ABSTRACT
The evidence emerged from the TOURANDOT trial encourages evaluating the role of anthropometric determinants on treatment outcomes in HER2-negative metastatic breast cancer patients treated with bevacizumab-including regimens. We thus analyzed data from a subgroup of these patients from a larger cohort previously assessed for treatment outcomes. Patients were included in the present analysis if body mass index values had been recorded at baseline. Clinical benefit rates, progression free survival and overall survival were assessed for the overall study population and subgroups defined upon molecular subtype. One hundred ninety six patients were included (N:196). Body mass index showed no impact on clinical benefit rates in the overall study sample and in the luminal cancer subset (p = 0.12 and p = 0.79, respectively), but did so in the triple negative subgroup, with higher rates in patients with body mass index ≥25 (p = 0.03). In the overall study sample, body mass index did no impact progression free or overall survival (p = 0.33 and p = 0.67, respectively). Conversely, in triple negative patients, progression free survival was significantly longer with body mass index ≥25 (6 vs 14 months, p = 0.04). In this subset, overall survival was more favorable (25 vs 19 months, p = 0.02). The impact of the molecular subtype was confirmed in multivariate models including the length of progression free survival, and number of metastatic sites (p < 0.0001). Further studies are warranted to confirm our findings in more adequately sized, ad hoc, prospective studies.
Introduction
HER2-negative, metastatic breast cancer (MBC) still represents one of the greatest challenges for medical oncologists. In these patients, the addition of bevacizumab, a humanized monoclonal antibody against VEGF-A, to first-line chemotherapy has significantly improved the overall response rate (ORR) and progression free survival (PFS), but has not impacted overall survival (OS).Citation1-Citation3
Overall survival has been recently addressed in the TURANDOT trial, a non-inferiority, randomized, phase III trial comparing outcomes from two approved bevacizumab-containing regimens for first-line treatment of HER2-negative MBC. The non-inferiority criterion was met in stratified analyses of data from both the per protocol and ITT population, while results from unstratified analyses were not supportive, either using a per protocol approach, or considering the ITT population. Data from the TURANDOT trial were also analyzed across subgroups differing by clinically relevant variables. The inherent results highlighted the role of body surface area and menopausal status as substantial source of heterogeneity of treatment effects in terms of OS.Citation4 A further attempt of identifying patients who may most benefit from bevacizumab-including regimens was based on the evaluation of the predictive role of plasma VEGF-A in HER2-negative MBC patients recruited within the MERiDian trial, a blind, randomized, placebo-controlled, phase III trial of bevacizumab plus paclitaxel versus placebo plus paclitaxel. No evidence in support of the predictive role of pVEGF-A emerged from the MERiDian trial or from the separately published analysis exclusively focused on the subgroup of Japanese patients from this same study.Citation5,Citation6
We have previously addressed treatment outcomes in a larger cohort of HER2-negative MBC patients treated with bevacizumab and paclitaxel in first-line chemotherapy.Citation7 In light of the evidence emerged from recent literature and within our previously established research pipeline on the role of anthropometric and metabolic determinants of treatment efficacy in breast and ovarian cancer, we have now focused on a more restricted subgroup of patients from the original cohort with available data on body mass index (BMI) values at baseline assessment.Citation8-Citation15 In this patient subgroup, data on several patient- and disease-related features were also analyzed and reinterpreted in light of the evidence emerging from the BMI-related analysis. This study was designed and implemented within a real world setting.Citation4-Citation6
Materials and methods
The present analysis was performed to explore the impact of several patient- and disease-related features, with a particularly specific focus on anthropometrics in a subset of patients from a larger study of HER2-negative MBC patients treated with paclitaxel and bevacizumab in first-line (N:314). Formal approval for the main and corollary studies was obtained from the institutional review boards of the coordinating and satellite centres. A written informed consent was secured from every study participant prior to study procedures. The study conduct was compliant with the Helsinki Declaration. The methods applied were extensively reported elsewhere.Citation7 Briefly, in the main study, HER2-negative MBC patients were identified and recruited from 12 Italian cancer centres. Treatment administration was compliant with the most updated indications and recommendations. Prior to data collection and medical records retrieving, an ad hoc database was set up and implemented. Collected data included patient anthropometrics (weight, height, BMI), demographics, morphological and clinical characteristics referred to the disease and patients of interest. Data on the administered therapy/ies and related outcomes were also made available for this study purposes. BMI values were obtained by dividing weight in kilograms by the square of the height in meters. This variable was addressed as categorical and a conventional cut off value, i.e., 25, was used to define its modalities.Citation16 In specific regard to outcome definition and assessment, objective response (OR) was reported using the conventional Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1. Clinical benefit rate (CBR) was codified as the percentage of patients with an objective response or stable disease for a minimum of 6 months. Progression free survival (PFS) was defined as the time window between treatment initiation and interruption due to disease progression or death from any cause. Overall survival (OS) was defined as the time from the start of treatment to patient death from any cause.
