ABSTRACT
Endoscopic ultrasound (EUS) have been not only a diagnostic tool, but also available in interventional therapy, which often previously needed surgical approaches to achieve. The study aimed to evaluate the effectiveness and safety of EUS-guided Nd:YAG laser ablation in unresectable tumors of the caudate lobe and left liver. We discussed ten cases of the caudate lobe and left liver tumors underwent laser ablation with EUS guidance. And we also have reviewed previous publication of EUS-guided thermal ablation for liver tumors in several decade years. EUS-guided Nd:YAG laser ablation (LA) of these tumors were successfully completed in ten patients, who had favourable prognosis with no complications in two-month follow-up. Based on our early observations, this suggested that EUS-guided LA might be technically feasible in selected patients with tumors of the caudate lobe and left liver. However, the safety of this technique need to be further confirmed in the future and if possible larger, prospective trials.
Abbreviations
| AFP | = | alpha fetal protein |
| CA12–5 | = | carbohydrate antigen 12–5 |
| CA19–9 | = | carbohydrate antigen 19–9 |
| CEA | = | carcinoembryonic antigen |
| CECT | = | contrast-enhanced computered tomography |
| EUS | = | Endoscopic ultrasound |
| LA | = | laser ablation |
| MRI | = | magnetic resonance imaging |
| MWA | = | microwave ablation. |
| RFA | = | radiofrequency ablation |
| TACE | = | transarterial chemoembolization |
Introduction
As the endoscope technique advance since the 1980s, endoscopic ultrasound have been not only a diagnostic tool, but also available in interventional therapy, which often previously needed surgical approaches to achieve. Thinner laser fibers in combination with EUS-needles could enable their potential application in deep abdominal organs like the pancreas, which are difficult through the percutaneous method. Previous preliminary studies in animals showed that EUS-guided LA could be conducted safely in the porcine pancreas,Citation1 and subsequently the new treatment way was successfully applied in recurrent pancreatic neuroendocrine tumor.Citation2 Interestingly, EUS-guided Nd:YAG LA of a hepatocellular carcinoma in the caudate lobe was ever reported. Follow-up of 2 months indicated that clinical examination and lab tests were normal, and CT scan suggested uniform hypoattenuation without enhancement in the ablated zone.Citation3 Here we report four successful cases of men with liver tumors by EUS-guided Nd:YAG LA, which further indicated the effectiveness and safety using this technique.
Methods and patients
This is a prospective, observational, open-label, single-arm clinical trial performed at one tertiary care center from May 2016 through October 2017, which was registered in Clinicaltrials.gov ID: NCT02816944. Patients were eligible if they met the following inclusion criteria:1) lesions caudate lobe or left liver that could not be easily detected by percutaneous ultrasound; 2) liver function evaluated with Child-Pugh classes A and B; 3) surgery was refused by the patient or not suitable assessed by surgeon. EUS-guided LA for targeting lesions would be not taken into account if any of the following criteria were included: 1) patients with suspicious upper gastrointestinal stenosis, esophagogastric varices or bleeding, existing severe cardiopulmonary dysfunction; 2) coagulopathy (platelets < 50, 000). In this study, ten patients who were unwilling to accept surgical resection for liver cancer were enrolled in the First Affiliated Hospital, College of Medicine, Zhejiang University. This trial was approved by the ethics committee of the First Affiliated Hospital of Zhejiang University. All authors had access to the study data and reviewed and approved the final manuscript. Both the enrolled patients had informed consent for the procedure. The basic information of the ten patients was listed in . Among the ten patients enrolled, 6 cases underwent laser ablation in the caudate lobe and 4 in the left liver, in whom 3 tumors came from colorectal carcinoma. In this study, case 1 was a 63-year-old man with recurrent hepatocellular carcinoma. He has a history of hepatectomy in IV segments in 2011. On admission, magnetic resonance imaging (MRI) presented a mildly enhancing mass ( and ) after combined treatment of TACE and RFA. EUS detected a homogenous hypoechoic lesion (2.2#1.7 cm) with a central hyperechoic focus in the caudate lobe (). He had experienced EUS-guided LA of the same nidus. Case 2 was a 70-year-old man who had colon surgery 2 years ago. One month later, he had liver resection and cholecystectomy. Recently, magnetic resonance imaging (MRI) (Philips, Amsterdam, Holland) showed T1 and T2 phase enhancement of the caudate lobe, suggesting a malignant metastasis ( and ). Case 3, a 57-year-old man, was admitted to this hospital because of recurrent liver cancer. The patient, who suffered from hepatocellular carcinoma, had left liver resection approximately 4 years ago. Then he underwent transarterial chemoembolization (TACE) because of recurrence 6 months later, and liver resection again this year. On admission, the patient had no obvious discomfort on examination such as fever, chills, abdominal distension, abdominal pain, nausea, vomiting, but an increased alpha fetal protein (AFP) level of 109.2 ng/mL. The latest MR images showed a tumor of 1.3#1.2 cm in size near the caudate lobe in contrast with previous images (). Case 4 had left liver resection and TACE three years ago. Preoperative T2 MR image showed a round tumor about 1.1#1.0 cm in the left liver ().
Table 1. Patient characteristics.
Figure 1. A 63-year-old man with hepatocellular carcinoma. Preoperative MR and CEUS images showed a mass of 2.2#1.7 cm in size in the caudate lobe (a, b and c) (white arrows). EUS suggested laser fiber inserted into the tumor (d) and then total enhancement of the lesion (e) (white arrows). One year later, substance phase MR image showed that the mass has a complete response (f) (white arrows).
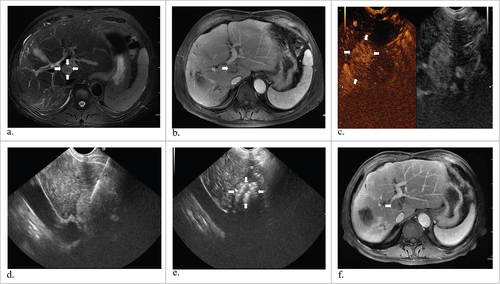
Figure 2. Representative images from a 70-year-old man diagnosed liver metastasis from colon cancer. T1 and T2 MR images revealed a tumor about 2.1#1.7 cm in the caudate lobe (a and b) (white arrows). One laser fiber was ablating the tumor with local enhancement (c) (white arrows). T2 MR image two months obtained after ablation showed complete response in the tumor (d) (white arrows). At the corresponding ultrasounography, it also showed a complete necrosis without any enhanced perfusion in CEUS (e and f) (white arrows).
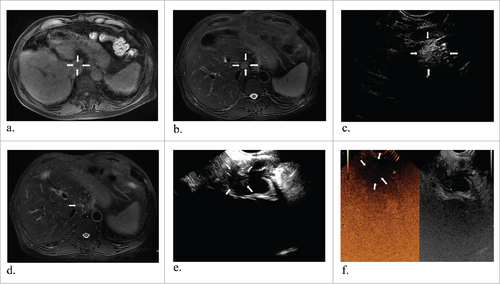
Figure 3. A 57-year-old man with hepatocellular carcinoma. MR obtained one month before ablation showed the tumor measuring 1.3#1.2 cm in diameter in the caudate lobe (a) (arrowheads). Preoperative EUS indicated a low echo area (b) (arrowheads) and it had increased echogenicity covering the whole mass after ablation (c) (arrowheads). After one month, enhanced MR revealed the lesion complete necrosis (d) (arrowheads).
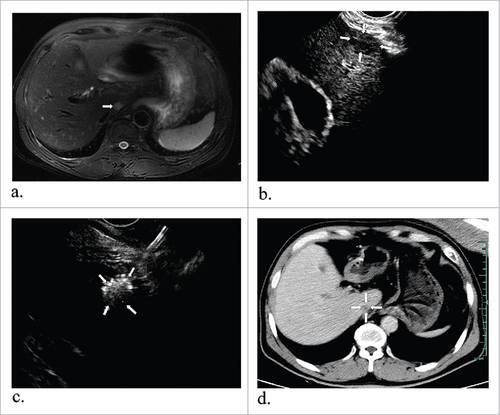
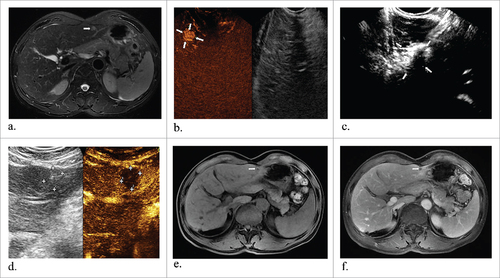
Figure 4. Representative images from a 54-year-old man diagnosed with hepatocellular carcinoma. Preoperative T2 MR image shows a round tumor about 1.1#1.0 cm in the left liver (a) (arrowheads). After enhanced perfusion of this tumor under CEUS guidance (b) (arrowheads), then a laser fiber ablated the target tumor (c) (arrowheads), and immediately CEUS showed no enhanced perfusion (d) (arrowheads). During six-month follow-up after ablation, T1 (e) and substance phase MR images (f) showed the mass was successfully ablated (arrowheads).
