ABSTRACT
Leptomeningeal metastases (LMs) were devastating metastatic complications of non-small cell lung cancer (NSCLC). Management of LMs relied on conventional therapy but with poor survival, lacking effective treatment strategies. We present the case of a 52-year-old female non-smoker with advanced lung adenocarcinoma and initially positive EGFR-mutation, who failed to the treatment of standard first-line chemotherapy (pemetrexed plus cisplatin) and bevacizumab (BEV), and maintenance therapy with pemetrexed plus BEV. Under the progression-free survival of 6 months, suffered from LMs, then erlotinib combined with BEV were delivered, and symptoms were significantly alleviated, remained stable and even relieved slightly for at least 18 months. The combination of bevacizumab and erlotinib could be an optional effective management strategy for patients with LMs from NSCLC and harboring EGFR-mutation.
Introduction
Leptomeningeal metastasis (LM) refers to the infiltration of malignant cells on the surface of meninges. It is also named carcinomatous meningitis when cancer cells originally come from the solid tumors, or lymphomatous/leukemic meningitis when cancer cells originate from a systemic lymphoma or leukemia. LMs were diagnosed in appropriately 5% of patients with malignant tumors.Citation1 However, the autopsy results revealed LM incidence might be 20% or more.Citation2 The incidence of LMs in non-small cell lung cancer (NSCLC) was 3.8%, being more frequent in lung adenocarcinoma. Because the activated pathways downstream of EGFR promoted malignant cells proliferation and metastasis, LM incidence is high especially in subgroup with EGFR mutations (9.4%) .Citation3,Citation4 The clinical data from a retrospective study including 1,127 NSCLC patients demonstrated that those with EGFR-mutations were more likely to develop LMs (30.8% vs 12.7%, p < 0.001).Citation5
LMs result in severe neurological adverse events reducing the life quality of patients. Regardless of the improvements of new therapeutics, management of LMs remains a challenge. Whole brain radiotherapy (WBRT), intrathecal methotrexate (MTX), systematic chemotherapy, surgery and best supportive care have been the selective approaches for LMs.Citation1,Citation6 The median survival time (MST) was only 5 months for NSCLC patients treated with chemoradiotherapy while survival was worse in patients treated with chemotherapy or radiotherapy alone.Citation7
In the modern treatment era, the application of targeted drugs greatly improved the survival of patients with LMs. Herein, we reported a case with LMs from advanced lung adenocarcinoma with EGFR mutations who exhibited a great response to second-line treatment of bevacizumab (BEV) and erlotinib.
Case presentation
Diagnosis
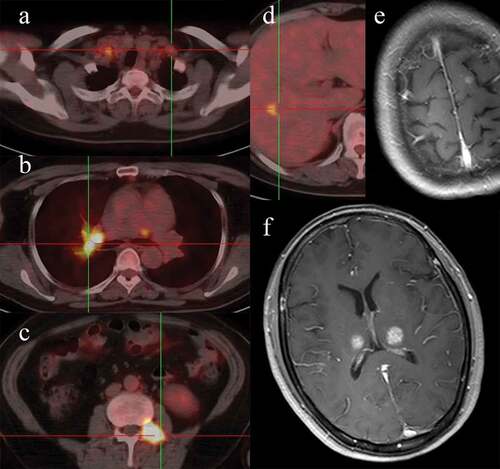
A 52-year-old female never-smoker was diagnosed moderately differentiated lung adenocarcinoma by computed tomography (CT)-guided percutaneous soft tissue core biopsy in July 2015. Systematic evaluation before treatment was taken, including performance status (PS), bronchoscopy, transbronchial needle aspiration, brain contrast-enhanced MRI, thoracic and abdominal CT, fluorodeoxyglucose positron emission tomography (PET)/CT, and indicated that multiple lymph nodes, bone, brain, and oligo-liver-metastasis were involved (). The examination of EGFR mutations by amplification refractory mutation system demonstrated EGFR with exon 19 deletion mutations. Then the patient was diagnosed with stage IV T4N3M1 NSCLC, accompanied with EGFR positive mutation and negative ALK-gene arrangement in accordance with the 7th edition of the TNM staging system.
