ABSTRACT
Topoisomerase 1 (TOPO-1) and carboxylesterase 2 (CES-2) are found to play crucial roles in the pathogenesis of various cancers. The prognostic role of TOPO-1 and CES-2 in patients with metastatic colorectal cancer (mCRC) who underwent irinotecan chemotherapy was largely unknown. In the current study, we assessed the expression of TOPO-1 and CES-2 in mCRC and analyzed its potential relevance to irinotecan based therapy. A total of 98 patients with mCRC were included in this study. The expression of TOPO-1 and CES-2 in mCRC tissues was evaluated by immunohistochemistry. For TOPO-1, 46 patients showed high expression and 52 patients showed low expression. For CES-2, 53 patients showed high expression and 45 patients showed low expression. The correlation between TOPO-1 or CES-2 expression and clinicopathological characteristics of mCRC patients was analyzed. Neither TOPO-1 nor CES-2 had significant correlation with age, gender, tumor site, tumor grade and metastatic sites in mCRC patients. However, high expression of CES-2 but not TOP-1 was positively correlated with better curative effect. Kaplan-Meier and log-rank test were applied to assess the correlation between progression-free survival (PFS)/overall survival (OS) and TOPO-1 or CES-2 expression in mCRC patients. High expression of TOPO-1 and CES-2 are correlated with longer PFS and OS. In summary, our findings suggest that TOPO-1 and CES-2 may play important roles irinotecan sensitivity in mCRC patients. Evaluation of expression of TOPO-1 and CES-2 may provide preliminary clinical evidence for the management of irinotecan-based therapy in mCRC patients.
Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors in the gastrointestinal tract.Citation1 About 50% CRC patients received surgical treatment when diagnosed. However, a large proportion of CRC patients received surgical treatment had local recurrence and distant metastasis.Citation2 For the patients with late-stage CRC, chemotherapy was commonly employed to improve patient survival and the quality of life.Citation3 Due to the differential gene expression profiles in individual patients, there was a large difference in terms of sensitivity to the same chemotherapeutic regimens among different patients.Citation3 Therefore, it is necessary to find out the differential gene expression related to chemo-sensitivity, which may help us to determine individualized chemotherapeutic strategies.
Irinotecan is a novel water soluble camptothecin derivative selected for clinical use in primary and chemo-resistant tumors based on various in vitro and in vivo experimental results,Citation4 Irinotecan is activated by hydrolysis via carboxylesterase (CES) to SN-38, an inhibitor of topoisomerase 1 (TOPO-1).Citation5The inhibition of TOPO-1 by the active SN-38 eventually leads to inhibition of both DNA replication and transcription, which in turn exerts its cytotoxic effects.Citation6 CESs are mainly distributed in the cytoplasm and endoplasmic reticulum and are important for drug metabolism. CESs are regulated by various nuclear receptors.Citation7 In humans, two carboxylesterases, CES-1 and CES-2 are identified, and both are expressed in the liver, but levels of CES-1 greatly exceed those of CES-2. In the intestine, only high levels of CES-2 are expressed, and CES-2 was also found to be more important in activating irinotecan than CES-1..Citation8Studies also demonstrated that CES-2 expression is correlated with irinotecan hydrolysis in CRC. CES-2 is important for the activation of irinotecan in various types of tumors. Studies have shown that CES-2 was up-regulated in tumor tissues and served as a biomarker for predicting the chemotherapeutic effect of irinotecan and prognosis, particularly in pancreatic cancer, neuroblastoma, non-small cell lung cancer and small cell lung cancer.Citation9-11 However, in CRC, the role of CES-2 for predicting the chemotherapeutic effect of irinotecan and prognosis has not been reported. TOPO-1 is an important nuclear enzyme, which involves diverse processes such as DNA replication, transcription, recombination, and repair. The increased activity of TOPO-1 was found in a large number of cancers, including ovarian cancer, breast cancer and lung cancer.Citation12–15 In this regard, the anti-tumor effect of irinotecan may be associated with the expression of CES-2 and TOPO-1.
In the present study, we determined the expression of TOPO-1 and CES-2 in mCRC and examined if expression of TOPO-1 and CES-2 were correlated with chemotherapeutic effect of irinotecan and prognosis in mCRC patients. This study will provide clinical preliminary evidence for application of irinotecan in mCRC patients.