After a 8-hour supervised fast, all cases underwent EUS-guided LA in supine position by orally taking lidocaine hydrochloride under conscious sedation. A curved linear array echoendoscope (Olympus Ltd, Tokyo, Japan) was used to have examinations by orally inserting probe. Under the echoendoscope, the deep lesions in the caudate lobe or left liver were easily visualized from the cardia of stomach, and punctured with a 22-gauge aspiration needle (Wilson Cook Medical, Bloomington, United States). Then the needle core was withdrawn and the laser fiber inserted into the needle sheath outside of the end within 1 cm. The distance between fiber tip and lesion wall was ranged from 1.0 cm to 2.0 cm. During the procedures, the nidus was ablated by a Nd:YAG laser fiber (300 μm in diameter) with a wavelength of 1064 nm, each of which has an output power of 5W for 1500–1800 J (ESAOTE, Genova, Italy). The ablation was not stopped until hyperechoic area overlaying the entire lesions. After each ablation, especially for the tumor larger than 2 cm, immediate CEUS images (2.4 ml SonoVue mixed with 10.0 ml 0.9% sodium chloride solution) were required to closely monitor whether any potential residual lesions left. If found, then additional punctures and fibers ablation were repeated orally by angle adjustments within EUS guidance.
These patients underwent followed-up using ultrasound, CT (GE, Fairfield, United States), MRI images every 3 months after the operation Hematological examinations included: carcinoembryonic antigen (CEA) levels, AFP levels, carbohydrate antigen 19–9 (CA19–9) levels, and carbohydrate antigen 12–5 (CA12–5) levels.
Furthermore, we seriously performed a systematic review using keywords of endoscopic ultrasonography, liver, tumor, and thermal ablation by searching from PubMed, Scoups and Web of science before November 2016 without language limitations (Appendix 1 and Fig. S1).
Results
The detailed information of EUS-guided tumor ablation techniques in the ten patients was listed in . The total energy in each patient ranged from 1800 J to 11950 J under 5 w power. After laser ablation by EUS guidance, cases had significant decrease in AFP level. The results from CT or MR scan at least one or three months later showed completed response with a homogeneous nonenhancing (). On the second day after ablation, only case 3 complained of a sense of mild nausea then relieved. There were no major procedure-related complications.
Table 2. The detailed description of EUS-guided laser ablation for tumors.
During a systematic review, we found 4 studies presenting abdominal tumors following EUS-guided RFA or cryoablation, of which the summary was listed in .Citation3-6 There were 3 studies about EUS-guided RFA on porcine livers and 1 for EUS-guided LA on a HCC in the caudate lobe. They both showed a favourable prognosis.
Table 3. Summary of EUS-guided interventional treatment of tumors in 4 published literatures.
Discussion
Recently, traditional routes of ablation included percutaneous and surgical applications,Citation7,Citation8 but using flexible endoscopy and EUS-guided needles was still limited. In a porcine model, the lesions from liver and lymph node were successfully treated using RFA under EUS guidance.Citation9-12 Previous studies had reported that percutaneous laser ablation with chemotherapy is effective for colorectal cancer liver metastases, and the median overall survival time was 19.1 months.Citation13 EUS-guided RFA has been used for the pancreatic cystic neoplasms and neuroendocrine tumors.Citation14 Complications included retroperitoneal fibrosis, adhesions of the pancreas.Citation12 It was also showed that complications like postinterventional abdominal pain, minor duodenal bleeding, jaundice, duodenal stricture appeared after EUS-guided cryoablation for locally advanced pancreatic cancer.Citation15 Several technical problems might occur regarding the prototype RFA probe, including bending of the tip of the catheter probe and mild stripping of the probe by moving the EUS needle forward or backward.Citation10
However, percutaneous laser thermal ablation of liver malignancies is a well-established treatment for both primary and secondary liver tumors, with its effectiveness and safety being proven over the last several years,Citation16 and with a rate of complications lower than those of other thermal techniques such as RFA and MWA.Citation17,Citation18 Among all thermal treatment modalities, LA enabled the use of finer needles than RFA and MWA, and allowed to tailor the ablation volume by using one to four laser fibers, and thus preserving the normal parenchyma as such as possible. In addition, as is well-known, the high ablation temperature enables the tissue dehydration and strong coagulability. LA provided the highest central and surrounding temperature avoiding the splitting or falling off of target lesions. Moreover, LA provided unambiguous edge between coagulation area and adjacent normal structure rather than irregular and thick using RFA and MWA.Citation19 Moreover, emerging studies showed the possible malignant tumor relapse and rapid expansionCitation20-22 if excessive energy input. LA has the lower power of 1 W to 7 W while 10 W to 100 W in RFA and MWA, which brought better prognosis based on the destroyed tumor. These attributes made LA an attractive option for the treatment of nodules in high-risk, or multiple nodules in different size.Citation23,Citation24 Furthermore, CEUS image was used to immediately assess the size of the ablation zone, defined by the non-enhanced area, which was deemed better than US in monitoring the efficacy of ablation.Citation25 Thus it would make it possible to repeatedly ablate targeting lesions. In the light of the above, the endoscopic application of this technique should be reserved for selected cases not eligible for surgery or percutaneous treatment with severe comorbidity and/or advanced Child-Pugh (B or C), and would benefit patients with arrhythmia and cardiac pacemaker.
This study has several limitations present as follows. First, prognosis is limited by the small number of patients and the relatively short follow-up time. Second, this preliminary study just focused on the lesions in the caudate lobe and left liver. For lesions adjacent to large vessels, the efficacy of thermal ablation including LA might be sometimes influenced by the “heat-sink” effect, in which blood perfusion of vessels cooled surrounding tissue.Citation26,Citation27 Preliminary porcine model by PLA showed that portal regions had more heat sink than hepatic veins. The impact reduced with the distance between vessel and tip, but it was less for portal regions.Citation26
Conclusion
In conclusion, the study has indicated that the EUS-guided LA might be technically feasible in selected patients with the caudate lobe and left liver tumors. However, the safety of this technique need to be further confirmed in the future and if possible larger, prospective trials.
Disclosure of Potential Conflicts of Interest
The authors state that there is no conflict of interests regarding the publication of this paper.
Financial support
This study was supported by the opening foundation of the State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital of Medical College, Zhejiang University [grant number 2015KF06]; the Foundation of Zhejiang Health Committee [grant number 2017KY346].
Author contributions
| 1. | Study concept and design: Tian'an Jiang | ||||
| 2. | Acquisition of data: Tian'an Jiang, Fen Chen, Zhuang Deng, Ju Li, Weilu Chai | ||||
| 3. | Analysis and interpretation of data: Tian'an Jiang, Fen Chen | ||||
| 4. | Drafting of the manuscript: Tian'an Jiang | ||||
| 5. | Critical revision of the manuscript for important intellectual content: Tian'an Jiang, Guo Tian, Fen Chen | ||||
| 6. | Statistical analysis: Guo Tian, Haiwei Bao, Weilu Chai | ||||
| 7. | Obtained funding: Tian'an Jiang | ||||
| 8. | Technical, or material support: Zhuang Deng, Ju Li | ||||
| 9. | Study supervision: Tian'an Jiang | ||||
2017CBT10503R-s02.tif
Download ()Additional information
Funding
References
- Matteo FD, Martino M, Rea R, Pandolfi M, Rabitti C, Masselli GM, Silvestri S, Pacella CM, Papini E, Panzera F, Valeri S, Coppola R, Costamagna G. EUS-guided Nd:YAG laser ablation of normal pancreatic tissue: a pilot study in a pig model. Gastrointest Endosc. 2010;72:358–63, http://www.ncbi.nlm.nih.gov/pubmed/20541187. doi:10.1016/j.gie.2010.02.027.
- Matteo FD, Picconi F, Martino M, Pandolfi M, Pacella CM, Schena E, Costamagna G. Endoscopic ultrasound-guided Nd:YAG laser ablation of recurrent pancreatic neuroendocrine tumor: a promising revolution? Endoscopy. 2014;46 Suppl 1 UCTN:E380–1, http://www.ncbi.nlm.nih.gov/pubmed/25254586.
- Matteo FD, Grasso R, Pacella CM, Martino M, Pandolfi M, Rea R, Luppi G, Silvestri S, Zardi E, Costamagna G. EUS-guided Nd:YAG laser ablation of a hepatocellular carcinoma in the caudate lobe. Gastrointestinal Endoscopy. 2011;73:632–6. doi:10.1016/j.gie.2010.08.019.
- Carrara S, Arcidiacono PG, Albarello L, Addis A, Enderle MD, Boemo C, Neugebauer A, Campagnol M, Doglioni C, Testoni PA. Endoscopic ultrasound-guided application of a new internally gas-cooled radiofrequency ablation probe in the liver and spleen of an animal model: a preliminary study. Endoscopy 2008;40:759–63, http://www.ncbi.nlm.nih.gov/pubmed/18702032. doi:10.1055/s-2008-1077520.
- Varadarajulu S, Jhala NC, Drelichman ER. EUS-guided radiofrequency ablation with a prototype electrode array system in an animal model (with video). Gastrointestinal Endoscopy 2009;70:372–6. doi:10.1016/j.gie.2009.03.008.
- Park JS, Seo DW, Song TJ, Park DH, Sang SL, Lee SK, Kim MH. Endoscopic ultrasound-guided ablation of branch-duct intraductal papillary mucinous neoplasms: Feasibility and safety tests using porcine gallbladders. Digestive Endoscopy, 2016;28:599–606 doi:10.1111/den.12628.
- Yun D, Kim S, Song I, Chun K. Comparative analysis of Laparoscopic versus open surgical radiofrequency ablation for malignant liver tumors. Korean J Hepatobiliary Pancreat Surg 2014;18:122–8, http://www.ncbi.nlm.nih.gov/pubmed/26155264. doi:10.14701/kjhbps.2014.18.4.122.
- Zhang X, Yan L, Li B, Wen T, Wang W, Xu M, Wei Y, Yang J. Comparison of laparoscopic radiofrequency ablation versus open resection in the treatment of symptomatic-enlarging hepatic hemangiomas: a prospective study. Surg Endosc 2016;30:756–63, http://www.ncbi.nlm.nih.gov/pubmed/26123327. doi:10.1007/s00464-015-4274-y.
- Varadarajulu S, Jhala NC, Drelichman ER. EUS-guided radiofrequency ablation with a prototype electrode array system in an animal model (with video). Gastrointest Endosc 2009;70:372–6, http://www.ncbi.nlm.nih.gov/pubmed/19560138. doi:10.1016/j.gie.2009.03.008.
- Sethi A, Ellrichmann M, Dhar S, Hadeler KG, Kahle E, Seehusen F, Klapper W, Habib N, Fritscher-Ravens A. Endoscopic ultrasound-guided lymph node ablation with a novel radiofrequency ablation probe: feasibility study in an acute porcine model. Endoscopy 2014;46:411–5, http://www.ncbi.nlm.nih.gov/pubmed/24505019. doi:10.1055/s-0034-1364933.
- Goldberg SN, Mallery S, Gazelle GS, Brugge WR. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc 1999;50:392–401, http://www.ncbi.nlm.nih.gov/pubmed/10462663. doi:10.1053/ge.1999.v50.98847.
- Kim HJ, Seo DW, Hassanuddin A, Kim SH, Chae HJ, Jang JW, Park do H, Lee SS, Lee SK, Kim MH. EUS-guided radiofrequency ablation of the porcine pancreas. Gastrointest Endosc 2012;76:1039–43, http://www.ncbi.nlm.nih.gov/pubmed/23078928. doi:10.1016/j.gie.2012.07.015.
- Deng L, Zou D, Shen W, Liu X, Sheng H, Xi L, Zeng J, Cao X. The clinical observation of laser ablation combined with chemotherapy in post-operative colorectal cancers with liver metastasis. Minerva Chir. 2017;72:18–23, http://www.ncbi.nlm.nih.gov/pubmed/27787480. doi: 10.23736/S0026-4733.16.07204-7.
- Pai M, Habib N, Senturk H, Lakhtakia S, Reddy N, Cicinnati VR, Kaba I, Beckebaum S, Drymousis P, Kahaleh M, Brugge W. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015;7:52–9, http://www.ncbi.nlm.nih.gov/pubmed/25914783. doi:10.4240/wjgs.v7.i4.52.