First-line treatment and maintenance therapy
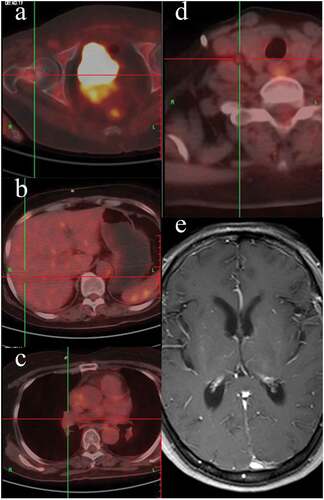
Because the tyrosine kinase inhibitor (TKI) was not covered by the health insurance in China at that time, so chemotherapy as the first-line standard agents were administrated: cisplatin 30 mg/m2 day 1–3, pemetrexed 500 mg/m2 day1 in combination with bevacizumab (BEV) 7.5 mg/Kg day 0 every 3 weeks (Q3W) for 4 cycles. Thoracic and abdominal CT were conducted to evaluate the response of treatment every two cycles and showed great partial responses for lung sites, lymph nodes and other extracranial lesions (complete response for brain situs) after 4 cycles (). Maintenance chemotherapy with pemetrexed (500 mg/m2 day1) and bevacizumab (7.5 mg/Kg day 0) were conducted every 3 weeks.
Targeted for leptomeningeal metastases
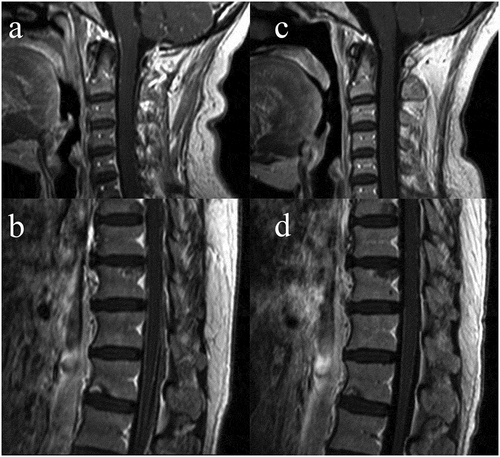
During maintenance treatment, the patient experienced progressive headache at the time of 6 months after the initial treatment on Jan 2016, and remarkably weak for both lower limbs. Intracranial pressure increased to 320 mm H2O with normal white blood cells, glucose and protein in cerebrospinal fluid (CSF), and cytopathology was negative for tumor cells in the first time of lumbar puncture. However, the tumor biomarker CEA of CSF was significantly increased (544.9 ng/ml) while NSE and Cyfra21-1 were remained normal. The brain and spine contrast-enhanced MRI was performed and showed an atypical enhanced and thickening thoracic pia mater and setuliform enhanced cauda equine (,). Given clinical symptoms, apparently increased CEA in CSF and pathological enhancement in MRI findings, LM was confirmed. Extracranial lesions were still stable demonstrated by PET/CT (). After placing subcutaneous reservoir and ventricular catheter (SRVC), the routine examination of CSF was still normal but malignant cells were not found in CSF after being tested twice. Next-generation sequencing (NGS) of peripheral blood samples, CSF and tissue was performed and exon 19 deletion was demonstrated in all samples. Considering the failure of maintenance therapy, erlotinib 150mg was taken orally once daily in combination with bevacizumab (7.5mg/kg, Q3W) intravenously, and 2 weeks later, the symptom relieved. After 2 months, MRI indicated leptomeningeal lesions relieved than ever (,). Additionally, CSF CEA significantly decreased to 50.8ng/ml and remained stable approximately at this level.
Figure 3. The MRI images at the initial time of LM diagnosis and after 2 months erlotinib given. The enhanced-contrast MRI showed an atypical thickness setuliform enhanced cervical and thoracic pia mater (a, b); after 2 months for erlotinib and bevacizumab administered, the degree of enhanced decreased and the thickness was thinner than the initial MRI image (c, d).
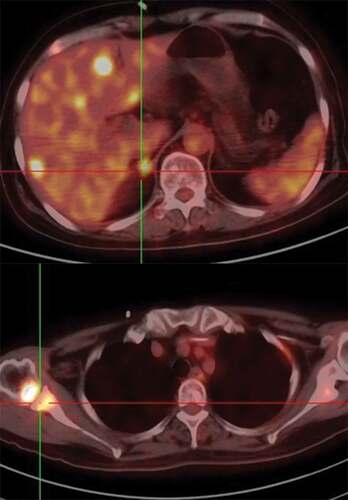
Ten months later, disease recurrence was found at the site of liver, bone, lung and brain which were confirmed by PET/CT and MRI (), with decreased KPS. MRI revealed BMs were recurrence but LMs remained stable. Eventually, the case died in May 2017. From the time of LM diagnosis to death of the patient, LMs have been stable and even slightly relieve.
