ABSTRACT
Attempts for identifying targeted therapy strategies in metastatic gastric and gastroesopheal junction cancer (upper-GI) revealed that the inhibition of human epidermal growth factor receptor-2 (HER2) by monoclonal antibody trastuzumab improves survival of these patients. Hence, adding trastuzumab to doublet chemotherapy has become the standard treatment in this setting. Although the patient survival is extended among clinical trials, the knowledge on the real-time setting is limited. With this retrospective, single center analysis of the patient data of the Medical University of Vienna, we sought to investigate the clinical characteristics and outcome of patients, who received trastuzumab-based chemotherapy for metastatic upper-GI tumor. All patients, who received trastzumab at least once were included to the analysis. Clinical and pathological data were recorded. This search revealed 33 patients. The demographic data was comparable with that of the previous clinical trials. Progression free survival (PFS) was 11 months, whereas overall survival (OS) was 21 months. OS was significantly associated with initially favorable response to treatment. Thirteen patients (39%) received trastuzumab as maintenance treatment with a median cycle number of 6. Toxicity profile was acceptable with only one patient detected to have cardiotoxicity. Taken together, trastuzumab based treatment induced a considerable PFS and OS in metastatic or advanced upper-GI tumors with acceptable toxicity profile. The maintenance therapy with trastuzumab was safe and effective in patients who had initially a favorable response to chemotherapy. The optimal duration of the maintenance therapy should be tested in future clinical trials.
Introduction
Gastric cancer is the fourth most commonly diagnosed cancer and the second most common cause of cancer related deaths worldwide.Citation1 Most patients present with inoperable advanced or metastatic disease requiring palliative treatment, whereas early detection is more common in Asia than in other regions. In the UK MAGIC study showed a 5 year survival of 36% in patients with operable disease who were administered to perioperative chemotherapy.Citation2 However, 5-year survival for advanced or metastatic gastric and gastroesophageal junction (GEJ) cancer (together upper-GI tumors) is approximately 5–20%, with median overall survival (OS) being around 1 year.Citation1,Citation3
There is currently no single well-established standard of care, but fluoropyrimidine-based and platinum-based combinations with or without a third drug (usually taxane or anthracycline) are the most widely used combinations in Europe and the USA.
Targeted therapies are introduced for clinical use in patients with advanced upper-GI tumors. Up to 20% of gastric tumors overexpress human epidermal growth receptor 2 (HER2).Citation4-6 There exists varying information on the expression of HER2 and the prognosis of upper-GI tumor patients. Poor outcomes and aggressive disease is mainly described,Citation7-9 whereas comparable survival times with HER2 negative patients were also shown.Citation10 Recently, Gu et al performed a meta-analysis of the prognosis of HER2 positive patients, who were diagnosed according to ToGA (Trastuzumab for Gastric Cancer) criteria, where no survival difference between negative and positive patients were observed.Citation11
The pivotal ToGA trial was the first randomized, prospective, multicenter phase III trial to study the efficacy of first-line trastuzumab (a monoclonal antibody against HER2) in patients with HER2 positive advanced upper-GI tumors.Citation5 Patients were randomly assigned to receive standard chemotherapy (cisplatin plus fluorouracil or capecitabine) or chemotherapy plus trastuzumab. Of 3665 patients originally screened for HER2, 810 (22%) had HER2 positive tumors. Five hundred eighty four were enrolled and received study treatment at least once. Median OS was 13.8 months in the trastuzumab group, compared with 11.1 months in the chemotherapy group. The longest survival (median 16 months) was seen in patients with highest HER2 protein overexpression and HER2 amplification. On the basis of this study, trastuzumab in combination with cisplatin and a fluoropyrimidine has been approved for the first-line treatment of advanced HER2-positive upper-GI tumors.
Since our understanding of trastuzumab mainly comes from clinical trial setting, we would like to answer the questions, how real-life cohort looks like, whether survival data is comparable with that from clinical trials and whether toxicity profile of trastuzumab differs than that, which is reported till now. For this purpose, we conducted a retrospective investigation of the patients with HER2 positive upper-GI tumor under trastuzumab-based chemotherapy, who are available within the records of the Medical University of Vienna.
Results
shows the demographics and the baseline characteristics of the patients included to the analysis. A total of 33 patients in Medical University of Vienna received trastuzumab for the treatment of advanced or metastatic upper-GI tumors between 2010 and 2016. The median age was 57, ranging between 30 and 74. All patients were white Caucasians. Regarding the location of the primary tumor, 11 patients had gastric cancer, whereas 22 were diagnosed with GEJ tumors. Eight patients were women. Seven patients had a positive family histology for cancer, whereby 4 patients had already a positive cancer histology. Two patients received trastuzumab due to the locally advanced disease; all other patients had metastatic disease. There were 15 and 12 patients with 1 and 2 metastatic sites, respectively. Four patients showed 3 or more metastatic sites.
Table 1. Patients demographics and baseline characteristics.
Almost the half of the patients received a prior gastrectomy (48%). Four patients were treated with radiation therapy. Seven patients received a prior chemotherapy.
HER2 status was obtained from immunohistochemical staining of the tumor tissue, where except 1 patient had 3+ positive tumor according to the Hoffmann Criteria.Citation8 The only one patient with 2+ positivity had the gene amplified in FISH analyses.
Treatment with trastuzumab in combination with chemotherapy was the first line strategy for 23 patients, whereas 7 and 3 patients received trastuzumab as second and third line, respectively. Trastuzumab was administered based on the ToGA Schema in 23 patients, whereas combinations with DCF, EOX, FOLFOX, FOLFIRI as well as monotherapy administrations were observed. The number of cycles for the administered trastuzumab treatment was 9 (ranging between 1–82). 13 patients received trastuzumab as maintenance after completion of the chemotherapy. The median number of trastuzumab cycles of this group was 6 (ranging between 1–78) (). Currently, 6 patients continue to receive trastuzumab monotherapy as maintenance.
Table 2. Characteristics of trastuzumab therapy and side effects.
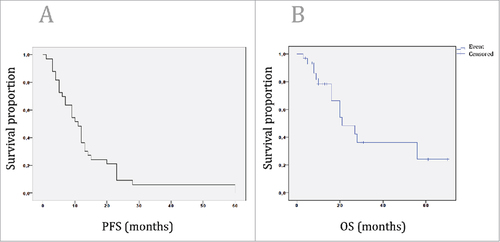
Complete response was observed in 4 patients, whereas partial response was present in 2 cases (). Ten patients had a stable disease and 2 patients showed a mixed response. Fifteen patients had a progressive disease. PFS was 11 months (7.8–14.1, 95% CI) among the whole cohort (), whereas OS was calculated to be 21 months (10.6–31.3, 95% CI) ().
Table 3. Efficacy analysis.
Figure 1. A: Kaplan-Meier survival curve of the PFS of the patients, who are treated with trastuzumab based treatment due to metastasized or recurrent upper-GI tumors. PFS was 11 months (7.8–14.1, 95% CI) among the whole cohort. 1B: Kaplan-Meier survival curve of the OS of the patients, who are treated with trastuzumab based treatment due to metastasized or recurrent upper-GI tumors. OS was 21 months (10.6–31.3, 95% CI) among the whole cohort.
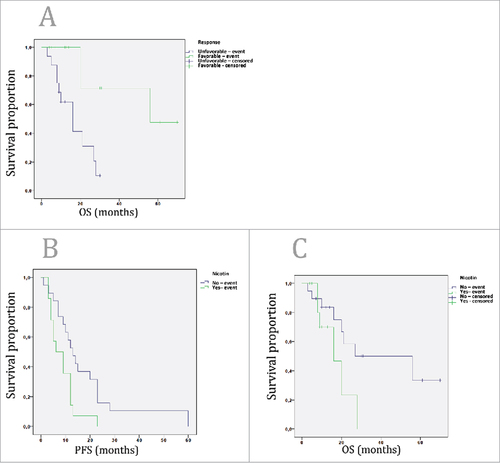
Using Cox Regression analysis, none of the parameters including, family history of cancer, location of the tumor, histological classification, number of metastasis, trastuzumab line, previous resection of the tumor or performance status associated with OS or PFS. However, as expected, number of trastuzumab cycles correlated significantly with OS and PFS (Pearson`s correlation coefficient 0.8 (p < 0.001) and 0.7 (p < 0.001), respectively. Tumor response was classified into favorable (complete response, partial response, stable disease) and unfavorable response (progressive disease, mixed response). Favorable tumor response associated with extended OS (56 vs. 16 months, Log Rank Test, p < 0.003) (). Nicotine consumption was associated with significantly lower PFS (6 vs. 13 months, Log Rank Test, p < 0.02) (). Furthermore, there was a non-significant association of nicotine consumption and decreased OS (16 vs. 56 months, Log Rank Test, p = 0.09) ().
Figure 2. A: Kaplan-Meier survival curve of the patients, who are treated with trastuzumab based treatment due to metastasized or recurrent upper-GI tumors, based on the response rates. Favorable response was defined as complete remission, partial response and stable disease. Unfavorable response was defined as progressive disease and mixed response. Favorable response patients had significantly higher OS than the unfavorable response patients (56 vs. 16 months, respectively; Log Rank Test, p<0.003). 2B: Patients with nicotine consumption had significantly lower PFS than that of without consummation (6 vs. 13 months, Log Rank Test, p<0.02). 2C: Patients with nicotine consumption had non-significantly lower OS than that of without consummation (16 vs. 56 months, Log Rank Test, p = 0.09).
The adverse events profile was acceptable including acute kidney injury, nausea, polyneuropathy and hematological toxicities (). These adverse events were most probably due to the administration of the chemotherapy. Cardiac adverse events were rare. Only one patient demonstrated with reduced ejaculation fraction upon trastuzumab treatment, where trastuzumab was discontinued due to the cardiotoxicity.
Seventeen patients were treated for a further line chemotherapy due to tumor progression.
Discussion
Over recent years, several targeted therapy approaches were tested for the treatment of the metastatic adenocarcinoma of the upper-GI. However, HER2 remained to be the only targeted therapy strategy for the first line setting in patients with HER2 positive in immunohistochemistry or FISH as served its biomarker. Therefore, trastuzumab, as first generation anti-HER2 monoclonal antibody, became the standard treatment together with chemotherapy for the metastasized or advanced upper-GI tumors. In the current report, we aimed to demonstrate our single center experience and clinical characteristics of a cohort, which consisted of western advanced upper-GI cancer patients treated with trastuzumab. Among this cohort, trastuzumab showed no unexpected toxicity profile. PFS and OS of the patients were notably higher than that of the ToGA protocol of the patients, where trastuzumab was initially tested in a phase III trial setting.Citation5
When compared to ToGA, current report exhibits some differences regarding to the patients characteristics, which might lead to different outcomes on patients` PFS and OS. Ten percent of the ToGA patients had a performance status of 2, whereas this was only 3% in our cohort including a high percentage of patients with best performance status (ECOG 0: 79%). It is a known fact, that patients with good performance status tolerate the chemotherapy well with having better outcome. In line with this fact, the subgroup analysis of the ToGA showed, that patients with better performance status had higher hazard ratios of OS when compared to that of bad performance statusCitation5.
Another explanation might be the proportional higher percentage of the patients with 3+ HER2 positivity in immunohistochemistry in our cohort (97%). The level of HER2 gene amplification and positivity in immunohistochemistry significantly predicts sensitivity to therapy and OS in patients with trastuzumab based chemotherapy.Citation4,Citation12,Citation13 Among post-hoc analysis of the initial ToGA trial, patients whose tumor specimen was HER2 highly positive had an OS of 16 months, which is still lower than that of what we observed in our cohort, but was significantly higher than the patients who had lower HER2 levelsCitation14.
Ethnicity and regional differences in outcome is increasingly considered in terms of prognosis and treatment of upper-GI tumors. Generally, Western patients have worse clinical outcome after surgery and adjuvant chemotherapy in resectable gastric cancer.Citation2,Citation15 Due to the high incidence of upper-GI tumors in East Asia, preventive strategies and screening investigations are well established, which leads to an early diagnosis and early initiation of the treatment of the upper-GI tumors. Apart from the high incidence of the disease, response rates to different agents alters between Asia and Western countries as well, which suggests a potential variation of the pathobiology of the tumorCitation16. Among ToGA trial, almost the halves of the patients' cohort were western patients, with having higher hazard ratio of OS from trastuzumab-based treatment when compared to Asian patients (n = 275, 49%).Citation5 Motivated by this fact, a subgroup analysis of the ToGA Study has been done for the Japanese patients, where the same benefit of adding trastuzumab to chemotherapy was observed in this group when compared to entire population.Citation17 Among the literature, varying survival times are available for patients treated with trastuzumab based treatment, both for Western and Asian patients. A previous report investigated 213 archival patients, where 15 cases received trastuzumab-containing chemotherapy. Patients with trastuzumab had an OS of 22.9 vs. 11.6 months of patients who received chemotherapy alone.Citation18 Recently, a Spanish group published the data of 90 patients, where the HER2 expression was divided based on the amplification score. Patients with >4.45 HER2/CEP17 score had significantly higher survival with 21.3 versus 13.6 months.Citation12 Another Asian cohort reported an OS of 18.5 months in 168 upper-GI patients treated in 12 different institutions.Citation19 Taken together, the observation of an OS of 21 months within the current cohort was in line with the previous findings, where different ethnicities of patients were included. The only exception exhibits the report from Ock et all, where highly extended OS times of 26.9 months were reported.Citation13 It is important to mention that 20% of this study population was treated with another anti-HER2 agent as second line treatment in the frame of various clinical trials. Although changing the anti-HER2 treatment modality in second line setting using lapatinib or TDM-1 failed to show a survival benefit in 2 phase III trials,Citation20,Citation21 further biomarker analysis identifying the appropriate patient population is necessary.
There exists no evidence on the optimal duration of the trastuzumab therapy in gastric cancer patients. Trastuzumab is applied in breast cancer patients in adjuvant setting over 1 year. In case of metastatic disease, trastuzumab might be further applied. However, there exist no generally applied recommendation among guidelines.Citation22 A working group from MD Anderson Cancer Center described a trastuzumab treatment beyond progression, which showed a considerable OS of the patients.Citation23 Another prospective study found a significant extension of PFS when trastuzumab was given beyond progression compared to chemotherapy alone, whereas OS was not different.Citation20 In our cohort, we identified 13 (39% of the whole population) patients with trastuzumab as maintenance treatment after a diagnosis of favorable response identified as stable disease, partial response or complete response. Trastuzumab treatment did not induce any additional toxicity including cardiac events in this population of patients, where a meaningful duration of response could be achieved. Those patients had longer survival times, 56 versus 20 months, which however did not reach statistical significance most probably due to smaller sample size. Still, information on the treatment duration of the trastuzumab in gastric cancer patients, who had a favorable response to initial trastuzumab treatment, is lacking. Further prospective clinical trials with appropriate biomarker analysis, specifically designed to answer this question is needed.
The addition of trastuzumab to chemotherapy did not increase toxicity profile of the patients. The most feared toxic effect of trastuzumab, namely the cardiotoxicity was observed only in 1 patient, which was manifested with lower EF values in echocardiography. Trastuzumab was subsequently discontinued after this diagnosis, and a second line treatment with ramicurumab was initiated. No prolonged cardiotoxic manifestations were observed. Other toxicities including vomiting, PNP, AKI could mainly be attributed to the chemotherapy and were manageable with symptomatic treatment options.
It is important to mention, that this report includes some limitations. Due to the retrospective nature of the study, not all patients are treated with homogenous backbone chemotherapy regimen. However, apart from 3 patients who received trastuzumab as mono-treatment, all other patients received a chemotherapy on the platinum and fluoroprimidine basis. Another limitation is the non-central radiological evaluation of the response rate of the patients, which might bias the PFS. Since the patients are usually discussed within interdisciplinary tumor board in our institution, we think that this bias is also mainly minimized.
To sum up, we demonstrate in this descriptive study our single institution characteristics of the upper-GI tumor patients who are treated with trastuzumab based therapy based on their HER2 positivity in tumor tissue. Toxicity profile of the treatment was acceptable, whereas a remarkable OS of 21 months could be found. A meaningful proportion of the patients received trastuzumab as maintenance therapy, however the optimal duration of the trastuzumab treatment should further be investigated in future prospective trials.
Patients and methods
Patient collection
The patients are selected by a comprehensive search of the chart data of the Medical University of Vienna for the patients who received trastuzumab due to an upper-GI tumor. Patients should have received trastuzumab at least once. Men or women older than 18 years of age are included. ECOG performance status was between 0 and 2. As routine practice, an adequate heart function (ejection fraction over 60%) was obligatory for the administration of trastuzumab. Patients received trastuzumab either as first line due to their locally advanced or metastatic disease, or as palliative treatment after failure of the first line adjuvant treatment.
A computed tomography scan was performed every 3 months for the evaluation of the response to the treatment. In the absence of disease progression during or after chemotherapy, patients received trastuzumab maintenance treatment up to the progress.
This study was approved by the local ethic committee in accordance of with the Helsinki Declaration of 1975.
Trastuzumab was given by intravenous infusion at a dose of 8 mg/kg on day 1 of the first cycle, followed by 6 mg/kg every 3 weeks until disease progression, unacceptable toxicity, or patient's refusal.
Demographic, clinical, pathological and survival parameters were recruited from chart data. Patients were followed up until death according to the hospital records or loss to follow-up.
Response evaluation
After administration of 3 cycles of trastuzumab containing treatment, the amount of tumor shrinkage was investigated based on computed tomography images and the tumor response was classified in accordance with RECIST24: complete response, disappearance of all target lesions; partial response, ≥ 30% decrement in the combined diameters of all target lesions; progressive disease, ≥20% increase in the combined diameters of target lesions; and stable disease, neither sufficient shrinkage to identify partial response nor increase to qualify for progressive disease.
HER2 analysis
Tumors are tested for HER2 status with immunohistochemistry (Hercep Test, Dako, Denmark) and fluorescence in-situ hybridization (FISH, HER2 IFSH pharmDx, Dako). For FISH, HER2 gene copy number and centromere enumerator probe 17 (CEP17) were investigated. The pathologists reported average copy numbers of HER2 and CEP17 in each case. The diagnosis criteria was based on the study of Hofmann and colleagues.Citation8 Patients were administered to trastuzumab if their tumour samples were scored as 3+ on immunohistochemistry or in case of 2+, if they were FISH positive (HER2:CEP17 ratio >2).
Statistical analysis
All patients receiving trastuzumab therapy once are included to this retrospective survey. Patients without an event (death) were censored at the date that they were last known to be alive. Kaplan-Meier survival estimates with log rank test and Cox regression analyses of OS and progression free survival (PFS) were done. All reported values are two sided and p value was considered to be significant when <0.05.
References
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–2150. doi:10.1200/JCO.2005.05.2308
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. doi:10.1056/NEJMoa055531
- Cunningham SC, Kamangar F, Kim MP, Hammoud S, Haque R, Maitra A, Montgomery E, Heitmiller RE, Choti MA, Lillemoe KD, et al. Survival after gastric adenocarcinoma resection: eighteen-year experience at a single institution. J Gastrointest Surg. 2005;9(5):718–725. doi:10.1016/j.gassur.2004.12.002
- Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, Chong JL, Lopez-Sanchez RI, Price T, Gladkov O, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18(3):476–484. doi:10.1007/s10120-014-0402-y
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi:10.1016/S0140-6736(10)61121-X
- Jorgensen JT. Targeted HER2 treatment in advanced gastric cancer. Oncology. 2010;78(1):26–33. doi:10.1159/000288295
- Kim KC, Koh YW, Chang HM, Kim TH, Yook JH, Kim BS, Jang SJ, Park YS. Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol. 2011;18(10):2833–2840. doi:10.1245/s10434-011-1695-2
- Hofmann M, Stoss O, Shi D, Buttner R, van de Vijver M, Kim W, Ochiai A, Ruschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52(7):797–805. doi:10.1111/j.1365-2559.2008.03028.x
- Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19(9):1523–1529. 10.1093/annonc/mdn169
- Shitara K, Yatabe Y, Matsuo K, Sugano M, Kondo C, Takahari D, Ura T, Tajika M, Ito S, Muro K. Prognosis of patients with advanced gastric cancer by HER2 status and trastuzumab treatment. Gastric Cancer. 2013;16(2):261–267. doi:10.1007/s10120-012-0179-9
- Gu J, Zheng L, Wang Y, Zhu M, Wang Q, Li X. Prognostic significance of HER2 expression based on trastuzumab for gastric cancer (ToGA) criteria in gastric cancer: an updated meta-analysis. Tumour Biol. 2014;35(6):5315–5321. doi:10.1007/s13277-014-1693-7
- Gomez-Martin C, Plaza JC, Pazo-Cid R, Salud A, Pons F, Fonseca P, Leon A, Alsina M, Visa L, Rivera F, et al. Level of HER2 gene amplification predicts response and overall survival in HER2-positive advanced gastric cancer treated with trastuzumab. J Clin Oncol. 2013;31(35):4445–4452. doi:10.1200/JCO.2013.48.9070
- Ock CY, Lee KW, Kim JW, Kim JS, Kim TY, Lee KH, Han SW, Im SA, Kim TY, Kim WH, et al. Optimal patient selection for trastuzumab treatment in HER2-positive advanced gastric cancer. Clin Cancer Res. 2015;21(11):2520–2529. doi:10.1158/1078-0432.CCR-14-2659
- Van Cutsem E, de Haas S, Kang YK, Ohtsu A, Tebbutt NC, Ming Xu J, Peng Yong W, Langer B, Delmar P, Scherer SJ, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol. 2012;30(17):2119–2127. doi:10.1200/JCO.2011.39.9824
- Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–1820. doi:10.1056/NEJMoa072252
- Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–2664. doi:10.1016/S0140-6736(16)30354-3
- Sawaki A, Ohashi Y, Omuro Y, Satoh T, Hamamoto Y, Boku N, Miyata Y, Takiuchi H, Yamaguchi K, Sasaki Y, et al. Efficacy of trastuzumab in Japanese patients with HER2-positive advanced gastric or gastroesophageal junction cancer: a subgroup analysis of the Trastuzumab for Gastric Cancer (ToGA) study. Gastric Cancer. 2012;15(3):313–322. doi:10.1007/s10120-011-0118-1
- Namikawa T, Munekage E, Munekage M, Maeda H, Yatabe T, Kitagawa H, Sakamoto K, Obatake M, Kobayashi M, Hanazaki K. Evaluation of a trastuzumab-containing treatment regimen for patients with unresectable advanced or recurrent gastric cancer. Mol Clin Oncol. 2016;5(1):74–78. doi:10.3892/mco.2016.892
- Yi JH, Kang JH, Hwang IG, Ahn HK, Baek HJ, Lee SI, Lim DH, Won YW, Ji JH, Kim HS, et al. A retrospective analysis for patients with HER2-positive gastric cancer who were treated with trastuzumab-based chemotherapy: In the perspectives of ethnicity and histology. Cancer Res Treat. 2016;48(2):553–560. doi:10.4143/crt.2015.155
- Li Q, Jiang H, Li H, Xu R, Shen L, Yu Y, Wang Y, Cui Y, Li W, Yu S, et al. Efficacy of trastuzumab beyond progression in HER2 positive advanced gastric cancer: a multicenter prospective observational cohort study. Oncotarget. 2016;7(31):50656–50665. doi:10.18632/oncotarget.10456
- Hecht JR, Bang YJ, Qin SK, Chung HC, Xu JM, Park JO, Jeziorski K, Shparyk Y, Hoff PM, Sobrero A, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC–A randomized Phase III trial. J Clin Oncol. 2016;34(5):443–451. doi:10.1200/JCO.2015.62.6598
- Cardoso F, Costa A, Senkus E, Aapro M, Andre F, Barrios CH, Bergh J, Bhattacharyya G, Biganzoli L, Cardoso MJ, et al. 3rd ESO-ESMO international consensus guidelines for Advanced Breast Cancer (ABC 3). Ann Oncol. 2017;28(1):16–33. doi:10.1093/annonc/mdw544
- Al-Shamsi HO, Fahmawi Y, Dahbour I, Tabash A, Rogers JE, Mares JE, Blum MA, Estrella J, Matamoros A, Jr, Sagebiel T, et al. Continuation of trastuzumab beyond disease progression in HER2-positive metastatic gastric cancer: the MD Anderson experience. J Gastrointest Oncol. 2016;7(4):499–505. doi:10.21037/jgo.2016.06.16
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
