Wang Y, Gao W, Fu Z, Zhou Q, Jiang Y, Lin Q, Tan L, Ye H et al. Long non-coding RNA LOC389641 promotes progression of pancreatic ductal adenocarcinoma and increases cell invasion by regulating E-cadherin in a TNFRSF10A-related manner. Cancer letters. 2016;371(2):354–365. doi:10.1016/j.canlet.2015.12.010.
- Zhan HX, Wang Y, Li C, Xu JW, Zhou B, Zhu JK, Han HF, Wang L, Wang YS, Hu SY. LincRNA-ROR promotes invasion, metastasis and tumor growth in pancreatic cancer through activating ZEB1 pathway. Cancer letters. 2016;374(2):261–271. doi:10.1016/j.canlet.2016.02.018.
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi:10.1038/35102167.
- Wang Y, Xu Z, Jiang J, Xu C, Kang J, Xiao L, Wu M, Xiong J, Guo X, Liu H. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev Cell. 2013;25(1):69–80. doi:10.1016/j.devcel.2013.03.002.
- Zhou X, Gao Q, Wang J, Zhang X, Liu K, Duan Z. Linc-RNA-RoR acts as a “sponge” against mediation of the differentiation of endometrial cancer stem cells by microRNA-145. Gynecol Oncol. 2014;133(2):333–339. doi:10.1016/j.ygyno.2014.02.033.
- Peng F, Li TT, Wang KL, Xiao GQ, Wang JH, Zhao HD, Kang ZJ, Fan WJ, Zhu LL, Li M et al. H19/let-7/LIN28 reciprocal negative regulatory circuit promotes breast cancer stem cell maintenance. Cell Death Dis. 2017;8(1):e2569. doi:10.1038/cddis.2016.438.
- Di Cecilia S, Zhang F, Sancho A, Li S, Aguilo F, Sun Y, Rengasamy M, Zhang W, Del Vecchio L, Salvatore F et al. RBM5-AS1 Is Critical for Self-Renewal of Colon Cancer Stem-like Cells. Cancer research. 2016;76(19):5615–5627. doi:10.1158/0008-5472.CAN-15-1824.
- Lee CJ, Dosch J, Simeone DM. Pancreatic cancer stem cells. J Clin Oncol: official journal of the American Society of Clinical Oncology. 2008;26(17):2806–2812. doi:10.1200/JCO.2008.16.6702.
- Kim MP, Fleming JB, Wang H, Abbruzzese JL, Choi W, Kopetz S, McConkey DJ, Evans DB, Gallick GE. ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PLoS One. 2011;6(6):e20636. doi:10.1371/journal.pone.0020636.
- Maeda S, Shinchi H, Kurahara H, Mataki Y, Maemura K, Sato M, Natsugoe S, Aikou T, Takao S. CD133 expression is correlated with lymph node metastasis and vascular endothelial growth factor-C expression in pancreatic cancer. Br J Cancer. 2008, 98(8):1389–1397. doi:10.1038/sj.bjc.6604307.
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–1037. doi:10.1158/0008-5472.CAN-06-2030.
- Jiao F, Hu H, Han T, Yuan CC, Wang L, Jin ZL, Guo Z, Wang LW. Long Noncoding RNA MALAT-1 Enhances Stem Cell-Like Phenotypes in Pancreatic Cancer Cells. Int J Mol Sci. 2015;16(4):6677–6693. doi:10.3390/ijms16046677.
- Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8(3):180–192.
- Koong AC, Mehta VK, Le QT, Fisher GA, Terris DJ, Brown JM, Bastidas AJ, Vierra M. Pancreatic tumors show high levels of hypoxia. Int J Radi Oncol, Biol, Phy. 2000;48(4):919–922. doi:10.1016/S0360-3016(00)00803-8.
- LaGory EL, Giaccia AJ. The ever-expanding role of HIF in tumour and stromal biology. Nat Cell Biol. 2016;18(4):356–365. doi:10.1038/ncb3330.
- Erickson LA, Highsmith WE, Jr., Fei P, Zhang J. Targeting the hypoxia pathway to treat pancreatic cancer. Drug Des, Devel Ther. 2015;9:2029–2031.
- Zhu G, Wang S, Chen J, Wang Z, Liang X, Wang X, Jiang J, Lang J, Li L. Long noncoding RNA HAS2-AS1 mediates hypoxia-induced invasiveness of oral squamous cell carcinoma. Mol Carcinog. 2017;56(10):2210–2222. doi:10.1002/mc.22674.
- Sun YW, Chen YF, Li J, Huo YM, Liu DJ, Hua R, Zhang JF, Liu W, Yang JY, Fu XL et al. A novel long non-coding RNA ENST00000480739 suppresses tumour cell invasion by regulating OS-9 and HIF-1 alpha in pancreatic ductal adenocarcinoma. Br J Cancer. 2014;111(11):2131–2141. doi:10.1038/bjc.2014.520.
- Roberts TC, Morris KV, Weinberg MS. Perspectives on the mechanism of transcriptional regulation by long non-coding RNAs. Epigenetics. 2014;9(1):13–20. doi:10.4161/epi.26700.
- Faghihi MA, Wahlestedt C. Regulatory roles of natural antisense transcripts. Nat Rev Mol Cell Biol. 2009;10(9):637–643. doi:10.1038/nrm2738.
- Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Nat Acad Sci U S A. 2009;106(28):11667–11672. doi:10.1073/pnas.0904715106.
- Takayama K, Suzuki T, Tsutsumi S, Fujimura T, Urano T, Takahashi S, Homma Y, Aburatani H, Inoue S. RUNX1, an androgen- and EZH2-regulated gene, has differential roles in AR-dependent and -independent prostate cancer. Oncotarget. 2015;6(4):2263–2276. doi:10.18632/oncotarget.2949.
- Wang HF, Yang H, Hu LB, Lei YH, Qin Y, Li J, Bi CW, Wang JS, Huo Q. Effect of siRNA targeting EZH2 on cell viability and apoptosis of bladder cancer T24 cells. Genet Mol Res: GMR. 2014;13(4):9939–9950. doi:10.4238/2014.November.27.23.
- Abbosh PH, Montgomery JS, Starkey JA, Novotny M, Zuhowski EG, Egorin MJ, Moseman AP, Golas A, Brannon KM, Balch C et al. Dominant-negative histone H3 lysine 27 mutant derepresses silenced tumor suppressor genes and reverses the drug-resistant phenotype in cancer cells. Cancer Res. 2006;66(11):5582–5591. doi:10.1158/0008-5472.CAN-05-3575.
- Terashima M, Tange S, Ishimura A, Suzuki T. MEG3 Long Noncoding RNA Contributes to the Epigenetic Regulation of Epithelial-Mesenchymal Transition in Lung Cancer Cell Lines. J Biol Chem. 2017;292(1):82–99. doi:10.1074/jbc.M116.750950.
- Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY. Long noncoding RNA as modular scaffold of histone modification complexes. Science (New York, NY). 2010;329(5992):689–693. doi:10.1126/science.1192002.
- Li CH, Xiao ZG, Tong JHM, To KF, Fang XD, Cheng ASL, Chen YC. EZH2 coupled with HOTAIR to silence MicroRNA-34a by the induction of heterochromatin formation in human pancreatic ductal adenocarcinoma. Int J Cancer. 2017;140(1):120–129. doi:10.1002/ijc.30414.
- Cai H, An Y, Chen X, Sun D, Chen T, Peng Y, Zhu F, Jiang Y, He X. Epigenetic inhibition of miR-663b by long non-coding RNA HOTAIR promotes pancreatic cancer cell proliferation via up-regulation of insulin-like growth factor 2. Oncotarget. 2016;7(52):86857–86870.
- Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R, Kim S, Safe S. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32(13):1616–1625. doi:10.1038/onc.2012.193.
- Cheng YT, Jutooru I, Chadalapaka G, Corton JC, Safe S. The long non-coding RNA HOTTIP enhances pancreatic cancer cell proliferation, survival and migration. Oncotarget. 2015;6(13):10840–10852. doi:10.18632/oncotarget.3450.
- Feng J, Sun Y, Zhang EB, Lu XY, Jin SD, Guo RH. A novel long noncoding RNA IRAIN regulates cell proliferation in non small cell lung cancer. Int J Clin Exp Pathol. 2015;8(10):12268–12275.
- Lian YF, Wang J, Feng J, Ding J, Ma ZH, Li J, Peng P, De W, Wang KM. Long non-coding RNA IRAIN suppresses apoptosis and promotes proliferation by binding to LSD1 and EZH2 in pancreatic cancer. Tumor Biol. 2016;37(11):14929–14937. doi:10.1007/s13277-016-5380-8.
- Xu TP, Liu XX, Xia R, Yin L, Kong R, Chen WM, Huang MD, Shu YQ. SP1-induced upregulation of the long noncoding RNA TINCR regulates cell proliferation and apoptosis by affecting KLF2 mRNA stability in gastric cancer. Oncogene. 2015;34(45):5648–5661. doi:10.1038/onc.2015.18.
- Wolff L, Bies J. p15Ink4b Functions in determining hematopoietic cell fates: implications for its role as a tumor suppressor. Blood Cells, Mol Dis. 2013, 50(4):227–231. doi:10.1016/j.bcmd.2013.01.006.
- O'Leary VB, Ovsepian SV, Carrascosa LG, Buske FA, Radulovic V, Niyazi M, Moertl S, Trau M, Atkinson MJ, Anastasov N. PARTICLE, a Triplex-Forming Long ncRNA, Regulates Locus-Specific Methylation in Response to Low-Dose Irradiation. Cell Reports. 2015;11(3):474–485. doi:10.1016/j.celrep.2015.03.043.
- Liu Y, Xu N, Liu B, Huang Y, Zeng H, Yang Z, He Z, Guo H. Long noncoding RNA RP11–838N2.4 enhances the cytotoxic effects of temozolomide by inhibiting the functions of miR-10a in glioblastoma cell lines. Oncotarget. 2016;7(28):43835–43851. doi:10.18632/oncotarget.9699.
- Izumiya M, Kabashima A, Higuchi H, Igarashi T, Sakai G, Iizuka H, Nakamura S, Adachi M, Hamamoto Y, Funakoshi S et al. Chemoresistance is associated with cancer stem cell-like properties and epithelial-to-mesenchymal transition in pancreatic cancer cells. Anticancer Res. 2012;32(9):3847–3853.
- You L, Chang D, Du HZ, Zhao YP. Genome-wide screen identifies PVT1 as a regulator of Gemcitabine sensitivity in human pancreatic cancer cells. Biochem Biophy Res Commun. 2011;407(1):1–6. doi:10.1016/j.bbrc.2011.02.027.
- Yang YR, Zang SZ, Zhong CL, Li YX, Zhao SS, Feng XJ. Increased expression of the lncRNA PVT1 promotes tumorigenesis in non-small cell lung cancer. Int J Clin Exp Pathol. 2014;7(10):6929–6935.
- Fang XY, Pan HF, Leng RX, Ye DQ. Long noncoding RNAs: novel insights into gastric cancer. Cancer lett. 2015;356(2 Pt B):357–366. doi:10.1016/j.canlet.2014.11.005.
- Zhang XW, Bu P, Liu L, Zhang XZ, Li J. Overexpression of long non-coding RNA PVT1 in gastric cancer cells promotes the development of multidrug resistance. Biochem Biophy Res Commun. 2015;462(3):227–232. doi:10.1016/j.bbrc.2015.04.121.
- Fang H, Declerck YA. Targeting the tumor microenvironment: from understanding pathways to effective clinical trials. Cancer Res. 2013;73(16):4965–4977. doi:10.1158/0008-5472.CAN-13-0661.
- Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5(15):1597–1601. doi:10.4161/cc.5.15.3112.
- Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411(6835):375–379. doi:10.1038/35077241.
- Kuniyasu H, Abbruzzese JL, Cleary KR, Fidler IJ. Induction of ductal and stromal hyperplasia by basic fibroblast growth factor produced by human pancreatic carcinoma. Int J Oncol. 2001;19(4):681–685.
- Hartel M, Di Mola FF, Gardini A, Zimmermann A, Di Sebastiano P, Guweidhi A, Innocenti P, Giese T, Giese N, Buchler MW et al. Desmoplastic reaction influences pancreatic cancer growth behavior. World J Surg. 2004;28(8):818–825. doi:10.1007/s00268-004-7147-4.
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi:10.1038/nrc1877.
- McAllister SS, Weinberg RA. Tumor-host interactions: a far-reaching relationship. J Clin Oncol: official journal of the American Society of Clinical Oncology. 2010;28(26):4022–4028. doi:10.1200/JCO.2010.28.4257.
- Zhuang J, Lu Q, Shen B, Huang X, Shen L, Zheng X, Huang R, Yan J, Guo H. TGFbeta1 secreted by cancer-associated fibroblasts induces epithelial-mesenchymal transition of bladder cancer cells through lncRNA-ZEB2NAT. Sci Rep. 2015;5:11924. doi:10.1038/srep11924.
- Nelles L, Van de Putte T, van Grunsven L, Huylebroeck D, Verschueren K. Organization of the mouse Zfhx1b gene encoding the two-handed zinc finger repressor Smad-interacting protein-1. Genomics. 2003;82(4):460–469. doi:10.1016/S0888-7543(03)00169-1.
- Zhao L, Ji G, Le X, Wang C, Xu L, Feng M, Zhang Y, Yang H, Xuan Y, Yang Y et al. Long Noncoding RNA LINC00092 Acts in Cancer-Associated Fibroblasts to Drive Glycolysis and Progression of Ovarian Cancer. Cancer Res. 2017;77(6):1369–1382. doi:10.1158/0008-5472.CAN-16-1615.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011, 144(5):646–674. doi:10.1016/j.cell.2011.02.013.
- Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, Ouellette MM, Hollingsworth MA. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res: an official journal of the American Association for Cancer Research. 2008;14(19):5995–6004. doi:10.1158/1078-0432.CCR-08-0291.
- Lohr M, Schmidt C, Ringel J, Kluth M, Muller P, Nizze H, Jesnowski R. Transforming growth factor-beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 2001;61(2):550–555.
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi:10.1038/35025220.
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–974. doi:10.1038/nature04483.
- Jia P, Cai H, Liu X, Chen J, Ma J, Wang P, Liu Y, Zheng J, Xue Y. Long non-coding RNA H19 regulates glioma angiogenesis and the biological behavior of glioma-associated endothelial cells by inhibiting microRNA-29a. Cancer lett. 2016;381(2):359–369. doi:10.1016/j.canlet.2016.08.009.
- Conigliaro A, Costa V, Lo Dico A, Saieva L, Buccheri S, Dieli F, Manno M, Raccosta S, Mancone C, Tripodi M et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol Cancer. 2015;14:155. doi:10.1186/s12943-015-0426-x.
- Kondo A, Nonaka A, Shimamura T, Yamamoto S, Yoshida T, Kodama T, Aburatani H, Osawa T. Long Noncoding RNA JHDM1D-AS1 Promotes Tumor Growth by Regulating Angiogenesis in Response to Nutrient Starvation. Mol Cell Biol. 2017;37(18). doi:10.1128/MCB.00125-17.
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. doi:10.1038/nrc1782.
- Ruffell B, DeNardo DG, Affara NI, Coussens LM. Lymphocytes in cancer development: polarization towards pro-tumor immunity. Cytokine Growth Factor Rev. 2010;21(1):3–10. doi:10.1016/j.cytogfr.2009.11.002.
- Noh KH, Kim BW, Song KH, Cho H, Lee YH, Kim JH, Chung JY, Kim JH, Hewitt SM, Seong SY et al. Nanog signaling in cancer promotes stem-like phenotype and immune evasion. J Clin Invest. 2012;122(11):4077–4093. doi:10.1172/JCI64057.
- Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol (Orlando, Fla). 2014;153(1):145–152. doi:10.1016/j.clim.2014.04.010.
- Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139(3):667–676. doi:10.1007/s10549-013-2581-3.
- Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res: an official journal of the American Association for Cancer Research. 2007;13(6):1757–1761. doi:10.1158/1078-0432.CCR-06-2599.
- Chapon M, Randriamampita C, Maubec E, Badoual C, Fouquet S, Wang SF, Marinho E, Farhi D, Garcette M, Jacobelli S et al. Progressive upregulation of PD-1 in primary and metastatic melanomas associated with blunted TCR signaling in infiltrating T lymphocytes. J Invest Dermatol. 2011;131(6):1300–1307. doi:10.1038/jid.2011.30.
- Tang Y, He Y, Shi L, Yang L, Wang J, Lian Y, Fan C, Zhang P, Guo C, Zhang S et al. Co-expression of AFAP1-AS1 and PD-1 predicts poor prognosis in nasopharyngeal carcinoma. Oncotarget. 2017;8(24):39001–39011.
- Ye Y, Chen J, Zhou Y, Fu Z, Zhou Q, Wang Y, Gao W, Zheng S, Zhao X, Chen T et al. High expression of AFAP1-AS1 is associated with poor survival and short-term recurrence in pancreatic ductal adenocarcinoma. J Transl Med. 2015;13:137. doi:10.1186/s12967-015-0490-4.
- Lu C, Paschall AV, Shi H, Savage N, Waller JL, Sabbatini ME, Oberlies NH, Pearce C, Liu K. The MLL1-H3K4me3 Axis-Mediated PD-L1 Expression and Pancreatic Cancer Immune Evasion. JNCI-J Natl Cancer Inst. 2017;109(6):12. doi:10.1093/jnci/djw283.