Statistical analysis. Descriptive analyses were performed for all the variables of interest. Medians and ranges were assessed for continuous variables, while frequencies and percentages were computed for categorical variables. Patient characteristics were first described for the overall study population and then for subgroups defined upon molecular characteristics and compared across BMI strata using X2 or Fisher test, with this choice being driven by the size and number of groups compared. Two main subgroups were defined and distinguished on the basis of the immunohistochemical assessment of estrogen and/or progesterone receptor/s (ER/PgR), namely, luminal cancer cases and triple negative (TN) cancer cases. The Hazard Ratio (HR) and 95% confidence interval (CI) were estimated for each variable. The impact of specific determinants on PFS and OS was evaluated by Cox univariate model. The Kaplan–Meier method and the log-rank test were used to analyze and assess differences in survival. Kaplan-Meier curves were estimated for the entire study population and for subgroups defined upon BMI and molecular subtypes. Multivariate models included variables testing significant at univariate analysis and/or being considered on the basis of their biological plausibility and/or literature data. Interactions terms were added to the multivariate models when appropriate. Significance was defined at the p≤0.05 level. The SPSS software (SPSS version 21.0, SPSS Inc., Chicago, IL) was used for all statistical evaluations.
Results
This analysis was carried out in 196 patients (N:196) diagnosed and treated at the Institutions involved from 2008 through 2015. Patients were eligible to contribute data to our analysis if having received at least one cycle of paclitaxel-bevacizumab and with BMI data recorded at baseline. In , the main patient and tumor characteristics are shown for the overall study population. When globally considered, median age at treatment start was 56 years (31-82). The ECOG PS assigned was 0 in most of our patients (121; 61.7%). The most common histology and molecular subclass were ductal invasive breast carcinoma (166; 84.7%) and luminal breast cancer (146; 74.5%), respectively. In 134 (68.4%) patients the Ki-67% expression was higher than 14. As concerns previous treatments, neo/adjuvant chemotherapy was administered in 138 (70.4%) women, 120 (61.2%) patients received adjuvant endocrine agents, while 109 (55.6%) patients received adjuvant radiotherapy. Thirty-six patients (18.4%) were metastatic at diagnosis. The metastatic spread was limited to one single site in 67 patients (34.2%). Two 2 metastatic sites were identified in 62 women (31.6%), and 67 (34.2%) patients showed metastatic involvement of 3 or more sites. Visceral involvement was present in 119 (60.7%) patients, while an exclusive bone involvement was represented in 23 (11.7%) patients only.
Table 1. Main patient and tumor characteristics (N: 196 pts).
In , patient and disease characteristics were compared across subgroups defined upon BMI, using a 25 cut off value. Overall, leaner patients tended to be slightly younger (p = 0.004) and had less commonly received neo-/adjuvant chemotherapy or adjuvant radiotherapy (p = 0.03 and 0.009, respectively).
Table 2. Main patient and tumor characteristics by categories of body mass index (N: 196 pts).
As shown in , all 196 patients were evaluable for response. No significant differences in response rates were observed by BMI, with an overall response rate (ORR) of 64% in patients with BMI < 25 and of 67% in patients with BMI≥25 (p = 0.64). Disease control rate (DCR), defined as CR, PR and SD lasting ≥6 months, was recorded in 83% of patients with BMI<25 and 91% of patients with BMI ≥ 25 (p = 0.12). ORR did not significantly differ in patient subgroups defined upon molecular subtypes across BMI strata (p = 0.99 and p = 0.49). Conversely, DCR was significantly influenced by BMI in TN tumor cases, with better outcomes in patients with higher BMI (p = 0.03).