Figure 4. Representative images after the disease progresses. PET/CT indicated the new metastatic lesions of liver and bone.
In conclusion, the PFS1 which was defined by the initial chemotherapy and BEV was 6 months. The PFS2 which was incurred by erlotinib and BEV was 12 months. The PFS of LM was 18 months. The OS was 22 months.
The dynamic change of CEA in serum and CSF
The serum CEA level was detected at the whole course and that in the CSF was monitored when the LM occurred. The kinetic change of CEA and the disease was consistent highly, and we summarized them in .
Table 1. The level of CEA in serum and CSF during the course of disease.
Discussion
LM is a devastating metastatic complication of malignant tumor and decreases survival. Due to the shortage of randomized trials, high-level evidence-based data is insufficient to guide the clinical treatment of patients with LMs. In this case with advanced lung adenocarcinoma initially with EGFR-mutations, first-line chemotherapy plus bevacizumab followed by maintenance therapy (pemetrexed plus BEV) was performed. BMs were almost reached complete response (CR). However, atypical LMs were highly suspected based on imaging findings, high level of CEA in CSF, NGS on CSF samples showed EGFR 19 exon mutation. Concurrently erlotinib and bevacizumab were administered to relieve symptoms and control the disease. LMs remained stable for at least 16 months until the patient eventually died of the progression of BMs with a survival time of 22 months.
A positive CSF cytology remains the gold standard for the diagnosis of leptomeningeal metastasis. But the sensitivity of the initial lumbar puncture is only 50%, which could increase to 75% – 85% with a second CSF analysis.Citation8 A gadolinium-enhanced MRI of the brain and spine is the best imaging technique for the evaluation of leptomeningeal metastasis. The characters of leptomeningeal metastasis in MRI finding are the pathological enhancement of the leptomeninges of the brain, cranial nerves, and spinal cord, which appears as nodular, linear, arched, focal, or diffuse intensification. But Remon J showed that about 20 – 30% of patients with leptomeningeal metastasis had a normal or false-negative MRI.Citation6 A liquid biopsy of the CSF has been used successfully for the diagnosis of leptomeningeal metastasis, the detection of genomic alterations, and the observation of treatment responses, and its excellent role has been evaluated in the osimertinib cohort of the BLOOM study.Citation9 In this case, the diagnosis was confirmed by the MRI positive findings and the NGS of CSF with exon 19 deletion, but the CSF cytology was negative.
Before tyrosine kinase inhibitors (TKIs) and anti-angiogenesis drugs were used in NSCLC, the management of LMs relied on chemoradiotherapy but with great heterogeneity and outcomes were disappointing.Citation7,Citation10–Citation14 The data from a retrospective study including 519 cases with LMs from solid tumors indicated MST was only 3 months with 10% of 1-year survival rate.Citation7 Even for patients treated with chemoradiotherapy, MST was just 5 months. Due to the presence of blood-brain barrier (BBB) and blood-tumor barrier, chemo-drugs are difficult to penetrate into the intracranial space to the tumor sites. Theoretically, radiotherapy could block BBB that make a time window for chemotherapy to be possible. However, WBRT was failed to improve survival compared to those who were not treated with WBRT (p = 0.84).Citation13 The negative results may be caused by low percentage of intrathecal chemotherapy (6%, 7/119) and systematic chemotherapy (16%, 20/119). However, the results from a 2-phase prospective study which evaluated the role of involved-field irradiation combined with concomitant intrathecal MTX in cases with LMs from solid tumors were also pessimistic.Citation14 Regardless of 86.4% (51/59) of overall response rate, MST was 6.5 months (range, 0.4 – 36.7 months) with 21.3% of 1-year survival rate. High dose intrathecal MTX or systematic chemotherapy could lead to severe toxicity, further limiting the anti-tumor effect.
In the era of targeted therapy, BEV and TKIs for patients with LMs from NSCLC provide a new paradigm shift, particularly for those with sensitive EGFR-mutations.Citation3-Citation15 Erlotinib was more likely to penetrate BBB than gefitinib.Citation16 The CSF concentration and penetration rate of erlotinib were significantly higher than those of gefitinib (p = 0.0008 and < 0.0001, respectively). Moreover, cytologic conversion rate of LMs was better in patients treated with erlotinib than those with gefitinib (64.3% vs 9.1%, p = 0.012).Citation17 Furthermore, for patients who failed to first-generation TKIs, improving the dose of TKIs or switching to the second or third generation TKIs for the appropriate patients, such as the patients with T790M mutation, was still valid.Citation18,Citation19 The results from an EGFR-mutant BM xenograft mouse model indicated osimertinib distribution into the brain was 10-fold higher than gefitinib and resulted in a marked shrinkage of brain lesions.Citation20 Preliminary results presented at the 2016 ASCO meeting also showed an impressive response for osimertinib in patients with BMs/LMs from EGFR-mutant NSCLC.Citation21 Among 12 patients reaching intracranial image assessment, 7 and 2 had radiological improvement and stable disease, respectively, and 3 were not evaluable. However, other possible resistant mutations, such as T790M, were not tested in this case after the second progression of the disease due osimertinib was not available in mainland China. Additionally, the high price of osimertinib limits its clinical application. Erlotinib plus bevacizumab would be therefore an optimal choice for those patients who do not have the conditions to use osimertinib.
