ABSTRACT
Purpose: The benefits of additional use of nimotuzumab (NTZ) in the treatment of locoregionally advanced nasopharyngeal carcinoma (LA-NPC) is largely unclear. We aim to compare LA-NPC treatment outcomes in patients that received CCRT with nimotuzumab (NTZ) to patients that received CCRT only.
Materials and Methods: Between October 2009 and January 2012, 31 previously untreated and newly diagnosed LA-NPC patients were administered CCRT (3 cycles of 100 mg/m2 cisplatin every third week with intensity-modulated radiotherapy) plus NTZ according to an IRB-approved institutional research protocol. A well-balanced cohort of 62 patients who received CCRT alone was created by matching each patient who received CCRT plus NTZ via propensity-matched analysis in a 2:1 ratio.
Results: Compared with CCRT only, CCRT plus NTZ was significantly associated with superior overall survival (5-year OS; 96.8% vs. 82.3%; P = 0.001), superior distant metastasis-free survival (5-year DMFS; 90.3% vs. 80.6%, P = 0.012) and superior progression-free survival (5-year PFS; 83.9% vs. 71.0%, P = 0.006). In multivariate analysis, the inclusion of NTZ to CCRT was confirmed to be a favorable factor for OS (HR, 0.31; 95% CI, 0.02–0.71; P = 0.027), DMFS (HR, 0.45; 95% CI, 0.13–0.77; P = 0.034), and PFS (HR, 0.38; 95% CI, 0.11–0.89; P = 0.041). In addition, no significant differences in hematology parameters, dermatitis, nausea, vomiting, xerostomia, nephrotoxicity or neurotoxicity were found between the two arms (all P > 0.05).
Conclusion: The inclusion of NTZ to CCRT is more effective for long-term survival among LA-NPC patients than CCRT only.
Introduction
Nasopharyngeal carcinoma (NPC) is a relatively rare head and neck tumor in North America and Europe,Citation1 however, endemic in southern China.Citation2 Prior studies reported thatCitation3 up to 70% of NPC patients are diagnosed with locoregionally advanced (Stage III-IVB) NPC at presentation. Based on guidelines set by the National Comprehensive Cancer Network (NCCN), treatment standards for locoregionally advanced NPC (LA-NPC) is concurrent chemoradiotherapy (CCRT).Citation4 However, for approximately thirty percent of NPC patients, this therapy can be ineffective, as majority of these failures in treatment are because of distant metastasis.Citation5,Citation6 Thus, novel treatment strategies are needed for further improvement in outcomes of patients with LA-NPC.
In most human epithelial carcinomas, epidermal growth factor receptor (EGFR) is greatly expressed, and has shown to correlate with poor prognosis.Citation7,Citation8 Moreover, previous studies have reported that EGFR is overexpressed in 80% of NPC patients,Citation9 and has potential promise for new therapeutic target in NPC patients. Ideally, addition of EGFR-targeting antibody to the standard CCRT regimen could improve the outcomes of patients with NPC. However, the RTOG 0522 studyCitation10 showed the combination of cetuximab (the first EGFR-targeting antibody to be approved by United States Food and Drug Administration) with CCRT cannot be recommended for head and neck carcinoma (not included NPC), as this regimen increased acute toxicities without improving survival outcomes. Nimotuzumab (NTZ) is another EGFR-targeting antibody that does not exert intrinsic stimulating activity.Citation11 A phase II trial found the addition of NTZ to CCRT was associated with encouraging survival outcomes in LA-NPC, though only 23 patients were enrolled to be administered CCRT and NTZ, with a follow-up median of 24.1 months.Citation12 Considering the absence of randomized controlled trial to determine the efficacy of CCRT plus NTZ vs. CCRT alone in patients with LA-NPC, it remains under debate if patients with LA-NPC benefit from the addition of NTZ for the recommended standard treatment of CCRT.
To address this knowledge gap, we reviewed data on 93 patients with LA-NPC who received the standard CCRT regimen with or without NTZ to provide additional information on the benefit of NTZ in the treatment of LA-NPC.
Materials and methods
Patients
Between October 2009 and January 2012, patients with histologically-confirmed NPC treated at Sun Yat-sen University Cancer Center were retrospectively reviewed. All included patients had to meet the following inclusion criteria: (1) disease classified as stage III-IVB in accordance to the American Joint Committee on Cancer/International Union Against Cancer (AJCC/UICC) staging system (7th edition, 2009); (2) histologically confirmed NPC; (3) patient received the standard CCRT regimen with or without NTZ; (4) radiation delivery technique was intensity-modulated radiotherapy (IMRT); (5) molecularly-targeted drug was NTZ.