Table 3. Response and disease control rate in the overall study population and subgroups defined upon molecular subtype across BMI strata (N:196).
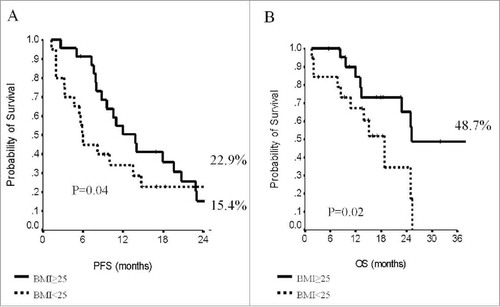
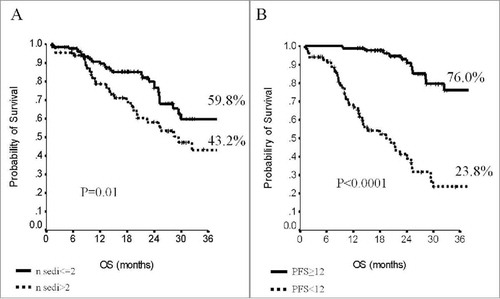
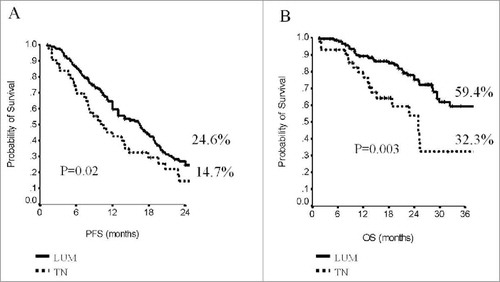
When addressing survival outcomes in the overall patient population, median PFS was 14 months (95% CI, 11–17), and median OS was 40 months (95% CI, 28–52). Survival outcomes were both affected by the molecular subtype (). At 24 months, 24.6% of patients within the luminal subtype group were free from disease progression compared to 14.7% of patients from the TN subgroup (p = 0.02). In regard to OS, the estimates showed significantly better outcomes in patients from the luminal subgroup (59 vs 32.3%, p = 0.003). No differences in terms of PFS or OS were observed in the overall population when analyzing data by BMI (p = 0.33 and p = 0.67, respectively) (supplementary Table S1). When stratifying by molecular subtype, in the subset of patients with hormone-receptor positive tumors, PFS and OS were independent on BMI (p = 0.85 and p = 0.41, respectively). Conversely, in the TN subgroup, median PFS was 6 months (95% CI, 5–7) in patients with BMI<25 and 14 months (95% CI, 10–18) in patients with BMI≥ (p = 0.04). Similarly, median OS was substantially longer in TN patients with BMI≥25 (25 vs 19 months, p = 0.02). Survival data in patients from the TN subgroup across BMI strata are displayed in . In univariate models, OS was further affected by the number of metastatic sites (p = 0.01) and length of PFS (p<0.0001), with more favorable outcomes in patients with the lowest metastatic burden and PFS≥12 month interval ( and ). The impact of molecular subtype, length of PFS, and number of metastatic sites was confirmed in multivariate models ().
Table 4. Multivariate analysis of factors impacting treatment outcomes in the overall study population (N:196).
Figure 1. Progression-Free Survival (A) and Overall Survival (B) by molecular subtype in the overall study population (N:196).
Discussion
In this multicenter, observational study, we analyzed data from 196 patients with HER2-negative MBC treated with paclitaxel and bevacizumab in first-line with available BMI data at baseline assessment. This patient subgroup originated from a larger cohort, whose treatment outcomes had been addressed in previous work.Citation7 In the present manuscript, data where analyzed to assess the impact of BMI on treatment outcomes. In the overall patient cohort, stratification by BMI did not affect ORR or DCR, neither did we observe any effect in the luminal type subgroup. Conversely, in TN patients (N:43), we found significantly higher DCR associated with BMI values ≥25. In the overall study sample (N:196), BMI did not influence PFS, which was solely affected by the molecular subtype. In analysis stratified by molecular subtype, we observed significantly more favorable PFS and OS in TN patients with BMI ≥25. In the overall study sample, a lower number of metastatic sites and longer length of PFS were associated with significantly longer OS.
In our study, differences by BMI did not significantly affect treatment outcomes in the overall study sample, but did so in analysis of DCR stratified by molecular subtype and in uni- and multivariate Cox models of survival. Both DCR and survival outcomes appeared to be positively influenced by higher values of BMI. Current knowledge on the role of overweight and obesity in metastatic breast cancer is limited. Gennari and colleagues analyzed data from 489 MBC cancer patients treated with regimens including anthracyclines and taxanes in three trials of first-line chemotherapy. No association was detected between BMI and PFS or OS.Citation17 However, the relevant differences in terms of treatment administered may at least partly explain the inconsistency between these findings and those from our study. As previously mentioned, in the TURANDOT trial, treatment effects on survival showed significant differences by body surface area and menopausal status. Among patients randomized to paclitaxel plus bevacizumab, those who were premenopausal and with a higher body surface area (≥1.8 m2) exhibited the most favorable outcomes.Citation4 Unfortunately, data on menopausal status were not available for our study population. When using “age in years” as a proxy of menopausal status, no significant differences emerged in the overall study population or subgroups defined upon the molecular subtype for any of the outcomes considered (data available upon request). Although findings from the metastatic setting do not necessarily apply to the early setting, in discussing this study results we are also considering evidence on BMI and treatment outcomes from previous studies carried out in the neoadjuvant setting. In a phase II clinical trial exploring efficacy and toxicity outcomes of the combined use of epirubicin and trastuzumab in 45 HER2-positive breast cancer patients with locally advanced operable disease, we observed better outcomes in patients with BMI≥25 and whose tumors did not express hormone receptors.Citation10 Beyond the not negligible differences in terms of patients characteristics, disease features and study design, these findings are fully consistent with what reported in the study herein presented. Conversely, in young breast cancer patients, i.e., women aged 45 or less, treated with neoadjuvant CT, we observed that lower values of BMI were associated with longer OS, along with non-TN molecular subtype and adjuvant RT.Citation12 However, this latter study population included a limited number of HER2-positive cases, i.e. 19/45, while the vast majority of tumors expressed hormone receptors, i.e. 52/86. In addition, differences in treatment outcomes may also be driven by a more aggressive behavior, which is well documented in women diagnosed at a young age.Citation18,Citation19
Our study has some limitations. Firstly, the observational, retrospective design increases the chance for bias and confounding, which we attempted to contain by statistical methods, e.g. data stratification and adjustment in multivariate models. Secondarily, missing data of potentially relevant variables have limited our ability to compare findings from this study to those from the TURANDOT, as well exemplified by the lack of information on menopausal status, although we considered age as a proxy variable with no significant results. Thirdly, the sample size is limited, still, potentially acceptable given the “operating” nature of our setting, i.e. a real world setting, and the difficulties related to the conduct of such a study in a real world population.
Our study also has strengths. Current knowledge on the role of BMI on treatment outcomes in MBC is scant and the extent to which the available evidence from the early setting may be translated to these patients is openly questionable. Furthermore, the interest of the scientific community towards life-style related factors with an impact on cancer is fuelled by their modifiable nature, which may translate into improved outcomes in breast and other cancers.Citation20-Citation22 In this view, investigating the role of BMI on treatment outcomes of HER2-negative metastatic breast cancer patients may appear particularly appealing, since this disease is still considered incurable.Citation23 Our main finding is represented by the impact of BMI in the subgroup of patients with TN disease, with reflections in terms of both DCR and survival. In multivariate Cox models, the addition of an interaction terms for BMI and molecular subgroups yielded significant results. However, given the hypothesis-generating nature of our study and the limited size of patients with TN MBC (N:43), our findings warrant confirmation in adequately sized, prospective studies of HER2-negative MBC patients treated with first-line paclitaxel and bevacizumab. When designing such studies, researchers will hopefully consider difficulties stemming from the current limitations of the inherent literature, not only from a quantitative, but also from a qualitative standpoint. Indeed, comparing evidence from studies differing significantly by design, patient-/disease characteristics and administered treatment may be misleading and methodologically incorrect.
In conclusions, in this multicentric, observational study of HER2-negative MBC patients treated with paclitaxel and bevacizumab in first-line we found evidence of the role of BMI on DCR, PFS and OS in the subgroup of TN patients. This finding is of difficult interpretation, given the limitations of the currently available evidence from our research group and from other researchers. Given the still incurable character of the disease of interest and the interest towards BMI and, more generally, factors related to life-style with a potential impact on treatment outcomes, further studies are warranted to confirm our findings and allow a deeper comprehension of the underlying mechanisms.
Ethics approval and consent to participate
The study protocol was evaluated for approval by the Institutional Review Board of the coordinating centre, i.e. the Regina Elena National Cancer Institute of Rome. The approved documents were then transmitted for further examination to each of the participating centers. Written consents were secured from the study participants.
Consent for publication
Individual patient data were anonymized prior to inclusion in our analyses. The institutional consent form will be made available upon request at any stage (eventually including publication).
Availability of data and material
The datasets during and/or analyses during the current study available from the corresponding author on reasonable request.
Disclosure Statement
The authors declare they have no conflict of interest.
sup_mat_KCBT_1416938.docx
Download MS Word (14.8 KB)Acknowledgments
We thank Ana Maria Edlisca and Dr Rosa Carbone for the technical and administrative support to our work.
Additional information
Funding
References
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–76. doi:10.1056/NEJMoa072113. PMID:18160686.
- Miles DW, Chan A, Dirix LY, Cortés J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–47. doi:10.1200/JCO.2008.21.6457. PMID:20498403.
- Robert NJ, Diéras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, et al. RIBBON-1: Randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–60. doi:10.1200/JCO.2010.28.0982. PMID:21383283.
- Zielinski C, Láng I, Inbar M, Kahán Z, Greil R, Beslija S, Stemmer SM, Zvirbule Z, Steger GG, Melichar B, et al. Bevacizumab plus paclitaxel versus bevacizumab plus capecitabine as first-line treatment for HER2-negative metastatic breast cancer (TURANDOT): Primary endpoint results of a randomised, open-label, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17:1230–9. doi:10.1016/S1470-2045(16)30154-1. PMID:27501767.
- Miles D, Cameron D, Bondarenko I, Manzyuk L, Alcedo JC, Lopez RI, Im SA, Canon JL, Shparyk Y, Yardley DA, et al. Bevacizumab plus paclitaxel versus placebo plus paclitaxel as first-line therapy for HER2-negative metastatic breast cancer (MERiDiAN): A double-blind placebo-controlled randomised phase III trial with prospective biomarker evaluation. Eur J Cancer. 2017;70:146–55. doi:10.1016/j.ejca.2016.09.024. PMID:27817944.
- Masuda N, Takahashi M, Nakagami K, Okumura Y, Nakayama T, Sato N, Kanatani K, Tajima K, Kashiwaba M, et al. First-line bevacizumab plus paclitaxel in Japanese patients with HER2-negative metastatic breast cancer: Subgroup results from the randomized Phase III MERiDiAN trial. Jpn J Clin Oncol. 2017;47:385–92. doi:10.1093/jjco/hyx001. PMID:28158579.
- Gamucci T, Mentuccia L, Natoli C, Sperduti I, Cassano A, Michelotti A, Di Lauro L, Sergi D, Fabi A, Sarobba MG, et al. A Real-World Multicentre Retrospective Study of Paclitaxel-Bevacizumab and Maintenance Therapy as First-Line for HER2-Negative Metastatic Breast Cancer. J Cell Physiol. 2017;232:1571–78. doi:10.1002/jcp.25685. PMID:27861874.
- Pizzuti L, Marchetti P, Natoli C, Gamucci T, Santini D, Scinto AF, Iezzi L, Mentuccia L, D'Onofrio L, Botticelli A, et al. Fasting glucose and body mass index as predictors of activity in breast cancer patients treated with everolimus-exemestane: The EverExt study. Sci Rep. 2017;7:10597. doi:10.1038/s41598-017-10061-2. PMID:28878375.
- Barba M, Vici P, Pizzuti L, Di Lauro L, Sergi D, Di Benedetto A, Ercolani C, Sperati F, Terrenato I, Botti C, et al. Body mass index modifies the relationship between γ-H2AX, a DNA damage biomarker, and pathological complete response in triple-negative breast cancer. BMC Cancer. 2017;17:101. doi:10.1186/s12885-016-3045-z. PMID:28166748.
- Pizzuti L, Barba M, Giannarelli D, Sergi D, Botti C, Marchetti P, Anzà M, Maugeri-Saccà M, Natoli C, Di Filippo S, et al. Neoadjuvant Sequential Docetaxel Followed by High-Dose Epirubicin in Combination With Cyclophosphamide Administered Concurrently With Trastuzumab. The DECT Trial. J Cell Physiol. 2016;231:2541–7. doi:10.1002/jcp.25432. PMID:27187274.
- Vici P, Pizzuti L, Di Lauro L, Conti L, Mandoj C, Antenucci A, Digiesi G, Sergi D, Amodio A, Marchetti P, et al. Metabolic Determinants and Anthropometric Indicators Impact Clinical-pathological Features in Epithelial Ovarian Cancer Patients. J Cancer. 2016;7:516–22. doi:10.7150/jca.13578. PMID:26958087.
- D'Aiuto M, Chirico A, De Riggi MA, Frasci G, De Laurentiis M, Di Bonito M, Vici P, Pizzuti L, Sergi D, Maugeri-Saccà M, et al. Body mass index and treatment outcomes following neoadjuvant therapy in women aged 45 years or younger: Evidence from a historic cohort. Cancer Biol Ther. 2016;17:470–6. doi:10.1080/15384047.2016.1156265. PMID:26934127.
- Barba M, Pizzuti L, Sperduti I, Natoli C, Gamucci T, Sergi D, Di Lauro L, Moscetti L, Izzo F, Rinaldi M, et al. Body Mass Index and Treatment Outcomes in Metastatic Breast Cancer Patients Treated With Eribulin. J Cell Physiol. 2016;231:986–91. doi:10.1002/jcp.25213. PMID:26449308.
- Vici P, Crispo A, Giordano A, Di Lauro L, Sperati F, Terrenato I, Pizzuti L, Sergi D, Mottolese M, Botti C, et al. Anthropometric, metabolic and molecular determinants of human epidermal growth factor receptor 2 expression in luminal B breast cancer. J Cell Physiol. 2015;230:1708–12. doi:10.1002/jcp.24891. PMID:25510909.
- Vici P, Sperati F, Maugeri-Saccà M, Melucci E, Di Benedetto A, Di Lauro L, Pizzuti L, Sergi D, Terrenato I, Esposito L, et al. p53 status as effect modifier of the association between pre-treatment fasting glucose and breast cancer outcomes in non diabetic, HER2 positive patients treated with trastuzumab. Oncotarget. 2014;5:10382–92. doi:10.18632/oncotarget.2060. PMID:25071015.
- http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi.
- Gennari A, Nanni O, Puntoni M, DeCensi A, Scarpi E, Conte P, Antonucci G, Amadori D, Bruzzi P. Body mass index and prognosis of metastatic breast cancer patients receiving first-line chemotherapy. Cancer Epidemiol Biomarkers Prev. 2013;22:1862–67. doi:10.1158/1055-9965.EPI-13-0595. PMID:23945199.
- Acharya CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, Febbo PG, Nevins JR, Potti A, Blackwell KL, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–30. doi:10.1200/JCO.2007.14.2471. PMID:18612148.
- Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. 2009;36:237–49. doi:10.1053/j.seminoncol.2009.03.001. PMID:19460581.
- Renehan AG, Soerjomataram I. Obesity as an Avoidable Cause of Cancer (Attributable Risks). Recent Results Cancer Res. 2016;208:243–256. doi:10.1007/978-3-319-42542-9_13. PMID:27909911.
- Bittoni MA, Fisher JL, Fowler JM, Maxwell GL, Paskett ED. Assessment of the effects of severe obesity and lifestyle risk factors on stage of endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:76–81. doi:10.1158/1055-9965.EPI-12-0843. PMID:23118146.
- Morimoto LM, White E, Chen Z, Chlebowski RT, Hays J, Kuller L, Lopez AM, Manson J, Margolis KL, Muti PC, et al. Obesity, body size, and risk of postmenopausal breast cancer: The Women's Health Initiative (United States). Cancer Causes Control. 2002;13:741–51. doi:10.1023/A:1020239211145. PMID:12420953.
- Reddy S, Raffin M, Kaklamani V. Targeting angiogenesis in metastatic breast cancer. Oncologist 2012. 17:1014–26.