At the present point of view, immunotherapy plus radiotherapy or chemoradiotherapy was helpful in the treatment of advanced NSCLC. However, the clinical benefits of checkpoint inhibitors have been less frequently observed in patients with EGFR mutations compared to those patients with wild-type EGFR. On the other hand, the patients with central nerve system (CNS) metastases have generally been excluded from immunotherapy studies that leads few studies focus on the topic of immunotherapy and brain metastases in advanced NSCLC. A retrospective study reported in 2018 ASCO meeting indicated the response rate, median PFS and OS in advanced NSCLC patients with CNS metastases treated with checkpoint inhibitors was 16.9%, 2.0 months and 10.3 months, respectively, which were not different from those of patients without CNS metastasis.Citation22 No EGFR mutation status were reported and PD-L1 expression was accessed in 27 patients (0%: 2 pts, 1–49%: 10 pts, 50%< 15 pts). Only good performance status (p = 0.0004) and local radiotherapy to CNS lesion (p = 0.004) were prognostic factors for OS in multivariate analysis. In this case study, the tumor tissue specimens were obtained from mediastinal metastatic lymph nodes through a bronchoscopic needle aspiration. After placing SRVC, NGS was performed in the sample of peripheral blood, CSF and tumor tissue. Due to stable disease of extracranial lesions, no secondary biopsy for primary lung lesions were conducted. Furthermore, LM sites were not suitable for biopsy that made this case in danger easily. Moreover, the gold standard for the diagnosis of LM was to verify malignant cells in CSF. Therefore, no LM sites were used to biopsy. In addition, due to high price of immunotherapy agents and difficult to access these antibodies, immunotherapy was not considered for the patient and her family. No PD-L1 status and tumor mutation burden thus were detected.
Previous study indicated VEGF concentration in CSF may be higher and associated with poor survival.Citation23 The addition of BEV prolonged survival compared to those of erlotinib alone (16.0 vs 9.7 months, p = 0.0015).Citation15 After using BEV and erlotinib, intolerable headache was significantly relieved in this case. The updated safety results of JO25567 showed high frequency of hypertension and bleeding occurred in patients treated with bevacizumab plus erlotinib but were manageable.Citation24 Consistent with JO25567, the hypertension was in control in present case, and there were no other serious adverse events.
Additionally, serum CEA is a useful indicator of OS and progression-free survival in patients with colorectal cancer and non-SCLC, especially adenocarcinoma. And it could be used to monitor the disease progression. Interestingly, the kinetic change of CEA in serum and CSF was related to the disease course in this case.
Conclusion
In summary, this case gave us some interesting hints in the diagnosis and treatment of LM. Firstly, CSF liquid biopsy, especially with the technique of NGS, could be a promising method for the diagnosis of leptomeningeal metastasis. Secondly, Erlotinib in combination with bevacizumab should be considered as one of the therapeutic options for patients with EGFR-mutant lung adenocarcinoma. Finally, the dynamic changes of CEA in CSF can be used as indicators for monitoring the treatment response of LMs, especially for atypical LMs.
Disclosure of interest
The authors report no conflict of interest.
Ethics approval and consent to participate
Informed consent was provided, and the patient has signed it. This study was approved by the institutional review board and ethics committee at the Shandong Cancer Hospital Affiliated to Shandong University.
Acknowledgments
We would like to thank the patient for participating in this research and allowing us to share the findings.
Additional information
Funding
References
- Pavlidis N. The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann Oncol. 2004; 15(Suppl 4):iv285–291. doi:10.1093/annonc/mdh941.
- Beauchesne P. Intrathecal chemotherapy for treatment of leptomeningeal dissemination of metastatic tumours. Lancet Oncol. 2010; 11(9):871–879. doi:10.1016/S1470-2045(10)70034-6.