Treatment
IMRT treatment details have been previously reported.Citation13 Target volumes were delineated according to a previously described treatment protocol by our institution,Citation12 in agreement with International Commission on Radiation Units (ICRU) and Measurements reports 62Citation14 and 83.Citation15 The prescribed radiation dose was defined as follows: a total dose of 68–70 Gy at 2.12–2.27 Gy/fraction to the planning target volume (PTV) of the GTV-P (primary gross tumor volume), 60 Gy to the PTV of CTV-1 (high-risk regions) and 54 Gy to the PTV of CTV-2 (low-risk regions). All patients were treated with one fraction daily, five days per week. Concomitant chemotherapy consisted of cisplatin (100 mg/m2) every three weeks, or cisplatin (40 mg/m2) weekly. Reasons for non-compliance included refusal by individual patients, age, or organ dysfunction suggestive of intolerance. NTZ was administered concomitantly with IMRT at a dose of 200 mg weekly, commencing on the first day of radiotherapy. NTZ was diluted in 250 mL saline and intravenously infused over 1 h.
Ethical statement
This study was approved by our institutional review board (IRB-approved number, YB2017-040). As this was a retrospective analysis of routine clinical data, a waiver of the requirement for individual informed consent was granted by the institutional ethics committee.
Follow-up
During treatment, evaluation of patients occurred at least one time a week. After treatment, patients were evaluated every third month during the first three years, every six months the following two years, and every year afterward. During this period, for each follow-up visit, patients underwent a sequence of conventional examinations to detect potential relapse or distant metastasis. Biopsy, MRI (magnetic resonance imaging), or both was used to confirm local relapse. Clinical examination and MRI of the neck was used to diagnosis regional relapse, and in doubtful cases, by fine needle aspiration of lymph nodes. To diagnosis distant metastasis, physical examinations, clinical symptoms, and imaging methods with bone scan, chest radiography, abdominal sonography, and MRI was used. Further investigations were arranged if indicated.
Statistical analysis
We used propensity score matching method to select patients treated with CCRT plus NTZ to match with CCRT treatment only.Citation16 According to presumed covariates, including age, Karnofsky score, pathology, total radiation dose, sex, chemotherapy, T category, N category and clinical stage, logistic regression was computed for each patient for propensity scores. At a 1:2 ratio, we matched patients without replacement using these scores, instead of by individual covariates.
Using χ2 test, the balance of covariates between both study arms were examined. Kaplan-Meier method was used to estimate overall survival (OS), distant metastasis-free survival (DMFS), progression-free survival (PFS), locoregional relapse-free survival (LRRFS).Citation17 Cox regression analysis was used to calculate both crude and adjusted hazard ratios with 95% confidence intervals (for CCRT plus NTZ, using CCRT as the reference).Citation18 All test were two-tailed and P values < 0.05 were deemed statistically significant. We performed all analyses using R 3.1.2 software (Vienna, Austria; https://mirrors.tuna.singhua. edu.cn/CRAN/).
Results
Patient characteristics
31 patients treated with CCRT plus NTZ and 62 patients treated with CCRT only remained in the current analysis after propensity score matching. The follow-up median was 59.2 months (49.5–72.4 months) for the CCRT plus NTZ arm and 57.4 months (6.5–69.4 months) for the CCRT only arm. In the CCRT plus NTZ arm, 3 (9.7%) patients suffered distant metastasis, and 4 patients (12.9%) developed locoregional recurrence. In the CCRT only arm, 13 (21.0%) patients suffered distant metastasis, and 7 patients (11.3%) developed locoregional recurrence. Interestingly, only 33% (2/6) patients suffered disease progression within 3 years after radical RT in the CCRT plus NTZ arm. In contrast, up to 90% (16/18) patients suffered disease progression within 3 years after radical RT the CCRT only arm. presents the characteristics at baseline for the two study arms. We did not observe a significant difference in respect to age, pathology, sex, Karnofsky score, total radiation dose, radiotherapy treatment time, chemotherapy, T category, N category and overall stage. Subsequent analyses reported derived from propensity-matched cohort.
Table 1. Patient and tumor characteristics.
Treatment and compliance
All patients completed the full course of radiotherapy. In the CCRT plus NTZ arm, 2/31 patients (6.5%) completed four cycles of NTZ, 5 (16.1%) completed five cycles, 21 (67.7%) completed six cycles, and 3 (9.7%) completed seven cycles. 21 of the 31 patients (67.7%) received two or three cycles of 100 mg/m2 cisplatin each third week, and 10 (32.3%) received 40 mg/m2 cisplatin weekly for 5–7 planned cycles. Median administered total dose of cisplatin during radiotherapy was 240 mg/m2 (IQR 200–280 mg/m2) in the CCRT plus NTZ arm. As shown in , propensity score matching ensured the chemotherapy regimens and cycles of the CCRT only arm were well-matched with those of the CCRT plus NTZ arm.
Survival outcomes and multivariate analysis
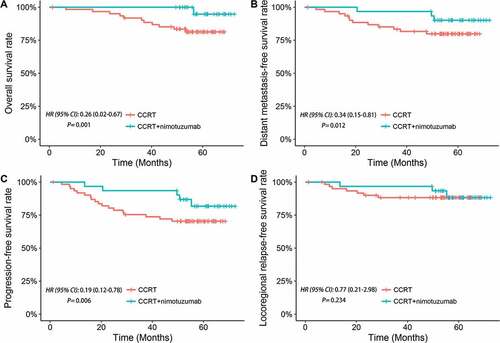
The differences in efficacy between these two arms are presented in . The 5-year OS was significantly higher for CCRT plus NTZ than those treated with CCRT only (96.8% vs. 82.3%; P = 0.001; ). The 5-year DMFS was significantly superior in CCRT plus NTZ treated patients than those treated with CCRT only (90.3% vs. 80.6%; P = 0.012; ). Similarly, the 5-year PFS was significantly higher in CCRT plus NTZ treated patients than those treated with CCRT only (83.9% vs. 71.0%; P = 0.006; ). There were trends towards improved LRRFS for the addition of NTZ over CRRT only, though no associated difference in LRRFS was observed between both arms (90.3% vs. 88.7%; P = 0.234; ). Consistent with univariate analysis, multivariate analysis showed that adding NTZ to CCRT was a favorable prognostic factor for OS ([hazard ratio (HR), 0.31; 95% confidence interval (CI), 0.02–0.71; P = 0.027), DMFS (HR, 0.45; 95% CI, 0.13–0.77; P = 0.034), and PFS (HR, 0.38; 95% CI, 0.11–0.89; P = 0.041) ().
Table 2. Multivariate analysis of treatment regimen status and other prognostic factors for treatment outcomes.
Figure 1. Kaplan-Meier curves of (A) overall survival, (B) distant metastasis-free survival, (C) progression-free survival and (D) locoregional relapse-free survival curves for the CCRT plus nimotuzumab arm versus CCRT arm.
Acute toxicities
displays the treatment toxicities of each arm. The most frequently observed toxicity was oral mucositis. The rates of grade 3–4 mucositis was 25.8% in CCRT plus NTZ group, and 19.4% in CCRT only group (P = 0.59). No significant differences in hematology parameters, dermatitis, nausea, vomiting, xerostomia, nephrotoxicity or neurotoxicity were found between the two arms (all P > 0.05). Overall, treatment toxicities during radiotherapy were well-tolerated, and no treatment-related deaths occurred in either arm.
Table 3. Grade 3–4 adverse events during radiotherapy.
Discussion
This is the first assessment of the clinical outcomes of LA-NPC treated patients with standard CCRT regimen plus NTZ in comparison to CCRT treated patients only. To balance the baseline characteristics of the two arms, a propensity score-matched method was applied. In comparison to CCRT treated patients only, CCRT plus NTZ was statistically associated with superior OS, DMFS, and PFS. Strikingly, NTZ addition to the standard CCRT regimen did not increase acute toxicities.
EGFR has a major part in tumorigenesis and survival of cancer cells. Several studiesCitation9,Citation19,Citation20 have demonstrated high EGFR expression is associated with an increased treatment resistance, elevated aggressive phenotype, and poorer prognosis in NPC. EGFR-blocking monoclonal antibodies may potentially exert encouraging effects in LA-NPC. Liu et al.Citation21 examined 42 cases of LA-NPC treated with NTZ plus CCRT. The follow-up median was twenty-five months (ranging 7–44 months), where only two patients had died by last follow-up. Recently, a preliminary clinical trialCitation11 of 23 patients with LA-NPC treated with CCRT plus NTZ reported an estimated 2-year OS rate of up to 95.0%. Consistent with these studies, we also found adding NTZ to CCRT resulted in encouraging survival outcomes in LA-NPC. Moreover, compared to CCRT only, including NTZ to CCRT was significantly associated with improved survival. This information suggest that combined NTZ with CCRT could maximize survival in LA-NPC patients compared to patients taking CCRT only.
Previous literature reported 90% control rates for NPC when using IMRT in combination with systematic chemotherapy even in patients presenting with LA-NPC.Citation22,Citation23 As a result of recent advances in IMRT, there was no observed significant difference in 5-year LRRFS (90.3% vs. 88.7%, P = 0.704) between the two arms. This seems reasonable, as IMRT provides excellent locoregional control,Citation24,Citation25 and concurrent chemotherapy has been confirmed to improve locoregional control.Citation26,Citation27 Collectively, these effects would limit the actual benefit of NTZ on LRRFS.
In the current study, the rate of grade 3–4 mucositis was comparable between patients receiving CCRT plus NTZ (25.8%) and patients receiving CCRT only (19.4%). A recent retrospective analysis by Liu and colleaguesCitation21 also concluded NTZ could be safely combined with CCRT for treatment of LA-NPC. However, Huang et al.Citation12 reported 34.8% of patients receiving CCRT plus NTZ experienced grade 3 to 4 mucositis. These inconsistencies could be due to obvious differences between chemotherapy regimens, where only patients receiving concurrent chemotherapy consisting of cisplatin (100 mg/m2) every three weeks or cisplatin (40 mg/m2) weekly were eligible for the current study. However, the study by Huang et al.Citation12 included patients who received more intensive concurrent chemotherapy regimens (75 mg/m2 docetaxel and 80 mg/m2 nedaplatin), which may have increased the incidence of mucositis during radiotherapy.
Nonetheless, we must note inherent limitations in the present study. First, this was a single-center observational study, and the number of patients who received CCRT plus NTZ was relatively low. We expect promising results will soon be available from the phase III clinical trial of NTZ in combination with chemoradiation for LA-NPC, even though this study is ongoing but not recruiting participants (ClinicalTrials. gov: NCT01074021). Second, we failed to incorporate data on plasma Epstein–Barr Virus (EBV) DNA, where prior studies demonstrated strong prediction for NPC survival.Citation28,Citation29 Further studies are urgently required to identify the individual patients who may benefit most from CCRT plus NTZ based on other biomarkers such as plasma EBV DNA, EGFR status or K-ras (kirsten rat sarcoma) gene levels before treatment, especially as NTZ is more expensive than conventional treatment.
In summary, including NTZ in addition to standard CCRT regimen is more effective for extending survival in LA-NPC patients in comparison to CCRT only. Additionally, CCRT plus NTZ was well-tolerated and did not increase acute toxicities. However, a prospective randomized clinical trial is required to validate the superiority of CCRT plus NTZ compared to CCRT only in LA-NPC.
Abbreviations
Conflict of Interest
Conflict of interest relevant to this article was not reported.
Additional information
Funding
References
- Ferlay J, Bray F, Pisani P, Parkin DM. Cancer incidence, mortality and prevalence worldwide. 2.0. Lyon: IARC Press; 2004.
- Torr e LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108. doi:10.3322/caac.21262.
- Li WF, Sun Y, Chen M, Tang LL, Liu LZ, Mao YP, Chen L, Zhou GQ, Li L, Ma J. Locoregional extension patterns of nasopharyngeal carcinoma and suggestions for clinical target volume delineation. Chin J Cancer. 2012;31(12):579–587. doi:10.5732/cjc.012.10095.
- Internet National Comprehensive Cancer Network. NCCN Guidelines: head and neck cancers; Version 2.2013. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- Lee AW, Sze WM, Au JS, Leung SF, Leung TW, Chua DT, Zee BC, Law SC, Teo PM, Tung SY, et al. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2005; 61:1107–1116. doi:10.1016/j.ijrobp.2004.07.702.
- Ma BB, Hui EP, Chan AT. Systemic approach to improving treatment outcome in nasopharyngeal carcinoma: current and future directions. Cancer Sci. 2008;99(7):1311–1318. doi:10.1111/j.1349-7006.
- Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001; 7:2958–2970.
- Mendelsohn J. Targeting the epidermal growth factor receptor for cancer therapy. J Clin Oncol. 2002; 20:1S–13S.
- Zhang P, Wu SK, Wang Y, Fan ZX, Li CR, Feng M, Xu P, Wang WD, Lang JY. p53, MDM2, eIF4E and EGFR expression in nasopharyngeal carcinoma and their correlation with clinicopathological characteristics and prognosis: A retrospective study. Oncology Letters. 2015; 9:113–118. doi:10.3892/ol.2014.2631.
- Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, Galvin JM, Bonner JA, Harris J, El-Naggar AK, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32(27):2940–2950. doi:10.1200/JCO.2013.53.5633.
- Talavera A, Friemann R, Gómez-Puerta S, Martinez-Fleites C, Garrido G, Rabasa A, López-Requena A, Pupo A, Johansen RF, Sánchez O, et al. Nimotuzumab, an antitumor antibody that targets the epidermal growth factor receptor, blocks ligand binding while permitting the active receptor conformation. Cancer Research. 2009; 69:5851–5859. doi:10.1158/0008-5472.CAN-08-4518.
- Huang JF, Zhang FZ, Zou QZ, Zhou LY, Yang B, Chu JJ, Yu JH, Zhang HW, Yuan XP, Tai GM, et al. Induction chemotherapy followed by concurrent chemoradiation and nimotuzumab for locoregionally advanced nasopharyngeal carcinoma: preliminary results from a phase II clinical trial. Oncotarget. 2017;8(2):2457–2465. doi:10.18632/oncotarget.13899.
- Yao JJ, Yu XL, Zhang F, Zhang WJ, Zhou GQ, Tang LL, Mao YP, Chen L, Ma J, Sun Y. Radiotherapy with neoadjuvant chemotherapy versus concurrent chemoradiotherapy for ascending-type nasopharyngeal carcinoma: a retrospective comparison of toxicity and prognosis. Chin J Cancer. 2017;36(1):26. doi:10.1186/s40880-017-0195-6.
- ICRU report. Vol. 62: prescribing, recording, and reporting photon beam therapy. Maryland: International Commission on Radiation Units and Measurements; 1999.
- ICRU Report. Vol. 83: prescribing, Recording, and Reporting Photon-Beam Intensity-Modulated Radiation Therapy (IMRT). Maryland: International Commission on Radiation Units and Measurements; 2010.
- D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998; 17:2265–2281. doi:10.1002/(SICI)1097-0258(19981015)17:19<2265::AID-SIM918>3.0.CO;2-B.
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958; 53:457–481. doi:10.1080/01621459.1958.10501452.
- Cox DR. Regression models and life tables. J Roy Stat Soc B. 1972; 34:187–220.
- Ma X, Huang J, Wu X, Li X, Zhang J, Xue L, Li P, Liu L. Epidermal growth factor receptor could play a prognostic role to predict the outcome of nasopharyngeal carcinoma: A meta-analysis. Cancer Biomark. 2014;14(4):267–277. doi:10.3233/CBM-140401.
- Sun W, Long G, Wang J, Mei Q, Liu D, Hu G. Prognostic role of epidermal growth factor receptor in nasopharyngeal carcinoma: a meta-analysis. Head Neck. 2014;36(10):1508–1516. doi:10.1002/hed.23481.
- Liu ZG, Zhao Y, Tang J, Zhou YJ, Yang WJ, Qiu YF, Wang H. Nimotuzumab combined with concurrent chemoradiotherapy in locally advanced nasopharyngeal carcinoma: a retrospective analysis. Oncotarget. 2016;7(17):24429–24435. doi:10.18632/oncotarget.8225.
- Peng G, Wang T, Yang KY, Zhang S, Zhang T, Li Q, Han J, Wu G. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104(3):286–293. doi:10.1016/j.radonc.
- Zhang MX, Li J, Shen GP, Zou X, Xu JJ, Jiang R, You R, Hua YJ, Sun Y, Ma J, et al. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: A 10-year experience with a large cohort and long follow-up. Eur J Cancer. 2015;51(17):2587–2595. doi:10.1016/j.ejca.2015.08.006.
- Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, Bosch W, Morrison WH, Quivey J, Thorstad W, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol. 2009;27(22):3684–3690. doi:10.1200/JCO.2008.19.9109.
- Lee N, Xia P, Quivey JM, Sultanem K, Poon I, Akazawa C, Akazawa P, Weinberg V, Fu KK. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys. 2002;53(1):12–22.
- Aw L, Wh L, Sy T, Dt C, Chappell R, Xu L, Siu L, Wm S, Tw L, Js S, et al., Nasopharyngeal Cancer Study Group. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol. 2005; 23: 6966–6975. doi:10.1200/JCO.2004.00.7542.
- Blanchard P, Lee A, Marguet S, Leclercq J, Wt N, Ma J, At C, Py H, Benhamou E, Zhu G, et al., MAC-NPC Collaborative Group. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16(6):645–655. doi:10.1016/S1470-2045(15)70126-9.
- Lin JC, Wang WY, Chen KY, Wei YH, Liang WM, Jan JS, Jiang RS. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004;350(24):2461–2470. doi:10.1056/NEJMoa032260.
- Lo YM, Chan AT, Chan LY, Leung SF, Lam CW, Huang DP, Johnson PJ. Molecular prognostication of nasopharyngeal carcinoma by quantitative analysis of circulating Epstein-Barr virus DNA. Cancer Res. 2000;60(24):6878–6881.