Materials and methods
Patients and clinical samples
CRC specimens were collected from mCRC patients who underwent resection for curative intent at the Fourth Affiliated Hospital of Guangxi Medical University from January 1, 2014 to December 31, 2014. All the patients provided written informed consent under the approval of the ethics committee of the Fourth Affiliated Hospital of Guangxi Medical University. Clinicopathological characteristics including age, gender, tumor site, tumor grade and metastatic sites were obtained from a clinical data base maintained by the Department of Oncology of the Fourth Affiliated Hospital of Guangxi Medical University.
Immunohistochemical analysis
All the CRC samples were collected from mCRC patients without any preoperative therapies. The immunohistochemical staining for TOPO-1 and CES-2 markers in CRC tissues was performed according to previous protocols.Citation16, Citation17 Stained slides for TOPO-1 and CES-2 expression were reviewed by two pathologists. For TOPO-1 grading, a score for intensity and distribution of nuclear staining was assigned according to a 4-tier system. The intensity ranged from 0 to 3 (0 = no staining, 1 = weakly positive, 2 = moderately positive, and 3 = strongly positive staining). The staining distribution considered the percentage of positive tumor cells and ranged from 0 to 3 (0 = 0 to 5%, 1 = 6% to 25%, 2 = 26% to 50%, 3 = 51% to 100%). An overall TOPO-1 expression score was calculated as the sum of the intensity and distribution scores in each case. Cases with a total score of at least 4 were considered high expression tumors (with altered pattern), whereas cases with a total score of 0–3 were considered negative or low expression tumors (with normal pattern). For CES-2 grading, high CES-2 expression: positive CES-2-stained cells are greater than 25%; low CES-2 expression: positive CES-2-stained cells are less than 25%.
Irinotecan based therapy for mCRC patients and follow up study
All the included patients completed at least four cycles of FOLFIRINOX treatment (oxiplatin 75 mg/m2 d1 + irinotecan 150 mg/m2 d1 + 5-FU 2000 mg/m2 46 h CI). The therapeutic effects were followed up every two weeks. After completing 4 cycles of FOLFIRINOX treatment, all the mCRC patients were subjected to radiographic evaluation. The recent curative effect of FOLFIRINOX treatment was evaluated using to the Response assessment in solid tumours (RECIST) 1.1 criteria. The overall response was divided into complete response (CR), partial response (PR), stable diseases (SD) and progression diseases (PD). Response rate (RR) = (CR + PR)/Total number of included patients. Progression-free survival (PFS) was defined as the time elapsed between treatment initiation and tumor recurrence or progression or death with censoring of patients who are lost to follow-up. Overall survival (OS) was defined as time elapsed between treatment initiation and death with censoring of patients who are lost to follow up. The cut-off date for the follow-up study was March 20, 2016.
Statistical analysis
All the statistical analysis was analyzed by using the GraphPad Prism (Version 6.0; GraphPad Software Inc., San Diego, CA). Categorical data was analyzed by Chi-square test. PFS curves were constructed using the Kaplan-Meier method. The log-rank test was used to evaluate the significant differences. Receiver operating characteristics (ROC) curves were constructed to evaluate the diagnostic values of TOPO-1 and CES-2, and p values-associated area under curve was computed by using the trapezoid rule. All hypothesis tests were two-sided, and P value less than 0.05 was considered to be statistically significant.
Results
Patient characteristics
A total of 105 patients from the Fourth Affiliated Hospital of Guangxi Medical University were recruited in this study, and all the patients were diagnosed with mCRC by pathological and radiographic examination. There were 5 patients who did not complete the chemotherapy and 2 patients who suffered from a second type of cancer. These 7 patients were excluded from the study, and therefore 98 patients were finally included in the analysis. All the 98 patients were followed up until March 20, 2016. summarized the primary clinicopathological characteristics of these patients.
Table 1. The primary characteristics of 98 patients with mCRC.
Immunohistochemical analysis of TOPO-1 and CES-2 expression in CRC tissues
Immunohistochemical staining showed positive staining of TOPO-1 localized in the nucleus of the CRC cells. The tumor specimens showed various levels of expression, with some tissues showed high expression of TOPO-1 () and some showed low expression of TOPO-1 (). The positive immunostaining of CES-2 was localized in the cell membrane and cytoplasm of the CRC cells. Similarly, some tumor tissues showed high expression of CES-2 (), and some showed low expression of CES-2 (). Using the median value of expression as a cut-off point, quantitative analysis of the staining indicated that 46 (46.94%) patients had high expression of TOPO-1, and 52 patients had low expression of TOPO-1 (43.06%). For CES-2, 53 (54.08%) patients had high expression and 45 (45.92%) patients had low expression. The correlation analysis showed that there was no significant association between TOPO-1 and CES-2 expression in CRC tissues ().
Figure 1. Immunohistochemistry of TOPO-1 in CRC tissues. (A) High expression of TOOPO-1. (B) Low expression of TOPO1. Scale bar = 100 µm.
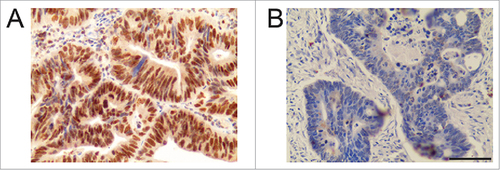
Figure 2. Immunohistochemistry of CES-2 in CRC tissues. (A) High expression of CES-2. (B) Low expression of CES-2. Scale bar = 100 µm.
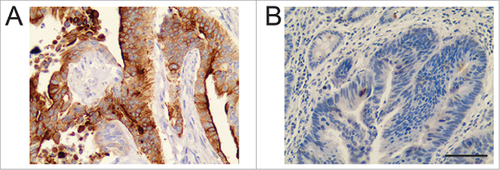
Table 2. The correlation of TOPO-1 and CES-2 expression in patients.
The correlation of the expression of TOPO-1 or CES-2 and clinicopathological characteristics in mCRC patients
We examined the differential expression of TOPO-1 or CES-2 and their correlation with the clinicopathological characteristics in mCRC patients. As shown in , there is no statistically significant association of TOPO-1 expression with age, gender, tumor site, tumor grade and metastatic sites. Similarly, no significant correlation was found in CES-2 expression with age, gender, tumor site, tumor grade and metastatic sites ().
Table 3. The correlation between TOPO-1 expression and clinicopathological characteristics in mCRC patients.
Table 4. The correlation between CES-2 expression and clinicopathological characteristics in mCRC patients.
The relationship between the TOPO-1 or CES-2 expression and the irinotecan curative effect in mCRC patients
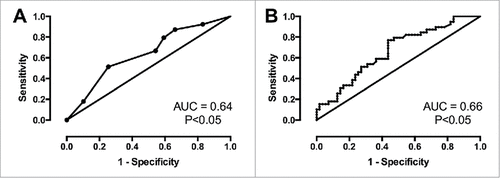
In the following-up of irinotecan treatment in these 98 mCRC patients, 4 patients achieved CR, 35 patients had PR, 33 patients had SD, and 26 patients had PD. The RR was 39.80%. As shown in , the RR in mCRC patients with high TOPO-1 expression was higher than that with low TOPO-1 expression, but was not statistically significant (P = 0.052). The RR in high CES-2 expression group was 49.06%, and the RR in the low CES-2 expression group was 28.89%, and there was a significantly difference in RR between these two groups (, P = 0.042). These results suggested that the high expression of TOPO-1 or CES-2 predicts higher RR in mCRC patients. The ROC curves for TOPO-1 and CES-2 were also constructed. The AUC for TOPO-1 curve was 0.641 with 0.6667 (95% confidence interval (CI): 0.4978–0.8091) sensitivity and 0.4576 specificity (95% CI: 0.3272–0.5925) when the cut-off values was 3.5 (, P = 0.019); the AUC for CES-2 curve was 0.6648 with 0.5897 (95% CI: 0.421–0.744) sensitivity and 0.5636 (95% CI: 0.4232–0.6970) specificity when the cut-off value was 24.55% (, P = 0.0067).
Table 5. The correlation of TOPO-1 and CES-2 expression in mCRC with curative effect.
Table 6. Compare the expression of TOPO-1, CES-2 in mCRC and the curative effect.
The correlation between TOPO-1 or CES-2 expression and PFS in mCRC patients
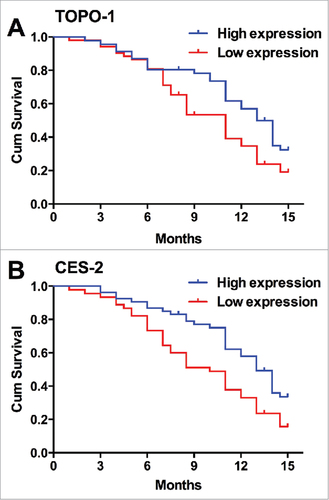
Based on the follow-up study, we constructed the Kaplan-Meier curves for PFS in mCRC patients with correlation with TOPO-1 or CES-2 expression in CRC tissues. Among all the patients, 5 patients progressed and 69 patients died. The patients with the high expression of TOPO-1 in CRC tissues had higher PFS than that with the low expression of TOPO-1 in CRC tissues (Median PFS: high expression, 14 months vs. low expression, 10 months, , P = 0.0101). Similarly, the patients with the high expression of CES-2 in CRC tissues also had higher PFS than that with the low expression of CES-2 in CRC tissues (Median PFS: high expression, 13 months vs. low expression, 9 months, , P = 0.046). The Kaplan-Meier curves for OS was also constructed based on the follow-up study, and consistently, patients with high expression of TOPO-1 or CES-2 in CRC tissues had higher OS than that with low expression of TOPO-1 (, P = 0.0383) or CES-2 (, P = 0.0077) in CRC tissues. These results suggest that the low expression of TOPO-1 or CES predicts poor prognosis in mCRC patients.
Figure 4. The relationship between TOPO-1 expression/CES-2 expression and the PFS in patients with mCRC. (A) Kaplan-Meier curves for PFS in mCRC patients correlation with TOPO-1 expression in CRC tissues (P = 0.00101). (B) Kaplan-Meier curves for PFS in mCRC patients correlation with CES-2 expression in CRC tissues (P = 0.046).
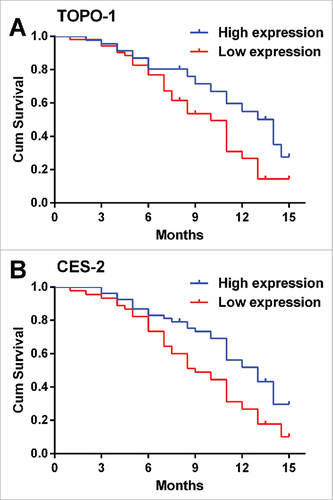
Figure 5. The relationship between TOPO-1 expression/CES-2 expression and the OS in patients with mCRC. (A) Kaplan-Meier curves for OS in mCRC patients correlation with TOPO-1 expression in CRC tissues (P = 0.00273). (B) Kaplan-Meier curves for OS in mCRC patients correlation with CES-2 expression in CRC tissues (P = 0.0077).
Discussion
Based on the cancer statistics 2016, CRC accounts for approximately 10% of all types of malignant tumors worldwide..Citation18In the last two decades, the incidence of CRC ranked the second among all the gastrointestinal malignant tumors.Citation18Among all the diagnosed CRC cases, 40–50% cases were at early stage, which can be cured by surgical resection. About 30% patients were diagnosed at late stage, and these CRC patients usually had recurrence and tumor metastasis after surgical resection, and these patients had a 5-year survival rate of only 5–8%.Citation19For these patients, chemotherapy was commonly used to improve the survival of the patients.
Recently, irinotecan in combination with other chemotherapeutic drugs were used in clinic for the management of mCRC. Irinotecan is a novel water soluble camptothecin derivative, and it specifically inhibits TOPO-1 activity resulting in irreversibly damage of DNA in tumor cells and leading to tumor cell death.Citation20Irinotecan can be hydrolyzed into SN-38 by CES. The anti-tumor effect of the active SN-38 is 100–1000 times more potent than irinotecan. Some clinical studies showed that the anti-tumor effects of irinotecan are due to the cytotoxic activity of SN-38.Citation21, 22 Studies also showed that treatment with irinotecan, fluorouracil, and leucovorin resulted in significantly longer PFS and longer overall survival, as compared with treatment with fluorouracil and leucovorin.Citation23In some multicenter randomized trials, the response rate and overall survival were significantly higher in patients in the irinotecan group than in those in the no-irinotecan group; time to progression was also significantly longer in the irinotecan group than in the no-irinotecan one.Citation24 These results suggest the effective role of irinotecan in the treatment for mCRC.
TOPO-1 are enzymes that cut of the two strands of double stranded DNA, relax and re-anneal the DNA strand. TOPO-1 has several functions including removal of DNA supercoils during transcription and DNA replication; strand breakage during recombination; chromosome condensation and disentangling intertwined DNA during mitosis. In the malignant tumors such as CRC, cervical cancer, ovarian cancer and neuroblastoma, the expression of TOPO-1 in these tumors cells were significantly higher than the respective control normal cells.Citation10, Citation12, Citation25 Thus, these tumors cells are more sensitive to the TOPO-1 inhibitors such as irinotecan, which selectively inhibits the TOPO-1 activity. Qiu et al., 2013 showed that mCRC patients with down-regulated ECC1 on Oxaliplatin or up-regulated TOPO-1 on irinotecan have longer survival and better curative effect.Citation26 In addition, the results of the MRC FOCUS study of 1313 patients with mCRC indicated that tumors with moderate or high levels of TOPO-1 expression as determined by immunohistochemistry showed the greatest benefit from adding irinotecan in the first-line metastatic setting.Citation27However, subsequent data from the similar ‘CAIRO’ study from the Dutch Colorectal Cancer Group failed to replicate these findings, showing no association seen between TOPO-1 expression (by immunohistochemistry) and response to irinotecan in 545 patients.Citation28 In the present study, we demonstrated that TOPO-1 expression was correlated with PFS and OS in mCRC patients, however, neither TOPO-1 had significant association with the investigated clinicopathological characteristics including age, gender, tumor site, tumor grade and metastatic sites. These contradictory findings suggest that although absolute TOPO-1 expression may play a role, it is likely that additional molecules might contribute to the sensitivity of irinotecan.
CESs are mainly distributed in the cytoplasm and endoplasmic reticulum and are important pathways of drug metabolism. CESs are regulated by various nuclear receptors. In humans, two carboxylesterases, CES-1 and CES-2 are identified, and both are expressed in the liver, but levels of CES-1 greatly exceed those of CES-2. In the intestine, only high levels of CES-2 are expressed, and CES-2 was also found to be more important in activating irinotecan than CES-1[29]. Studies have demonstrated the up-regulation of CES-2 in various types of tumor cells and suggested the potential role of CES-2 as a biomarker for chemotherapeutic effect of irinotecan and prognosis in human cancers. Studies from Ohtsuka et al, demonstrated that 70% of non-small cell lung cancers expressed CES-211. Uchida et al., showed that CES-2 expression in neuroblastoma cell lines was positively correlated with sensitivity to irinotecan, and CES-2 expression was found to be significantly higher in patients with a characteristics related to advanced disease[10]. One study also showed that 48 of 118 pancreatic ductal adenocarcinomas exhibiting high CES-2 expression and the high expression of CES-2 in tumor tissues was associated with longer overall survival in pancreatic ductal adenocarcinoma patients who underwent irinotecan treatment[9]. Consistently, in our study, we showed that CES-2 expression level was significantly associated with the curative effect and PFS and OS in mCRC patients. These results suggest that expression of CES-2 may be a contributor to irinotecan sensitivity in mCRC patients.
In summary, our results suggest both TOPO-1 and CES-2 may serve as biomarkers for predicting the chemotherapeutic effect of irinotecan and prognosis in mCRC patients. The lack of significant correlation between TOPO-1 and CES-2 suggest that both of them act on different pathways. In the present study, by suing single TOPO-1 or CES-2 as single biomarker present relative low sensitivities and specificities, suggesting that TOPO-1 or CES-2 alone may be inadequate to provide very powerful clinical evidence for irinotecan-based therapy. Using both TOPO-1 and CES-2 as prognostic factors can be more accurately predict the therapeutic effect of irinotecan in mCRC patients. However, because of the limited number of mCRC patients, the present findings warrants further study in more mCRC patients.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Sup_mat_1414754_KCBT.xlsx
Download ()Acknowledgments
This study was supported by the National Natural Science Foundation of China (No.81260340).
Additional information
Funding
References
- Marley AR, Nan H. Epidemiology of colorectal cancer. Int J Mol Epidemiol Genet. 2016;7:105–114. eCollection 2016. PMID:27766137.
- Yang N, Li S, Li G, Zhang S, Tang X, Ni S, Jian X, Xu C, Zhu J and Lu M. The role of extracellular vesicles in mediating progression, metastasis and potential treatment of hepatocellular carcinoma. Oncotarget. 2017;8:3683–3695; doi: 10.18632/oncotarget.12465. PMID:27713136.
- Chen G, Yang Z, Eshleman JR, Netto GJ and Lin MT. Molecular Diagnostics for Precision Medicine in Colorectal Cancer: Current Status and Future Perspective. Biomed Res Int. 2016;2016:9850690. doi: 10.1155/2016/9850690
- Wulaningsih W, Wardhana A, Watkins J, Yoshuantari N, Repana D, Van Hemelrijck M. Irinotecan chemotherapy combined with fluoropyrimidines versus irinotecan alone for overall survival and progression-free survival in patients with advanced and/or metastatic colorectal cancer. Cochrane Database Syst Rev. 2016;2:CD008593. doi:10.1002/14651858.CD008593.pub3
- Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010;17:421–33. doi:10.1016/j.chembiol.2010.04.012
- Yu M, Tong X, Qi B, Qu H, Dong S, Yu B, Zhang N, Tang N, Wang L, Zhang C. Berberine enhances chemosensitivity to irinotecan in colon cancer via inhibition of NFkappaB. Mol Med Rep. 2014;9:249–54. doi:10.3892/mmr.2013.1762. PMID:24173769.
- Fujita K, Kubota Y, Ishida H and Sasaki Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J Gastroenterol. 2015;21:12234–48. doi: 10.3748/wjg.v21.i43.12234
- Hsieh YT, Lin HP, Chen BM, Huang PT and Roffler SR. Effect of Cellular Location of Human Carboxylesterase 2 on CPT-11 Hydrolysis and Anticancer Activity. PLoS One. 2015;10:e0141088. doi: 10.1371/journal.pone.0141088
- Capello M, Lee M, Wang H, Babel I, Katz MH, Fleming JB, Maitra A, Wang H, Tian W, Taguchi A and Hanash SM. Carboxylesterase 2 as a Determinant of Response to Irinotecan and Neoadjuvant FOLFIRINOX Therapy in Pancreatic Ductal Adenocarcinoma. J Natl Cancer Inst. 2015;107. doi:10.1093/jnci/djv132. PMID:26025324,
- Uchida K, Otake K, Tanaka K, Hashimoto K, Saigusa S, Matsushita K, Koike Y, Inoue M, Ueeda M, Okugawa Y, Inoue Y, Mohri Y and Kusunoki M. Clinical implications of CES2 RNA expression in neuroblastoma. J Pediatr Surg. 2013;48:502–9. doi: 10.1016/j.jpedsurg.2012.10.004
- Ohtsuka K, Inoue S, Kameyama M, Kanetoshi A, Fujimoto T, Takaoka K, Araya Y and Shida A. Intracellular conversion of irinotecan to its active form, SN-38, by native carboxylesterase in human non-small cell lung cancer. Lung Cancer. 2003;41:187–98.
- Bar JK, Grelewski P, Noga L, Rabczynski J, Grybos M and Jelen M. The association between the p53/topoisomerase I and p53/ topoisomerase IIalpha immunophenotypes and the progression of ovarian carcinomas. Adv Clin Exp Med. 2012;21:35–42.
- Zhao M and Gjerset RA. Topoisomerase-I PS506 as a Dual Function Cancer Biomarker. PLoS One. 2015;10:e0134929. doi: 10.1371/journal.pone.0134929
- Liu YP, Chen HL, Tzeng CC, Lu PJ, Lo CW, Lee YC, Tseng CH, Chen YL and Yang CN. TCH-1030 targeting on topoisomerase I induces S-phase arrest, DNA fragmentation, and cell death of breast cancer cells. Breast Cancer Res Treat. 2013;138:383–93. doi: 10.1007/s10549-013-2441-1
- Lu B, Zhang H, Zhang T, Cai Y, Hu Y, Zheng H and Li B. Topoisomerase I expression is associated with prognosis in postoperative non-small cell lung cancer patients. Thorac Cancer. 2016;7:486–94. doi:10.1111/1759-7714.12359
- Silvestris N, Simone G, Partipilo G, Scarpi E, Lorusso V, Brunetti AE, Maiello E, Paradiso A and Mangia A. CES2, ABCG2, TS and Topo-I primary and synchronous metastasis expression and clinical outcome in metastatic colorectal cancer patients treated with first-line FOLFIRI regimen. Int J Mol Sci. 2014;15:15767–77. doi:10.3390/ijms150915767
- Azzoni C, Bottarelli L, Cecchini S, Ziccarelli A, Campanini N, Bordi C, Sarli L and Silini EM. Role of topoisomerase I and thymidylate synthase expression in sporadic colorectal cancer: associations with clinicopathological and molecular features. Pathol Res Pract. 2014;210:111–7. doi:10.1016/j.prp.2013.11.004
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: A Cancer Journal for Clinicians. 2016;66:7–30. doi:10.3322/caac.21332
- Nanji S, Tsang ME, Wei X, Booth CM. Outcomes after repeat hepatic resection for recurrent metastatic colorectal cancer: a population-based study. Am J Surg. 2017;3:1053–1059. doi:10.1016/j.amjsurg.2016.08.014. Epub 2016 Sep 6. PMID:27697193.
- Digkas E, Kareli D, Chrisafi S, Passadaki T, Mantadakis E, Hatzimichail A, Vargemezis V and Lialiaris T. Attenuation of cytogenetic effects by erythropoietin in human lymphocytes in vitro and P388 ascites tumor cells in vivo treated with irinotecan. CPT-11). Food Chem Toxicol. 2010;48:242–9. doi:10.1016/j.fct.2009.10.007. PMID:19819285.
- Cercek A, Boucher TM, Gluskin JS, Aguilo A, Chou JF, Connell LC, Capanu M, Reidy-Lagunes D, D'Angelica M and Kemeny NE. Response rates of hepatic arterial infusion pump therapy in patients with metastatic colorectal cancer liver metastases refractory to all standard chemotherapies. J Surg Oncol. 2016;114:655–663. doi:10.1002/jso.24399
- Meisenberg C, Ashour ME, El-Shafie L, Liao C, Hodgson A, Pilborough A, Khurram SA, Downs JA, Ward SE and El-Khamisy SF. Epigenetic changes in histone acetylation underpin resistance to the topoisomerase I inhibitor irinotecan. Nucleic Acids Res. 2017;45:1159–1176. doi: 10.1093/nar/gkw1026. PMID:28180300.
- Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL and Miller LL. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–14. doi:10.1056/nejm200009283431302
- Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L and Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–7.
- Musa F, Blank S and Muggia F. A pharmacokinetic evaluation of topotecan as a cervical cancer therapy. Expert Opin Drug Metab Toxicol. 2013;9:215–24. doi:10.1517/17425255.2013.758249
- Qiu JG, Shen DM, Huang CJ and Tang JX. [Clinical values of detecting excision repair cross complementing 1 and top-isomerase I in individualized therapies of metastatic colorectal cancer]. Zhonghua Yi Xue Za Zhi. 2013;93:3852–6.
- Braun MS, Richman SD, Quirke P, Daly C, Adlard JW, Elliott F, Barrett JH, Selby P, Meade AM, Stephens RJ, Parmar MK and Seymour MT. Predictive biomarkers of chemotherapy efficacy in colorectal cancer: results from the UK MRC FOCUS trial. J Clin Oncol. 2008;26:2690–8. doi:10.1200/jco.2007.15.5580
- Koopman M, Antonini NF, Douma J, Wals J, Honkoop AH, Erdkamp FL, de Jong RS, Rodenburg CJ, Vreugdenhil G, Loosveld OJ, van Bochove A, Sinnige HA, Creemers GJ, Tesselaar ME, Slee PH, Werter MJ, Mol L, Dalesio O and Punt CJ. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer. CAIRO): a phase III randomised controlled trial. Lancet. 2007;370:135–42. doi:10.1016/s0140-6736(07)61086-1
- Hatfield MJ, Tsurkan L, Garrett M, Shaver TM, Hyatt JL, Edwards CC, Hicks LD and Potter PM. Organ-specific carboxylesterase profiling identifies the small intestine and kidney as major contributors of activation of the anticancer prodrug CPT-11. Biochem Pharmacol. 2011;81:24–31. doi:10.1016/j.bcp.2010.09.001