- Liao BC, Lee JH, Lin CC, Chen YF, Chang CH, Ho CC, Shih JY, Yu CJ, Yang JC. Epidermal growth factor receptor tyrosine kinase inhibitors for non-small-cell lung cancer patients with leptomeningeal carcinomatosis. J Thorac Oncol. 2015; 10(12):1754–1761. doi:10.1097/JTO.0000000000000669.
- Li YS, Jiang BY, Yang JJ, Tu HY, Zhou Q, Guo WB, Yan HH, Wu YL. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol. 2016; 11(11):1962–1969. doi:10.1016/j.jtho.2016.06.029.
- Iuchi T, Shingyoji M, Itakura M, Yokoi S, Moriya Y, Tamura H, Yoshida Y, Ashinuma H, Kawasaki K, Hasegawa Y, et al. Frequency of brain metastases in non-small-cell lung cancer, and their association with epidermal growth factor receptor mutations. Int J Clin Oncol. 2015; 20(4):674–679. doi:10.1007/s10147-014-0760-9.
- Remon J, Le Rhun E, Besse B. 2017. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: A continuing challenge in the personalized treatment era. Cancer Treat Rev. 53:128–137. doi:10.1016/j.ctrv.2016.12.006.
- Hyun JW, Jeong IH, Joung A, Cho HJ, Kim SH, Kim HJ. 2016. Leptomeningeal metastasis: clinical experience of 519 cases. Eur J Cancer. 56:107–114. doi:10.1016/j.ejca.2015.12.021.
- Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999; 25(2):103–119. doi:10.1053/ctrv.1999.0119.
- Yang JCH, Kim DW, Kim SW Osimertinib activity in patients (pts) with leptomeningeal (LM) disease from non-small cell lung cancer (NSCLC): Updated results from BLOOM, a phase I study. 2016.
- Mack F, Baumert BG, Schafer N, Hattingen E, Scheffler B, Herrlinger U, Glas M. 2016. Therapy of leptomeningeal metastasis in solid tumors. Cancer Treat Rev. 43:83–91. doi:10.1016/j.ctrv.2015.12.004.
- Waki F, Ando M, Takashima A, Yonemori K, Nokihara H, Miyake M, Tateishi U, Tsuta K, Shimada Y, Fujiwara Y, et al. Prognostic factors and clinical outcomes in patients with leptomeningeal metastasis from solid tumors. J Neurooncol. 2009; 93(2):205–212. doi:10.1007/s11060-008-9758-3.
- Brower JV, Saha S, Rosenberg SA, Hullett CR, Ian Robins H. 2016. Management of leptomeningeal metastases: prognostic factors and associated outcomes. J Clin Neurosci. 27:130–137. doi:10.1016/j.jocn.2015.11.012.
- Morris PG, Reiner AS, Szenberg OR, Clarke JL, Panageas KS, Perez HR, Kris MG, Chan TA, DeAngelis LM, Omuro AM. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol. 2012; 7(2):382–385. doi:10.1097/JTO.0b013e3182398e4f.
- Pan Z, Yang G, He H, Zhao G, Yuan T, Li Y, Shi W, Gao P, Dong L, Li Y. Concurrent radiotherapy and intrathecal methotrexate for treating leptomeningeal metastasis from solid tumors with adverse prognostic factors: A prospective and single-arm study. Int J Cancer. 2016; doi: 10.1002/ijc.30214.
- Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, Yamamoto N, Hida T, Maemondo M, Nakagawa K, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014; 15(11):1236–1244. doi:10.1016/S1470-2045(14)70381-X.
- Togashi Y, Masago K, Masuda S, Mizuno T, Fukudo M, Ikemi Y, Sakamori Y, Nagai H, Kim YH, Katsura T, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012; 70(3):399–405. doi:10.1007/s00280-012-1929-4.
- Lee E, Keam B, Kim DW, Kim TM, Lee SH, Chung DH, Heo DS. Erlotinib versus gefitinib for control of leptomeningeal carcinomatosis in non-small-cell lung cancer. J Thorac Oncol. 2013; 8(8):1069–1074. doi:10.1097/JTO.0b013e318294c8e8.
- Hoffknecht P, Tufman A, Wehler T, Pelzer T, Wiewrodt R, Schutz M, Serke M, Stohlmacher-Williams J, Marten A, Maria Huber R, et al. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol. 2015; 10(1):156–163. doi:10.1097/JTO.0000000000000380.
- Nanjo S, Ebi H, Arai S, Takeuchi S, Yamada T, Mochizuki S, Okada Y, