ABSTRACT
Novel therapies including kinase inhibitors (KI) have led to high and durable response in patients with chronic lymphocytic leukemia (CLL), however, some patients stop therapy. This study evaluates reasons for treatment changes among CLL patients who stopped KI in real-world practice. Sixty-nine US oncologists/hematologists provided patient-level data abstracted from charts of CLL adult patients who initiated a KI and later (1) switched to another anti-neoplastic regimen (Switched cohort), (2) discontinued the KI and remained untreated (Discontinued cohort), or (3) restarted the same KI after an interruption of ≥60 days (Restarted cohort). Demographics, clinical/treatment characteristics, and reasons for stopping, restarting, and switching the KI therapy were described. In the Switched cohort, reasons for stopping included disease progression (72.5%), low/no disease activity (3.9%), adverse event [AE]/ intolerance/comorbidity (15.7%), and planned cellular therapies (7.9%). In the Discontinued cohort, approximately half (46.0%) of patients stopped KI therapy because they were terminally ill/died, or were moved to best supportive care — these patients were older, had more severe disease, and high comorbidity burden. The other half (54.0% of patients) stopped due to low/no disease activity (24.0%), AEs/toxicity (12.0%), or patient-requested drug holiday (18.0%). In the Restarted cohort, the most common reasons for stopping KIs were patient request (37.3%), AEs/intolerance (31.4%), and economic reasons (10%). Patients restarted when disease progressed (60.8%) or when they recovered from the AE (33%). Reasons for KI stop and subsequent treatment patterns were varied and multifactorial, suggesting heterogeneous disease management and a need for more evidence around supporting strategies and physician education.
Introduction
The treatment landscape for patients with chronic lymphocytic leukemia (CLL) has drastically changed with the approval of novel oral targeted therapies, including kinase inhibitors (KIs) and more recently a B-cell lymphoma-2 (BCL-2) inhibitor.Citation1 Two KIs are currently approved by the Food and Drug Administration for the treatment of CLL in the United States (US): ibrutinib, and idelalisib in combination with rituximab.Citation2,Citation3
KIs have been associated with high and durable response rates in clinical trials and are frequently prescribed in clinical practice.Citation4-6 However, despite their efficacy and guideline recommendation that treatment should be continued until disease progression, recent studies have shown that some patients receiving KIs discontinued therapy. This rate of discontinuation appears to be higher in patients who receive the KI in routine practice as opposed to clinical trial settings. Accordingly, there is a need to better understand reasons why patients discontinue KI therapies and the treatment patterns subsequent to KI discontinuation.
To date, a number of studies have examined reasons for KI therapy discontinuation and their associated outcomes.Citation7-13 Results have showed that intolerance/toxicity was the most common reason for KI therapy discontinuation in real-world settings, reported in up to half of discontinuation patients.Citation11,Citation12 Disease progression was generally the second most common reason.Citation7,Citation9,Citation11,Citation12 A recent study based on data collected from 9 US academic centers and the CLL Connect® registry provided a more thorough portrait of reasons for KI therapy discontinuation by exploring a wider spectrum of potential reasons.Citation11 Results showed that, although toxicity and disease progression were the most common reasons for KI therapy discontinuation, other reasons, such as patient preference, Richter's transformation, and planned stem cell transplant/chimeric antigen receptor (CAR) T cells may also drive KI therapy discontinuation.
While information on reasons for discontinuing KI therapy is emerging, more understanding on the drivers of discontinuation is needed as the potential follow-up for patients on KI therapies has increased since their approval in 2014. Additional data from a diverse sample of patients treated in academic and community settings are also needed as prior studies largely comprised patients in academic settings. Furthermore, patients may exhibit different treatment patterns after KI therapy discontinuation, with some patients remaining untreated, switching to another therapy, or stopping and later restarting the same KI therapy.
Using data from a diverse sample of patients treated in academic and community settings, this study aimed to assess drivers of treatment changes in patients with CLL who stopped KI therapy, stratified by treatment patterns after KI therapy stop.
