ABSTRACT
The tumor-shed antigen CA125 has recently been found to bind certain monoclonal antibodies (mAbs) and suppress immune-effector mediated killing through perturbation of the Fc domain with CD16a and CD32a Fc-γ activating receptors on immune-effector cells. Amatuximab is a mAb targeting mesothelin whose mechanism of action utilizes in part antibody-dependent cellular cytotoxicity (ADCC). It is being tested for its therapeutic activity in patients with mesothelioma in combination with first line standard-of-care. To determine if CA125 has immunosuppressive effects on amatuximab ADCC and associated clinical outcomes, post hoc subgroup analysis of patients from a Phase 2 study with primary diagnosed stage III/IV unresectable mesothelioma treated with amatuximab plus cisplatin and pemetrexed were conducted. Analysis found patients with baseline CA125 levels no greater than 57 U/m (∼3X the upper limit of normal) had a 2 month improvement in progression free survival (HR = 0.43, p = 0.0062) and a 7 month improvement in overall survival (HR = 0.40, p = 0.0022) as compared to those with CA125 above 57 U/mL. In vitro studies found that CA125 was able to bind amatuximab and perturb ADCC activity via decreased Fc-γ-receptor engagement. These data suggest that clinical trial designs of antibody-based drugs in cancers producing CA125, including mesothelioma, should consider stratifying patients on baseline CA125 levels for mAbs that are experimentally determined to be bound by CA125.
Introduction
Recent studies have found that the CA125 tumor-shed antigen (TSA) has immunosuppressive activities via binding to regulatory Siglec-type receptors, and most recently, through the direct binding to a subset of IgG1 isotype monoclonal antibodies (mAbs).Citation1-4 The latter effect suppresses mAb Fc domain engagement with Fc-γ activating receptors CD16a and CD32a thereby decreasing mAb-mediated antibody dependent cellular cytotoxicity (ADCC).Citation4,Citation5 This mechanism along with the tumor's ability to manipulate naturally occurring immune checkpoint pathways provide complementary ways by which tumors avert host immune responses. While agents that can overcome tumor-mediated immune checkpoint inhibition have gained broad interest due to their improvements in clinical response to various tumor types, few studies have been conducted that address TSA-mediated immune suppression. This alternative pathway is likely to be involved in tumor types or disease stages in which immune checkpoint inhibitors have little to no benefit. This hypothesis is supported by the observations reported from a Phase 3 clinical trial testing the experimental agent farletuzumab, a humanized mAb targeting folate receptor alpha, in patients with first-relapsed, platinum-sensitive ovarian cancer (NCT00849667). This trial found that a pre-specified subgroup of patients treated with farletuzumab plus standard-of-care (SOC) carboplatin/taxane with baseline serum CA125 levels no greater than 3X the upper limit of normal (≤ 3X ULN) demonstrated clinical improvements in both progression free survival (PFS) (hazard ratio [HR] 0.49, p = 0.0028) and overall survival (OS) (HR 0.44, p = 0.0108) as compared to patients treated with SOC and placebo.Citation4-6 This effect was not observed in the placebo control group (N = 357) when comparing patients exhibiting CA125 levels above or below the CA125 3X ULN threshold [PFS (HR 0.88, p = 0.481) and OS (HR 0.90, p = 0.638)], thus demonstrating that CA125 baseline levels are not a prognostic effect of SOC chemotherapy. Interestingly, the clinical utility of the checkpoint inhibitors pembrolizumab and nivolumab in ovarian cancer has not been as robust as seen in other cancer types.Citation7
Amatuximab is a chimerized high affinity monoclonal IgG1-isotype antibody targeting the GPI-anchored mesothelin cell surface protein.Citation8,Citation9 In vitro, amatuximab elicits anti-tumor effects via ADCC against mesothelin-expressing tumor cells and blocks heterotypic cell adhesion between mesothelin and cells expressing its cognate receptor MUC16/CA125.Citation10-12 In preclinical models, amatuximab was shown to be active against mesothelin-positive tumors as a single agent as well as in combination with standard chemotherapy. Amatuximab has been tested in an open-labeled, single-armed, Phase 2 clinical trial in patients with unresectable malignant pleural mesothelioma (MPM) in combination with first-line standard-of-care (SOC) cisplatin plus pemetrexed chemotherapy (NCT00738582).Citation13 While the study did not meet its targeted PFS primary endpoint as compared to historical control values, median OS and overall response rates (ORR) were favorable to the values reported in the Phase 3 cisplatin plus pemetrexed registration trial (14.8 vs 13.3 months and 33% vs 21%, respectively).Citation14 Moreover, responding patients (those with partial response or long term stable disease) exhibited an extended OS of greater than 24 months, suggesting that the combination of amatuximab plus cisplatin/pemetrexed may be effective in a subgroup of patients. As the recently concluded farletuzumab Phase 3 trial described above also showed evidence of clinical benefit in patients with low serum CA125Citation6 and an immuno-suppressive effect of CA125 on farletuzumab-mediated ADCC,Citation4 it was suggestive a similar effect may occur with amatuximab. To test this hypothesis, we conducted post hoc analyses on the amatuximab Phase 2 clinical specimens as well as in vitro cellular and molecular analysis of CA125 on amatuximab biological function. Analysis included clinical correlations of commonly monitored tumor-shed antigens (TSA), including CA125, as well as patient-specific correlations of baseline serum CA125 levels and tumor lesion size. Here we report that CA125 binds amatuximab and in turn elicits a negative effect on ADCC through perturbation of antibody-CD16a and -CD32a Fc-γ receptor engagement. Moreover, CA125 baseline levels were found to be predictive of amatuximab's clinical response in patients exhibiting less than 3X ULN. These findings are consistent with a role for CA125 in immunosuppressing antibody-mediated anti-tumor activity in a subset of tumor-targeting antibodies and patient clinical outcome.
Results
Baseline CA125 correlation and clinical outcome in patients treated with amatuximab plus cisplatin/pemetrexed
The sum of the longest diameter (SLD) of target lesions assessed by computerized tomography (CT) scan along with baseline serum CA125 levels quantified by electrochemiluminescence immunoassay (EIA) were measured in all patients entering the MORAb-009-003 Phase 2 clinical study. PFS was assessed using EORTC modified RECIST v1.0Citation16 documenting patient-specific SLD prior to first treatment by independent review. CA125 analysis found that 75 of the 89 enrolled subjects had measurable baseline CA125 levels. Values ranged from 1 to 1266 U/mL with 29 patients (38.7%) having CA125 levels above the normal range. Serum cut-point analysis of baseline CA125 using log rank tests and maximal chi square methodologyCitation15 were used to compare PFS and OS among CA125 subgroups.Citation17 Analyses identified 57 U/mL to be the optimal cut-point threshold of CA125 in patients within this population. shows the demographic profile of patients with greater or less/equal than 57 U/mL CA125. Median age and gender were similar in both groups. Kaplan-Meier (KM) analysis showed that patients with ≤ 57 U/mL CA125 had a statistically significant improvement in PFS (HR = 0.43, p = 0.0062) and OS (HR = 0.40, p = 0.0022) as compared to those with > 57 U/mL ( and ). Overall response rate remained favorable in the low vs high CA125 levels (33% vs 20%, respectively). This effect is unlikely due to CA125 being prognostic for cisplatin/pemetrexed in 1st line treatment as several prior studies have found no association of CA125 levels and outcome in patients treated with first line SOC therapy (19, 20, R. Hassan personal observation). Demographics revealed that patients with ≤ 57 U/mL CA125 consisted of nine patients with STAGE IB and STAGE II disease, which has been reported to be a positive prognostic factor in MPM.Citation21 To determine the impact of these patients on the overall CA125 clinical outcome relationship between the 57 U/mL groups, KM analysis was conducted on patients with only Stage III/IV disease. Results found that patients with ≤ 57 U/mL CA125 continued to exhibit a significant improvement in PFS (HR = 0.42, p = 0.0074) and OS (HR = 0.38, p = 0.0016) as compared to those above the threshold ( and ). Optimal cut-point analysis of two other prominent TSAs used in clinical assessment of mesothelioma, CA15-3 and CA19-9,Citation21 showed no correlation with response.
Table 1. Patient demographics in CA125 subgroups.
Figure 1. Clinical response of patients treated with amatuximab, cisplatin and pemetrexed above and below the optimal CA125 cut-point level. Kaplan Meier (KM) analysis of PFS (A) and OS (B) comparing amatuximab/cisplatin/pemetrexed treated patients with baseline serum CA125 levels. Red lines represent patient response with CA125 below the cut-point and green lines represent patients with CA125 above the cut-point. A significant linear increase in PFS and OS improvement [considered to be a HR value of approximately ≤ 0.5;Citation18] is observed in patients treated with amatuximab when sCA125 levels are less/equal to 57 U/mL. To confirm that the effect was not driven by the STAGE IB/II patients in the ≤ 57 U/mL CA125 subgroup, KM analysis of PFS (C) and OS (D) in STAGE III/IV only patients with > 57 U/mL or ≤ 57 U/mL CA125 was conducted. As shown STAGEIII/IV patients with CA125 levels ≤ 57 U/mL maintain a statistically improved PFS and OS. The circles represent censored subjects. All p values are two-sided.
![Figure 1. Clinical response of patients treated with amatuximab, cisplatin and pemetrexed above and below the optimal CA125 cut-point level. Kaplan Meier (KM) analysis of PFS (A) and OS (B) comparing amatuximab/cisplatin/pemetrexed treated patients with baseline serum CA125 levels. Red lines represent patient response with CA125 below the cut-point and green lines represent patients with CA125 above the cut-point. A significant linear increase in PFS and OS improvement [considered to be a HR value of approximately ≤ 0.5;Citation18] is observed in patients treated with amatuximab when sCA125 levels are less/equal to 57 U/mL. To confirm that the effect was not driven by the STAGE IB/II patients in the ≤ 57 U/mL CA125 subgroup, KM analysis of PFS (C) and OS (D) in STAGE III/IV only patients with > 57 U/mL or ≤ 57 U/mL CA125 was conducted. As shown STAGEIII/IV patients with CA125 levels ≤ 57 U/mL maintain a statistically improved PFS and OS. The circles represent censored subjects. All p values are two-sided.](/cms/asset/01b12909-d8eb-44b1-8538-039bbaa4285c/kcbt_a_1449614_f0001_oc.gif)
As reported by Kline et al., baseline CA125 levels did not correlate with measurable radiographic disease bulk in patients with ovarian cancer.Citation4 In this study, SLD at baseline ranged from 13 to 334 mm with a mean SLD of 95 mm. Analysis found no significant correlation to baseline CA125 levels (Pearson correlation coefficient of -0.112/0.046 for original/natural log scale, respectively) corroborating the findings in ovarian cancer and consistent with the scenario that MPM tumors may yield high or low levels of CA125 regardless of measurable disease as determined by radiology.
Soluble CA125 inhibits amatuximab-mediated ADCC
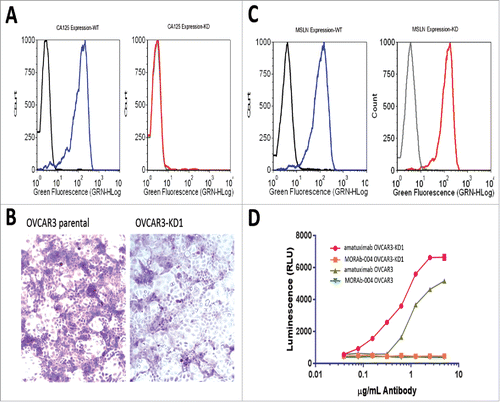
While CA125 has been previously reported to bind IgG1 isotype antibodies, it does not bind all IgG1 antibodies suggesting structural specificity is required for CA125-IgG1 interaction. To determine if soluble CA125 (sCA125) can bind and perturb amatuximab ADCC, we conducted ADCC assays using mesothelin-positive tumor cell lines and exogenously added sCA125 or tumor cell produced CA125. We first tested the ability of exogenously added sCA125 to perturb ADCC by adding patient-derived CA125 to amatuximab ADCC assays. As shown in , sCA125 was effective in suppressing the ADCC activity on mesothelin-positive tumor cell lines similar to that shown for farletuzumab.Citation4 This activity appeared non-cell specific as CA125 suppressed amatuximab-mediated ADCC in a dose-dependent manner when tested against a range of mesothelin-positive tumor cells including A431 cells ectopically expressing mesothelin at antibody concentrations ranging from 0.2 to 20 μg/mL (). This human epidermoid carcinoma cell line was previously shown by others to maintain high level mesothelin expression which was also observed for the engineered line employed here.Citation22 We next employed the isogenic human ovarian cancer cell lines OVCAR3 and OVCAR3-KD1, the latter of which has knocked-down levels of endogenous CA125 via shRNA as previously described and shown in .Citation4 Cells were stained with Periodic acid-Schiff (PAS) to determine if decreased CA125 expression had a meaningful effect on the overall glycoprotein profile of OVCAR3.Citation21 Interestingly, OVCAR3-KD1 cells had a significant reduction in staining when CA125 was suppressed, suggesting that it is one of the major glycan-containing proteins within these lines (). Flow cytometry found both lines express similar levels of mesothelin (). Amatuximab mediated significant ADCC signaling in the Jurkat-Luc reporter system with as little as 0.2 μg/mL amatuximab and maintained enhanced signaling up to 10 μg/mL against the CA125-knockdown OVCAR3-KD1 cells as compared to parental OVCAR3 cells (). As expected, no ADCC signaling was observed using this system when an irrelevant, non-target cell binding mAb (MORAb-004) was employed.
Figure 2. Effects of soluble CA125 on amatuximab ADCC. sCA125 effects on Jurkat-Luc effector cells. Jurkat-Luc cells were incubated with 6 µg/mL of amatuximab and CHO-mesothelin (panel A) or A431-mesothelin (panel B) cells with increasing concentrations of amatuximab. As shown, amatuximab inhibits amatuximab-mediated ADCC signaling in a dose-dependent manner. All data represent values of at least triplicate experiments and all meet p < 0.05.
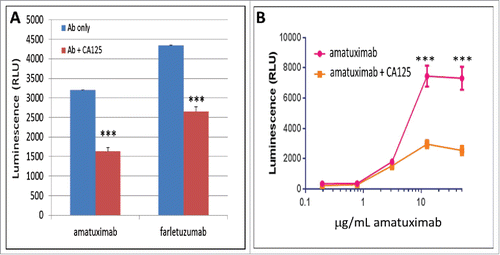
Figure 3. Effects of endogenously produced CA125 on amatuximab ADCC. The isogenic cell lines OVCAR3 and OVCAR3-KD1 were used to test the effects of membrane-bound CA125 on amatuximab-mediated ADCC. A) FACS analysis of parental vs CA125 knockdown (KD) cells using anti-CA125 antibody demonstrates robust cell surface expression of CA125 in OVCAR3 cells in contrast to significantly reduced CA125 in OVCAR3-KD1 cells. Black peak represents cells stained with secondary antibody only. B) PAS staining of both lines showed a global reduction of glycoproteins in the OVCAR-KD1 line suggesting CA125 is a major glycoprotein in these lines. C) FACS analysis of parental OVCAR3 and OVCAR3-KD1 cells using amatuximab demonstrates robust cell surface expression of mesothelin in both lines. Black peak represents cells stained with secondary antibody only. D) OVCAR3 and OVCAR3-KD1 cell lines were tested for amatuximab-mediated ADCC activity. Amatuximab-mediated ADCC activation occurred in OVCAR3 parental line but was significantly increased in shRNA-CA125 suppressed OVCAR-3 (OVCAR-KD1 cells (red line) when incubated with greater than 0.2 μg/mL amatuximab and maintained above 10 μg/mL. ****p < 0.00002; *****p < 0.000002.
CA125 binds to amatuximab and perturbs CD16a Fc-γ receptor binding
CA125 has been previously shown to bind to a subset of IgG1-type antibodies and alter their ability to engage with CD16a Fc-γ receptor on cells and at the molecular level.Citation4,Citation23,Citation24 To determine if CA125 exerts its suppression on ADCC activity via a similar mechanism, we tested if CA125 could bind amatuximab via ELISA assay using biotinylated amatuximab and derived fragments as probes. As shown in , amatuximab is able to bind to CA125 and appears to bind within the (Fab’)2 domain (middle set of bars) but not the Fc domain, which is similar to what was observed for the anti-folate receptor alpha mAb, farletuzumab.Citation4
Figure 4. Cell-based and ELISA-based assays to test for CA125 binding to amatuximab and effect on antibody- CD16a Fc-γ receptor engagement. A) Biotinylated amatuximab and its (Fab’)2 fragment, but not its Fc fragment bind immobilized CA125. 96-well plates were coated with 15 KU/mL CA125 or human serum albumin (control) and probed with biotin-labeled amatuxiumab, (Fab’)2 or Fc fragments. ***p < 0.00002; *****p < 0.000002. B) Testing the effect of CA125 on Jurkat-CD16a binding using BRA assay. Jurkat-CD16a cells were incubated in wells coated with amatuximab and incubated with or without sCA125. CA125 inhibited Jurkat-CD16a-amatuximab binding (top row). Farletuzumab (middle row) was used as a positive control and humanized anti-tissue factor (TF) antibody (bottom row) was used as a negative control for the assay. Shown are duplicate experiments for each condition. C) CA125 suppresses Fc receptor binding to amatuximab. Amatuximab was incubated alone or with sCA125 and probed with biotinylated-CD16a, -CD32a or -CD64a Fc-γ receptors. As shown, CA125 caused a significant decrease of CD16a binding to amatuximab (50%, p = 0.0000009). Reduction in amatuximab binding to CD32a Fc receptors was also significant (29%, p < 0.003) while no inhibition was observed by CA125 on amatuximab binding to CD64a Fc receptor (0%, p = 0.380). Similar reactions were probed with anti-human IgG-HRP to ensure incubation of amatuximab with CA125 did not result in less amatuximab binding to wells (last set of bars). D) FcRn which also binds to the Fc domain of amatuximab was biotinylated and used as probe to determine if CA125 interfered with its ability to bind amatuximab Fc domain. As shown, FcRn binding is not affected by CA125 binding to amatuximab.
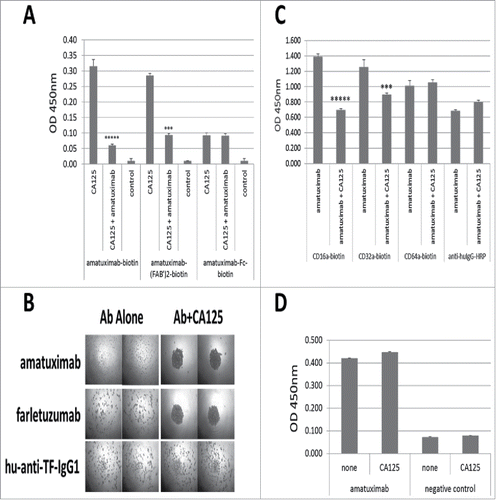
We next tested the effect of CA125 on blocking amatuximab-CD16a binding using cell- and ELISA-based assays. We employed the biological rolling assay (BRA) to determine if CA125 can perturb the engagement of amatuximab to CD16a, which is ectopically expressed by Jurkat-CD16a cells. The BRA assay utilizes microwell plates coated with amatuximab that are then seeded with Jurkat-CD16a with or without sCA125.Citation4 If CA125 perturbs cell surface Jurkat-CD16a-amatuxumab binding, the cells will not adhere to the amatuximab coated well walls and cluster at the bottom of the U-shaped well. As shown in , Jurkat-CD16a cells bind to amatuximab but are inhibited upon exposure to CA125 similar to farletuzumab (middle row), which was used as a positive control. The humanized anti-tissue factor antibody (hu-anti-TF-IgG1) that is not bound by CA125 in ELISA (not shown) and used as a negative control was unaffected by CA125 (bottom row). This inhibition appears to occur via binding to the (Fab’)2 domain as shown in and possibly allosterically inhibiting the binding to CD16a, and to a lesser extent CD32a as shown by ELISA (). The CA125 perturbation of CD16a and CD32a appeared specific and not a universal effect to Fc binding proteins as CA125 did not affect binding to the high affinity CD64a Fc-γ receptor () or to the FcRn receptor which binds to the C-terminal CH2 and CH3 motifs within the antibody Fc domain ().Citation25
Discussion
Here we demonstrate an association of baseline CA125 levels to clinical outcome in MPM patients treated with amatuximab plus cisplatin and pemetrexed. While the current study did not have a cisplatin/pemetrexed control arm, previous studies have reported that CA125 does not appear to be a prognostic indicator of response to SOC chemotherapyCitation19,Citation20,Citation26 (R. Hassan personal observation). The findings here parallel the effects of CA125 on the anti-folate receptor alpha antibody, farletuzumab, in patients with relapsed platinum-sensitive ovarian cancer and represents an independent study that reinforces the concept of CA125 as being a TSA with clinically relevant immuno-suppressive effects. Interestingly, reports have also found a negative clinical correlation of elevated CA125 serum levels in patients with refractory non-Hodgkin's Lymphoma,Citation27 suggesting that tumor-produced or induced CA125 may be a universal mechanism for a subset of tumors that avoid patient immune response via humoral immune suppression. While these antibodies have shown direct CA125 and Fc receptor perturbation, it should be noted that not all IgG1-type antibodies bind CA125.
A preliminary CA125 analysis from the amatuximab Phase 2 study using a linear CA125 cutoff analysis for maximal survival status previously identified 6 U/mL as an optimal cutoff value.Citation13 In this paper, a more typical statistical approach was used in which both survival status and survival time were derived using maximal chi square methodology and log rank tests for identifying the optimal baseline cut-point that identified 57 U/mL as the optimal threshold. Interestingly, 57 U/mL represents ∼2.7X the ULN using the diagnostic assay employed in our clinical study and trends closely to the value obtained in the farletuzumab Phase 3 MORAb-003-004 study, suggesting this threshold may be important for maximal antibody immune-effector suppression at the dose levels tested.Citation4,Citation6 Experimental studies using molecular and cellular based in vitro assays have suggested similar values.Citation4 Interestingly, while the CA125 ADCC suppression was maintained when levels of amatuximab were 10–20 μg/mL (), Gupta et al. reported that patients from the Phase 2 study, same as those tested in this study, with amatuximab Cmin levels > 32.9 μg/mL for PFS and > 38 μg/mL for OS had statistically improved outcomes as compared to those with levels below that value (log rank test 6.52 × 10−5 and 0.0202, respectively).Citation28 The fact that the ADCC suppression via CA125 reduces as antibody concentrations increase in in vitro assays and that 20% of patients had CA125 < 57 μ/mL suggests that a CA125/amatuximab threshold effect may occur that predicts effective exposure-response in context to serum CA125 levels. This hypothesis will need to be explored in future clinical studies that include larger patient populations to determine if a linear correlation exists.
While elevated levels of CA125 have been reported in a variety of solid cancer types,Citation29 few have reported on the prognostic and/or predictive levels in mesothelioma. Kabawat et al. were one of the first to report elevated CA125 levels in patients with malignant mesotheliomaCitation30 and Simsek et al. reported that CA125 levels appeared to correlate with tumor response to chemotherapy.Citation31 Immunohistochemistry (IHC) studies of mesothelioma tissue expression failed to detect reproducible homogeneous levels of CA125 directly on mesothelioma cells,Citation32 yet in vitro studies of primary human peritoneal mesothelial cells found them to produce CA125 at levels higher than observed for several prominent ovarian carcinoma cell lines for which CA125 is considered a marker of the disease.Citation33 This finding was consistent with those observed later on by Creaney et al. that found 44% of patients with malignant mesothelioma had CA125 serum levels above the upper limit of normal, which is in a similar range (39%) to patients within this study.Citation20 Their study also found that serum CA125 levels alone were not prognostic to SOC chemotherapy.
CA125 has been shown to bind cell surface-bound and soluble mesothelin produced by almost all mesotheliomas and ovarian cancers as well as some pancreatic lung cancers. This interaction is hypothesized to promote heterotypic cell adhesion as well as support tumor homeostasis and metastasis as part of its biological function.Citation11 Amatuximab (also known as MORAb-009) has been shown to block the interaction of mesothelin and CA125 in vitro and in vivo and has been found to be associated with elevated CA125 serum levels when administered to patients as a single agent in a reversible manner.Citation10,Citation12 This in vivo elevation may be a result of amatuximab blocking the binding of soluble CA125 to mesothelioma cells or a mechanism by which mesothelioma cells increase CA125 production as a means to overcome the blockade/anti-tumor effect(s) via immune-suppression at the lesion site. In the OVCAR-KD1 cells, suppression of CA125 led to a significant decrease in total glycan production as determined by PAS staining (). Interestingly, amatuximab bound both the OVCAR3 parental and OVCAR3-KD1 similarly (), demonstrating that CA125 does not diminish amatuximab-mediated ADCC activity by simply decreasing its ability to bind cell surface target. While this isogenic cell system has an ovarian cancer cell background, the amatuximab anti-tumor effect(s) on ovarian and mesothelioma/lung cancer cell lines behave similarly (kills via ADCC and blocks heterotypic adhesion) as the function of mesothelin is similar across naturally expressing cell types.
CA125 binding to the (Fab’)2 domain of amatuximab and its impact on molecular activity(s) in the Fc domain is similar to what was observed for farletuzumab.Citation4 While the exact mechanism by which this occurs is still being explored, previous reports have found similar effects of hapten-antibody binding that results in allosteric changes to antibody Fc domain.Citation34,Citation35 The fact that CA125 suppressed amatuximab-Fc-γ CD16a and CD32a receptor engagement but not CD64a suggests a structural function exists between these two receptors and amatuximab as well as a subset of other IgG1-type antibodies are affected by CA125 binding. As CD64a has approximately a two-log higher affinity for IgG1 binding, affinity may explain in part the difference of CA125 effect on the mAb Fc domain-Fc-γ receptor binding as CA125 effect on FCRG2A-131 and FCRG3A-158 polymorphic isotypes have shown a more pronounced suppression of Fc-γ receptor binding via CA125 on isotypes with the lower affinity allele.Citation5 Further structural analysis will aid in understanding the molecular alterations that occur when CA125 is bound to antibody that in turn alters Fc domain structure and/or function.
The findings reported here suggest CA125 may play a broad role in humoral immune suppression in a number of cancers via direct binding to a subset of tumor targeting antibodies and blockade of their immune-effector function. These findings also suggest that CA125 should be tested for therapeutic antibody binding and immune-suppression when designing clinical trials, and as in the case for amatuximab, may offer improved clinical outcome in patients with baseline CA125 levels of no greater than 57 U/mL.
Methods and materials
Quantitation of TSAs from patient serum
Baseline serum samples were evaluated for CA125, CA19-9 and CA15-3 levels using the Roche COBAS 6000 platform in a CLIA accredited laboratory at Aspira Labs, Austin, TX. The CA125 ULN for the clinical diagnostic assay used in the study is 21 U/mL.
All TSAs were measured using an EIA format that incorporates a streptavidin-coated microparticle in combination with a paired biotinylated-capture and ruthenylated-detection antibodies.
All patient informed consent documentation received institutional review board approval in accordance with the Declaration of Helsinki. All patients received clinical trial information and informed consent forms prior to any trial-specific screening. All patients consented to having post hoc biomolecular studies.
Statistical analysis of CA125 and clinical response
Maximal chi-square methodologyCitation15 was used to determine optimal cut-point analysis of CA125. Log-rank tests were used to compare clinical response between subgroups. Unless otherwise specified, all p-values are two-sided. Kaplan-Meier curves were generated on PFS and OS to derive medians. A Cox proportional hazards model is used to determine HR of response between >57 U/mL and ≤ 57 U/mL CA125 levels.
Cell culture
The human ovarian cancer cell line OVCAR3, the human epidermoid carcinoma cell line A431 and the human lymphocytic cell line Jurkat were all obtained from ATCC. All cells were grown in RPMI medium and maintained at 37°C/5% CO2. As A431 cells do not express mesothelin, the full length human mesothelin cDNA was cloned into the CMV promoter-driven expression plasmid pC+75IZ3 and subsequently used to transfect A431 cells using lipofectamine 2000 (Thermo-Fisher). Single cell clones were generated by 10 μg/mL zeocin selection. Clone A431-A3 was used in subsequent experiments due to the high mesothelin cell surface expression that was confirmed by flow cytometry.
CA125 preparations
Pooled patient ascites were used as a source for CA125. Samples were purified by size exclusion, gel filtration, affinity isolation as previously described.Citation4
Recombinant Jurkat CD16a ADCC reporter cell lines
Jurkat-ADCC reporter cell lines were generated by transfection using equivalent amounts of mammalian expression vectors containing the human CD16a and FcϵR cDNAs (combine to form the CD16 receptor complex) under control of a CMV promoter and selection markers. Recombinant clones were selected with 2.5 μg/mL blasticidin and 400 μg/mL G418. Flow cytometry was used to confirm CD16a cell surface expression as described.Citation4
shRNA knockdown of mCA125 expression in cancer cells
shRNA Mission Lentiviral particles were employed to knockdown CA125/MUC16 expression. OVCAR knockdown lines were generated as previously described.Citation4 Vectors covering non-overlapping regions were used to generate stable integrated cell lines and CA125 knockdown expression was confirmed as previously described.Citation4 Cell surface mesothelin expression was confirmed by flow cytometry using the MORAb-009 antibodyCitation10 followed by staining with FITC-conjugated secondary antibody. Cell lines were monitored for CA125 suppression over multiple passages. Effective shRNA constructs were TRCN0000262688 (KD1: 5′- CCGGTCACATCTCCAATGGTTATTACTCGAGTAATAACCATTGGAGATGTGATTTTTG-3′) and TRCN0000262686 (KD3: 5′- CCGGTGCCGTTCACCCTCAACTTTACTCGAGTAAAGTTGAGGGTGAACGGCATTTTTG-3′). Cell lines were characterized using assays previously described to confirm growth and morphological similarities between CA125 knockdown lines (OVCAR3-KD1) and parental OVCAR3 cells.Citation4
Periodic acid-Schiff (PAS) staining
OVCAR3 and OVCAR3-KD1 cells were grown to 80% confluence in 24 well plates, washed once in DPBS without Ca++ or Mg++ and fixed in 0.2% glutaraldehyde in DPBS for 30 minutes. All subsequent steps were done at room temperature. Wells were washed twice with DPBS and prepared for PAS staining as recommended by the vendor (ThermoFisher, Waltham, MA). Briefly, cells were incubated with 50% methanol / 50% DPBS for 30 min then washed twice with 3% acetic acid in DPBS for 10 min. Next, oxidation reagent was added for 15 min and cells were then washed three times with 3% acetic acid for 5 min each. Glycoprotein stain reagent was added for 15 min then removed and cells were treated with reduction agent for 5 minutes. Finally, cells were rinsed once with 3% acetic acid and then dH2O and imaged with the EVOS imaging system (Thermo-Fisher, Waltham, MA).
Antibody-dependent cellular cytotoxicity
Antibody dependent cellular cytotoxicity effects were conducted using Jurkat-Luc (Promega) effector reporter cells as described.Citation4 Percent inhibition was calculated as 1 – (sCA125-treated / untreated) x 100%.
Biological rolling assay (BRA assay)
BRA assays were conducted as described.Citation4 Briefly, U-bottom microwell plates were coated with 10 μg/mL of antibody or derived fragments in 0.2 M bicarbonate buffer, pH 9.4 coating buffer at 4°C. Wells were then blocked with PBS plus 1% BSA for 1 hr at room temperature. Jurkat-CD16a cells [25,000 in ADCC buffer (Promega)] were then added with or without CA125 to wells and plates were incubated for 5 hrs at 37°C/5% CO2 in a humidified incubator and imaged (EVOS System).
ELISA analysis of amatuximab-CA125 binding
ELISAs for antibodies, antibody fragments and CD16a, CD32a and CD64a receptors were carried out as previously described.Citation4 ELISA for FcRn was carried out similarly with the exception of probing and washing using powdered milk instead of BSA and PB buffer pH 5.5 instead of pH 7.2. Recombinant human CD16a-, CD32a- and CD64a-biotin probes were purchased from Sino Biological Inc. (Beijing, China). Recombinant human FcRn was purchased from R&D Systems. Antibodies, antibody fragments and FcRn were biotinylated using sulfo-tag conjugation as described (Meso Scale Diagnostics Inc). All reactions were done in at least triplicate.
Statistical analysis on laboratory experiments
Student's two-tailed t test was used to calculate all p values. All values are two-sided.
Abbreviations
| MPM | = | Malignant pleural mesothelioma |
| ADCC | = | antibody dependent cellular cytotoxicity |
| HR | = | hazard ratio |
| PFS | = | progression free survival |
| OS | = | overall survival |
| SLD | = | sum of the longest diameter |
| SOC | = | standard of care |
| ULN | = | upper limit of normal |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
N.C.N., L.G. and J.B.K. conducted experimental studies and drafted the manuscript. C.S., R.H. and E.B.S. supervised the project. E.N.R. and S.F. performed experiments. All authors contributed to manuscript preparation.
Acknowledgments
All authors except R. Hassan are employees of Morphotek and all have no ownership interests.
References
- Patankar MS, Jing Y, Morrison JC, Belisle JA, Lattanzio FA, Deng Y, Wong NK. Potent suppression of natural killer cell response. Gynecol Oncol. 2005;99:704–13. doi:10.1016/j.ygyno.2005.07.030. PMID:16126266.
- Gubbels JA, Felder M, Horibata S, Belisle JA, Kapur A, Holden H, Petrie S, Migneault M, Rancourt C, Connor JP, Patankar MS. MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol Cancer. 2010;9:11. doi:10.1186/1476-4598-9-11. PMID:20089172.
- Belisle JA, Gubbels JA, Raphael CA, Migneault M, Rancourt C, Connor JP, Patankar MS. Peritoneal natural killer cells from epithelial ovarian cancer patients show an altered phenotype and bind to the tumour marker MUC16. Immunology. 2007;122:418–29. doi:10.1111/j.1365-2567.2007.02660.x. PMID:17617155.
- Kline JB, Kennedy RP, Albone E, Chao Q, Fernando S, McDonough JM, Rybinski K, Wang W, Somers EB, Schweizer C, Grasso L, Nicolaides NC. Tumor antigen CA125 suppresses antibody-dependent cellular cytotoxicity (ADCC) via direct antibody binding and suppressed Fc receptor engagement. OncoTarget. 2017;8:52045–60. doi:10.18632/oncotarget.19090. PMID:28881712.
- Wang W, Somers EB, Ross EN, Kline JB, O'Shannessy DJ, Schweizer C., Weil S, Grasso L, Nicolaides NC. FCGR2A and FCGR3A genotype correlates with farletuzumab response in patients with first relapsed ovarian cancer exhibiting low CA125. Cytogenet Genome Res. 2017;152:169–79. doi:10.1159/000481213. PMID:29041009.
- Vergote I, Armstrong DJ, Scambia G, Teneriello M, Sehouli J, Schweizer C, et al.. A randomized, double-blind, placebo-controlled, phase III study to assess efficacy and safety of weekly farletuzumab in combination with carboplatin and taxane in patients with ovarian cancer in first platinum-sensitive relapse. J Clin Oncol. 2016;34:2271–8. doi:10.1200/JCO.2015.63.2596. PMID:27001568.
- Gaillard SL, Secord AA, Monk B. The role of immune checkpoint inhibition in the treatment of ovarian cancer. Gyn Oncol Res Prac. 2016;3:11–25. doi:10.1186/s40661-016-0033-6.
- Hassan R, Cohen SJ, Phillips M, Pastan I, Sharon E, Kelly RJ, Schweizer C, Weil S, Laheru D. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res. 2010;16:6132–8. doi:10.1158/1078-0432.CCR-10-2275. PMID:21037025.
- Fujisaka Y, Kurata T, Tanaka K, Kudo T, Okamoto K, Tsurutani J, Kaneda H, Okamoto I, Namiki M, Kitamura C, et al. Phase I study of amatuximab, a novel monoclonal antibody to mesothelin, in Japanese patients with advanced solid tumors. Invest New Drugs. 2015;33:380–8. doi:10.1007/s10637-014-0196-0. PMID:25502863.
- Hassan R, Ebel W, Routhier EL, Patel R, Kline JB, Zhang J, Chao Q, Jacob S, Turchin H, Gibbs L, et al. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007;7:20–30. PMID:18088084.
- Rump A, Morikawa Y, Tanaka M, Minami S, Umesaki N, Takeuchi M, Miyajima A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2014;279:9190–8. doi:10.1074/jbc.M312372200.
- Hassan R, Schweizer C, Lu KF, Schuler B, Remaley AT, Weil SC, Pastan, I. Inhibition of mesothelin-CA125 interaction in patients with mesothelioma by the anti-mesothelin monoclonal antibody MORAb-009: implications for cancer therapy. Lung Cancer. 2010;68:455–9. doi:10.1016/j.lungcan.2009.07.016. PMID:19744744.
- Hassan R, Kindler HL, Jahan T, Bazhenova L, Reck M, Thomas A, Pastan I, Parno J, O'Shannessy DJ, Fatato P, et al. Phase II clinical trial of amatuximab, a chimeric anti-mesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin Cancer Res. 2014;20:5927–36. doi:10.1158/1078-0432.CCR-14-0804. PMID:25231400.
- Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–44. doi:10.1200/JCO.2003.11.136. PMID:12860938.
- Miller R, Siegmund D. Maximally selected chi square statistics. Biometrics. 1982;38:1011–6. doi:10.2307/2529881.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi:10.1093/jnci/92.3.205. PMID:10655437.
- Kaplan EL, Meier P. Nonparametric-estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. doi:10.1080/01621459.1958.10501452.
- Ocana A, Tannock IF. When are “positive” clinical trials in oncology truly positive? J Natl Cancer Inst. 2011;103:16–20. doi:10.1093/jnci/djq463. PMID:21131576.
- Baratti D, Kusamura S, Martinetti A, Seregni E, Oliva DG, Laterza B, Deraco M. Circulating CA125 in patients with peritoneal mesothelioma treated with cytoreductive surgery and intraperitoneal hyperthermic perfusion. Ann Surg Oncol. 2007;14:500–8. doi:10.1245/s10434-006-9192-8. PMID:17151789.
- Creaney J, Dick IM, Dare H, Demelker Y, Nowak AK, Musk AW, Robinson BWS. Does CA125 binding to mesothelin impact the detection of malignant mesothelioma? Lung Cancer. 2013;80:39–44. doi:10.1016/j.lungcan.2012.12.008. PMID:23357461.
- Pass HI, Giroux D, Kennedy C, Ruffini E, Cangir AK, Rice D, Asamura H, Waller D, Edwards J, Weder W, et al. Supplementary prognostic variables for pleural mesothelioma: A report from the IASLC staging committee. J Thorac Oncol. 2014;9:856–64. doi:10.1097/JTO.0000000000000181. PMID:24807157.
- Feng Y, Xiao X, Zhu Z, Streaker E, Ho M, Pastan I, and Dimitrov DS. A novel human monoclonal antibody that binds with high affinity to mesothelin-expressing cells and kills them by antibody-dependent cell-mediated cytotoxicity. Mol Cancer Ther. 2009;8:1113–8. doi:10.1158/1535-7163.MCT-08-0945. PMID:19417159.
- Wong NK, Easton RL, Panico M, Sutton-Smith M, Morrison JC, Lattanzio FA, Morris HR, Clark GF, Dell A, Patankar MS. Characterization of the oligosaccharides associated with the human ovarian tumor marker CA125. J Biol Chem. 2003;278:28619–34. doi:10.1074/jbc.M302741200. PMID:12734200.
- Gunn B, Schneider J, Shansab M, Bastian AR, Fahrbach K, Smith A, et al.. Enhanced binding of antibodies generated during chronic HIV infection to mucus component MUC16. Mucosal Immunol. 2016;9:1549–58. doi:10.1038/mi.2016.8. PMID:26960182.
- O'Shannessy, DJ, Bendas K, Schweizer C, Wang W, Albone E, Somers EB, Weil S, Meredith RK, Wustner J, Grasso L, et al. Correlation of FCGRT genomic structure with serum immunoglobulin, albumin and farletuzumab pharmacokinetics in patients with first relapsed ovarian cancer. Genomics. 2017;109:251–7. doi:10.1016/j.ygeno.2017.04.006. PMID:28450240.
- Cheng X, Gou HF, Liu JY, Luo DY, Qiu M. Clinical significance of serum CA125 in diffuse malignant mesothelioma. SpringerPlus. 2016;5:368–76. doi:10.1186/s40064-016-1998-7. PMID:27066377.
- Fehm T, Beck E, Valerius T, Gramatzki M, Jager W. CA125 elevation in patients with malignant lymphomas. Tumour Biology. 1998;19:283–9. doi:10.1159/000030019. PMID:9679739.
- Gupta A, Hussein Z, Hassan R, Wustner J, Maltzman JD, Wallin BA. Population pharmacokinetics and exposure-response relationship of amatuximab, an anti-mesothelin monoclonal antibody, in patients with malignant pleural mesothelioma and its application in dose selection. Cancer Chemother Pharmacol. 2016;77:733–43. doi:10.1007/s00280-016-2984-z. PMID:26898299.
- Sturgeon CM, Duffy MJ, Stenman UH, Lilja H, Brunner N, Chan DW, et al.. National academy of clinical biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clinical Chem. 2008;54:e11–79. doi:10.1373/clinchem.2008.105601.
- Kabawat SE, Bast Jr RC, Bhan AK, Welch WR, Knapp RC, Colvin RB. Tissue distribution of a coelomic-epithelium-related antigen recognized by the monoclonal antibody OC125. Int J Gynecol Path. 1983;2:275–85 doi:10.1097/00004347-198303000-00005.
- Simsek H, Kadayifci A, Okan E. Importance of serum CA125 levels in malignant peritoneal mesothelioma. Tumour Biol. 1996;17:1–4. doi:10.1159/000217960. PMID:7501967.
- Bateman AC1, al-Talib RK, Newman T, Williams JH, Herbert A. Immunohistochemical phenotype of malignant mesothelioma: predictive value of CA125 and HBME-1 expression. Histopathology. 1997;30:49–56. doi:10.1046/j.1365-2559.1996.d01-562.x. PMID:9023557.
- Zeimet AG, Marth C, Offner FA, Obrist P, Uhl-Steidl M, Feichtinger H, Stadlmann S, Daxenbichler G, Dapunt O. Human peritoneal mesothelial cells are more potent than ovarian cancer cells in producing tumor marker CA-125. Gynecol Oncol. 1996;62:384–9. doi:10.1006/gyno.1996.0253. PMID:8812537.
- Oda M, Kozono H, Morii H, Azuma T. Evidence of allosteric conformational changes in the antibody constant region upon antigen binding. Int Immunol. 2003;15: 417–26. doi:10.1093/intimm/dxg036. PMID:12618486.
- anda A, Bowen A, Greenspan NS, Casadevall A. Ig constant region effects on variable region structure and function. Front Microbiol. 2016;7:1–10. doi:10.3389/fmicb.2016.00022. PMID:26834723.