- Arcidiacono PG, Carrara S, Reni M, Petrone MC, Cappio S, Balzano G, Boemo C, Cereda S, Nicoletti R, Enderle MD, Neugebauer A, von Renteln D, Eickhoff A, Testoni PA. Feasibility and safety of EUS-guided cryothermal ablation in patients with locally advanced pancreatic cancer. Gastrointest Endosc. 2012;76:1142–51, http://www.ncbi.nlm.nih.gov/pubmed/23021160. doi:10.1016/j.gie.2012.08.006.
- Pacella CM, Francica G, Di LF, Arienti V, Antico E, Caspani B, Magnolfi F, Megna AS, Pretolani S, Regine R. Long-term outcome of cirrhotic patients with early hepatocellular carcinoma treated with ultrasound-guided percutaneous laser ablation: a retrospective analysis. J Clin Oncol Official J Am Soc Clin Oncol. 2009;27:2615–21 doi:10.1200/JCO.2008.19.0082.
- Lahat E, Eshkenazy R, Zendel A, Zakai BB, Maor M, Dreznik Y, Ariche A. Complications after percutaneous ablation of liver tumors: a systematic review. Hepatobiliary Surg Nutri. 2014;3:317
- Arienti V, Pretolani S, Pacella CM, Magnolfi F, Caspani B, Francica G, Megna AS, Regine R, Sponza M, Antico E. Complications of laser ablation for hepatocellular carcinoma: a multicenter study. Radiology. 2008;246:947 doi:10.1148/radiol.2463070390.
- Ahmed M, Brace CL, Lee FT, Jr, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011;258:351–69, http://www.ncbi.nlm.nih.gov/pubmed/21273519. doi:10.1148/radiol.10081634.
- Dong S, Jian K, Kong F, Kong J, Gao J, Shan K, Wang S, Ding X, Sun W, Zheng L. Insufficient radiofrequency ablation promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells through Akt and ERK signaling pathways. J Translational Med. 2013;11:1–10. doi:10.1186/1479-5876-11-273.
- Ohno T, Kawano K, Yokoyama H, Tahara K, Sasaki A, Aramaki M, Kitano S. Microwave coagulation therapy accelerates growth of cancer in rat liver. J Hepatol. 2002;36:774–9. doi:10.1016/S0168-8278(02)00058-2.
- Franco B, Patrizia C, Silvia G, Emanuela R, Rita BP, Anna C, Giovannino C, Mario R. Local tumor progression of hepatocellular carcinoma after microwave percutaneous ablation: A preliminary report. Gastroenterology Res. 2012;5:28–32
- Tombesi P, Di VF, Sartori S. Laser ablation for hepatic metastases from neuroendocrine tumors. Ajr Am J Roentgenol. 2015;204:W732. doi:10.2214/AJR.14.14242.
- Pacella CM, Bizzarri G, Francica G, Bianchini A, De NS, Pacella S, Crescenzi A, Taccogna S, Forlini G, Rossi Z. Percutaneous laser ablation in the treatment of hepatocellular carcinoma with small tumors: analysis of factors affecting the achievement of tumor necrosis. J Vascular Interventional Radiol. 2005;16:1447–57. doi:10.1097/01.RVI.90000172121.82299.38.
- Alzaraa A, Gravante G, Wen YC, Al-Leswas D, Morgan B, Dennison A, Lloyd D. Contrast-enhanced ultrasound in the preoperative, intraoperative and postoperative assessment of liver lesions. Hepatol Res. 2013;43:809–19. doi:10.1111/hepr.12044.
- Frericks BB, Ritz JP, Albrecht T, Valdeig S, Schenk A, Wolf KJ, Lehmann K. Influence of intrahepatic vessels on volume and shape of percutaneous thermal ablation zones: in vivo evaluation in a porcine model. Invest Radiol. 2008;43:211–8, http://www.ncbi.nlm.nih.gov/pubmed/18340244. doi:10.1097/RLI.0b013e31815daf36.
- Yellin SA, LaBruna A, Anand VK. Nd:YAG laser treatment for laryngeal and hypopharyngeal hemangiomas: a new technique. Ann Otol Rhinol Laryngol 1996;105:510–5, http://www.ncbi.nlm.nih.gov/pubmed/8678425. doi:10.1177/000348949610500703.