Results
Physician characteristics
Data were collected from 69 oncologists/hematologists: 43 (62.3%) from community practice and 26 (37.7%) from academic practice. Oncologists/hematologists were predominantly from intermediate-sized practices (2–9 physicians) (49.3%) or large-sized practices (10 or more physicians) (47.8%). Participating oncologists/hematologists represented a geographically diverse mix with 23.2% from the Northeast, 20.3% from the Midwest, 31.9% from the South, and 24.6% from the West regions of the US.
Patient and treatment characteristics
Data were collected on 152 patients: 51 patients in the Switched cohort, 50 in the Discontinued cohort, and 51 in the Restarted cohort. The vast majority of patients were treated with ibrutinib (over 96% of patients in all three cohorts) and treated with the KI as front-line therapy for CLL (63% in the Switched cohort, 86% in the Discontinued cohort, and 59% in the Restarted cohort) (). The majority of patients were male, and the mean age at CLL diagnosis was 65, 69, and 66 for the Switched, Discontinued, and Restarted cohorts, respectively. At the time of KI initiation, the majority of patients had a clinical stage based on the Rai staging system of III–IV and an ECOG performance status of 0–1. Across the three cohorts, the most common comorbid conditions reported at the time of KI therapy initiation were cardiovascular (39.2–52.0%), respiratory (15.7–28.0%), and endocrine/metabolic disorders (10.0–25.5%). The proportion of patients deceased at the time of data collection varied largely across cohorts — 2.0% of patients in the Switched cohort, 32.0% in the Discontinued cohort, and 9.8% in the Restarted cohort.
Table 1. Patient and KI treatment characteristics.
Reasons for treatment changes
Reasons for stopping the KI therapy were varied, multifactorial, and often different from the reasons for switching to another therapy or restarting the same KI (). In the Switched cohort, 80.4% switched due to disease progression; among these patients who eventually switched due to disease progression, reasons for the original decision stopping the KI included low or no disease activity (3.9%), adverse event [AE] (15.7%), planned cellular therapies (7.9%), and disease progression (i.e., relapse or refractory to the KI) (72.5%) ( and ). Also in the Switched cohort, 13.7% of patients stopped and switched due to AEs/intolerance/severe comorbidities, and 5.9% stopped and switched because of planned cellular therapies (e.g., preparation for stem cell transplant). Median time from KI initiation to stop was 7.0 months, and most patients switched to another therapy shortly after KI stop (median time from KI stop to switch was 1.0 month).
Patients in the Discontinued cohort were a very heterogeneous group of patients. Approximately half of patients (46.0%) stopped KI therapy because they were terminally ill, died, or were moved to best supportive care () — median time to KI stop was 5.6 months. These 46.0% of patients were generally older (mean 72 years), had indicators of more severe disease (e.g., 69.6% had Rai stage of III–IV at KI initiation, 69.6% had ECOG status of 2 or higher), and had a high comorbidity burden ( and ). Among the subgroup of patients who died after KI stop, the median time from KI stop to death was 31 days. The other half of the Discontinued cohort (the remaining 54.0%) stopped KI therapy because of low or no disease activity (24.0%), AE or management of a severe comorbidity (12.0%), or the patient requested a drug holiday (18.0%). Among these patients, median time on therapy before KI stop was 11.1 months, and median time from KI stop to end of follow-up/patient's death was 2.5 months. Among the 18.0% of patients who stopped because they requested a drug holiday, 81.5% had a reported complete response to KI therapy, and the treating physician was supportive of the decision to stop the KI therapy in 77.3% of these patients.
Table 2. Comorbidities at KI Initiation and mortality status at the time of data collection.
Table 3. Description of rationale for treatment change.
Table 4. Adverse events that triggered KI stop.
Among patients in the Restarted cohort, the most common reasons for KI stop were patient request (i.e., patient requested a drug holiday or no longer perceived the benefit of the treatment) (37.3%), AEs/intolerance (31.4%), and economic reasons (9.8%). Median time from KI therapy initiation to stop was 5.0 months, and patients remained untreated for a median time of 3.1 months before restarting the same KI. Patients most commonly restarted the KI when the disease progressed (60.8%) or when they recovered from the AE/intolerance (33.3%). Among the 60.8% of patients who restarted the KI when the disease progressed, 87.1% had stopped after achieving at least a partial response (i.e., low or no disease activity or complete/partial response). Overall for the Restarted cohort, 68.6% of physicians were supportive of the decision to stop the KI therapy.
Time to KI stop varied across the three cohorts and by reason for stopping KI therapy. More specifically, across the three cohorts, median time from KI initiation to stop was 9.1 months for patients who stopped KI therapy due to disease progression and 4.1 and 5.6 months for patients who stopped due to a severe AE/intolerance and non-severe AE/intolerance, respectively.
Moreover, results from additional exploratory analyses across the three cohorts showed that reasons for stopping KI therapy varied between patients treated in front line versus later lines (), and that the variation was particularly pronounced for patients in the Discontinued cohort. In the Discontinued cohort, low/no disease activity and patient-requested drug holiday were reasons for stopping therapy in the front line for 27.9% and 20.9% of patients, respectively; these two reasons were not reported for patients in later lines. On the other hand, while AEs/intolerance was reported as the reason for KI stop in only 4.5% of patients in front line, this reason was reported in 57.1% of patients stopping in later lines. The proportion of patients who stopped KI therapy because they were terminally ill, died, or switched to best supportive care in the Discontinued cohort was similar between patients treated in front line versus later lines (46.5% in front line vs 43% in later lines).
Table 5. Reasons for stopping KI stratified by line of therapy.
Discussion
In this study, a large proportion of patients initiated KI therapy as a front-line therapy for CLL. Study findings showed that reasons for stopping, switching, or restarting were multifactorial and reflected clinical and non-clinical considerations. Reasons for KI therapy stop also varied between patients treated in front line versus later lines, notably among patients who stopped the KI therapy and remained untreated. Although intolerance and disease progression prompted the majority of patients to stop the KI therapy, many patients stopped for other reasons, such as patient-requested drug holiday or low or no disease activity, despite label recommendations to continue therapy until disease progression or unacceptable toxicity. The mixed profile of patients in the Discontinued cohort is notable. Some patients (46.0%) will never receive another therapy (e.g., terminally ill, died, or moved to best supportive care) while others will likely restart a KI or other therapy. Among patients who switched therapy due to disease progression, 39.2% switched to a chemo-based regimen whose effectiveness after a KI therapy has not been prospectively studied. Among patients who restarted the same KI therapy after an interruption, the most common reasons for the KI interruption were patient request and AEs/intolerance—patients most commonly restarted when the disease progressed. This suggests that, for some patients, intolerance to KI therapy is managed by stopping and restarting the same therapy. Among patients who restarted the KI therapy once the disease progressed, 87.1% of patients had originally stopped the KI after reportedly achieving at least a partial response (i.e., low or no disease activity or complete/partial response)—the median time from KI therapy stop to restart was 3.5 months, providing an estimate of durability of response.
Although previous clinical trial-based studies have assessed reasons for KI therapy discontinuation in patients with CLL, their findings are not directly comparable to those of the current study; while treatment use and discontinuation in clinical trials are per trial protocol, in this study KI use and discontinuation reflect clinical practice. Additionally, previous clinical trials have not examined treatment patterns subsequent to KI therapy discontinuation (stopping and restarting strategies, etc.).
Consistent with results from the current study, two recent multicenter retrospective studies assessing reasons for KI discontinuation among patients with CLL, reported that the most common reasons for KI discontinuation were toxicity (44.8% to 52.0% of discontinuations), followed by disease progression (20.5% to 31.0%).Citation11,Citation12 Richter transformation (3.5% to 8.0%), physician/patient preference (6.2% to 17.2%), stem cell transplant/CAR T cells (0% to 3.9%), and cost considerations (0% to 1.1%) were among other reasons reported for stopping KI therapy. Median time from KI initiation to stop ranged between 4.0 and 7.5 months depending on the study and type of KI therapy used—our study was consistent with these results with median time from the KI therapy initiation and stop ranging between 5.0 months for the Restarted cohort and 11.1 months for the Discontinued cohort.Citation11
The current study included ‘low or no disease activity’ as a reported reason for stopping KI therapy, which was a notable difference from prior studies. This was reported in 24.0% of patients in the Discontinued cohort, 3.9% in the Switched cohort, and 15.7% in the Restarted cohort. In addition, the proportion of patients who stopped KI therapy because of patient preference (i.e., patient-requested drug holiday or no longer perceived the benefit of treatment) also tended to be higher in the current study—18.0% of patients in the Discontinued cohort and 37.3% in the Restarted cohort. These discrepancies may reflect an increasing desire of patients to receive a time-limited duration of therapy or may reflect differences in management between patients treated in academic versus community settings. While prior studies largely comprised patients treated in academic settings, in the current study, the majority (59.2%) of patients were treated in community settings. Interestingly, 90.9% of patients who stopped because of low or no disease activity were treated in community settings.
The high proportion of patients who initiated a KI as front-line therapy suggests an increasing use of KIs in earlier lines of therapy in real-world practice. Further, the short time period between KI initiation and stop suggests that the first year is an important period to monitor patients for signs of CLL progression, disease transformation, or AEs/intolerance that may lead to therapy discontinuation. Findings from the current study also suggest that, although guidelines recommend KIs to be continued until disease progression or unacceptable toxicity, in clinical practice, additional patients' and physicians' considerations may also impact treatment strategy. Notably, results suggest that in real-world practice, a relatively high proportion of patients stop KI therapy when they reach a level of low disease activity or because of patient preference. Additional studies are warranted to understand why treatment is interrupted when patients reach a low level of disease activity and the clinical impact or appropriateness of such treatment strategy on outcomes.
The study findings also highlight an unmet need for many patients who stop KI therapy. Although prior retrospective studies showed better outcomes associated with switching patients to another KI or to venetoclax, versus chemotherapy-based regimens,Citation11,Citation14 in the current study, 39.2% of patients switched to a treatment regimen whose effectiveness after a KI therapy has not been studied prospectively (including CIT, CT or anti-CD20 antibody as monotherapy).Citation11 In addition, stopping and restarting KI therapy after an interruption was commonly observed among patients who experienced AEs/intolerance, and those whose disease progressed after stopping the KI. Evidence has shown that in presence of AEs, temporary short interruption (8–14 days) could be considered without affecting the patient's progression-free-survival.Citation13,Citation15 However, in the current study, patients stopped the KI therapy for a median of 3.1 months—the efficacy of re-initiating the same KI therapy, especially after longer treatment breaks, has not been clearly demonstrated. Switching to another therapy with a different mechanism of action or to a better tolerated agent might be a preferable alternative strategy for some of these patients.
Our results also suggest that reasons for KI stop varied greatly between patients treated in front line versus later lines, especially among those who stopped the KI therapy and remained untreated. This variation highlights the multifactorial aspect of treatment strategy for CLL patients. Further analyses are warranted to understand the considerations associated with the different strategies.
This study is subject to limitations. First, the number of patients for each cohort was determined a priori. Accordingly, the treatment pattern distribution reported in the current study does not reflect a naturalistic treatment pattern distribution. This study may also be subject to self-selection bias, potentially limiting the generalizability of the findings if the patients selected and the participating oncologists/hematologists had different characteristics and management practices than the overall population. Nevertheless, despite these limitations, this study provides important insight on drivers of treatment patterns among patients with CLL who stopped a KI therapy in a real-world setting.
In conclusion, this study describes reasons for treatment changes in real-world practice among CLL patients who stopped KI therapy. Consistent with prior studies, the majority of patients stopped KI therapy due to intolerance or disease progression. However, many patients stopped for other reasons including patient requests and low or no disease activity. Results suggest heterogeneous management in real-world practice and a need for more evidence around supporting strategies and physician education to manage patients with CLL.
Patients and methods
Data source and sample selection
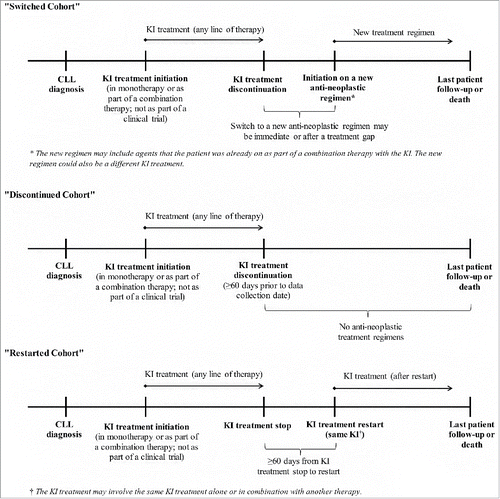
Data were collected from patient charts via an online data collection form completed by 69 US oncologists and hematologists. Physicians were recruited via email from a well-established ISO-certified panel, having one of the largest reaches of active oncologists and hematologists in the US. The panel represents a geographically diverse mix of physicians from both community and academic settings. Patients were eligible for this study if they were adults diagnosed with CLL who initiated a KI therapy and later stopped the KI. Patients were stratified into the following cohorts (): (1) Switched cohort (i.e., patients stopped the KI and then switched to a different KI or another anti-neoplastic agent/regimen), (2) Discontinued cohort (i.e., patients stopped the KI and did not receive any anti-neoplastic therapies until last follow-up or the patient's death) and (3) Restarted cohort (i.e., patients stopped the KI for a minimum of 60 days and later restarted the same KI). Patients were not eligible if they received the KI in a clinical trial or if their complete medical records were not available to the oncologist/hematologist who completed the data collection form. Patients were included in the study regardless of whether they were alive or deceased at the time of data collection.
Data Collection
The study aimed to collect data on 50 patients from each of the three study cohorts (). Data were collected between January 2017 and March 2017. Oncologists/hematologists were asked to provide information on their practice and patient-level information on up to three patients who met the inclusion criteria. The information collected was de-identified and an institutional review board exemption was obtained prior to study initiation. Quality control measures and checks were implemented at multiple stages of data collection and review. In the online data collection form, these measures included automatic checks to verify that patients met each eligibility criterion, along with range, date, and internal consistency checks across questions. Entry of questionable values in the form triggered soft or hard warnings to the physician, prompting them to confirm or revise their response. All completed forms also underwent additional review to assess data quality.
Study Measures and Statistical Analyses
For each cohort, demographic, clinical and treatment characteristics prior to KI therapy initiation, and reasons for stopping the KI (physicians were presented with a comprehensive list of possible reasons for stopping the KI and a free-text field allowed physicians to enter other reasons) were described. Reasons for treatment patterns after stopping the KI (e.g., reasons for restarting the same regimen or switching to another regimen), whether the physician was supportive of the decision to stop KI therapy, time from KI initiation to stop, and time from KI stop to switch/restart/end of follow-up were also described.
Patient demographic, clinical and treatment characteristics for the Discontinued cohort were also reported for patients stratified into two subgroups based on whether there was an intent to restart a CLL therapy after stopping the KI.
All analyses were descriptive. Means, medians, standard deviations, and ranges were reported for continuous variables; counts and percentages were reported for categorical variables.
Disclosure of interest
Jennifer Samp is an employee of AbbVie, Inc., and may own AbbVie and/or Abbott stock. Geneviève Gauthier and Emi Terasawa are employees of Analysis Group, Inc., which has received consultancy fees from AbbVie. Anthony R. Mato has received consultancy fees from AbbVie. Danielle M. Brander has received consultancy fees from AbbVie.
In collaboration with the other authors, AbbVie contributed to the design, study conduct, and data interpretation. All authors contributed to the development of the manuscript and maintained control over the final content.
Acknowledgments
We thank Claudia Cheung of Analysis Group, Inc., for her support in conducting the analyses and Sara Kaffashian of Analysis Group, Inc., for medical writing assistance. Their assistance was funded by AbbVie.
Additional information
Funding
References
- Owen C, Assouline S, Kuruvilla J, Uchida C, Bellingham C, Sehn L. Novel therapies for chronic lymphocytic leukemia: A Canadian perspective. Clin Lymphoma Myeloma Leuk. 2015;15(11):627–634.e625. doi:10.1016/j.clml.2015.07.649
- U.S. Food and Drug Administration. Zydelig (idelalisib) Tablets, Drug Apporval Package; 2014 [Accessed 5 October 2017]. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206545Orig1s000TOC.cfm
- U.S. Food and Drug Administration. Imbruvica (ibrutinib) Capsules, Drug Approval Package; 2015 [Accessed 5 Oct 2017]. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205552Orig2s000TOC.cfm
- Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi:10.1056/NEJMoa1215637
- Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, Barrientos JC, Zelenetz AD, Kipps TJ, Flinn I, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007. doi:10.1056/NEJMoa1315226
- Burger JA, Ghia P, Polliack A, Tam C, Suri D, Clow F, Kraljevic S, James D, Kipps TJ. Randomized, multicenter, open-label, phase III study of the BTK inhibitor ibrutinib versus chlorambucil in patients 65 years or older with treatment-naive CLL/SLL (RESONATE-2, PCYC-1115-CA). J Clin Oncol. 2013;31(15 suppl):TPS7130–TPS7130. doi:10.1200/jco.2013.31.15_suppl.tps7130
- Jain P, Keating M, Wierda W, Estrov Z, Ferrajoli A, Jain N, George B, James D, Kantarjian H, Burger J, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125(13):2062–7. doi:10.1182/blood-2014-09-603670
- Jain P, Thompson PA, Keating M, Estrov Z, Ferrajoli A, Jain N, Kantarjian H, Buggy JJ, O'Brien S, Wierda W. Causes of Discontinuation and Long-Term Outcomes of Patients with CLL after Discontinuing Ibrutinib. Blood. 2016;128(22):4390
- Jain P, Thompson PA, Keating M, Estrov Z, Ferrajoli A, Jain N, Kantarjian H, Burger JA, O'Brien S, Wierda WG. Long-term outcomes for patients with chronic lymphocytic leukemia who discontinue ibrutinib. Cancer. 2017;123(12):2268–73. doi:10.1002/cncr.30596
- Maddocks KJ, Ruppert AS, Lozanski G, Heerema NA, Zhao W, Abruzzo L, Lozanski A, Davis M, Gordon A, Smith LL, et al. Etiology of Ibrutinib therapy discontinuation and outcomes in patients with chronic Lymphocytic Leukemia. JAMA Oncol. 2015;1(1):80–87. doi:10.1001/jamaoncol.2014.218
- Mato AR, Hill BT, Lamanna N, Barr PM, Ujjani CS, Brander DM, Howlett C, Skarbnik AP, Cheson BD, Zent CS, et al. Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: results from a multicenter study of 683 patients. Ann Oncol. 2017;28(5):1050–6. doi:10.1093/annonc/mdx031
- Mato AR, Nabhan C, Barr PM, Ujjani CS, Hill BT, Lamanna N, Skarbnik AP, Howlett C, Pu JJ, Sehgal AR, et al. Outcomes of CLL patients treated with sequential kinase inhibitor therapy: a real world experience. Blood. 2016;128(18):2199–205. doi:10.1182/blood-2016-05-716977
- Forum UC. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica. 2016;101(12):1563–72. doi:10.3324/haematol.2016.147900
- Jones JA, Wierda W, Choi MY, Davids MD, Cheson BD, Furman RR. Venetoclax activity in CLL patients who have relapsed after or are refractory to ibrutinib or idelalisib. J Clin Oncol. 2016;34(15 suppl):7519. doi:10.1200/JCO.2016.34.15_suppl.7519
- Barr PM, Brown JR, Hillmen P, O'Brien S, Barrientos JC, Reddy NM, Coutre S, Mulligan SP, Jaeger U, Furman RR, et al. Impact of ibrutinib dose adherence on therapeutic efficacy in patients with previously treated CLL/SLL. Blood. 2017;129(19):2612–5. doi:10.1182/blood-2016-12-737346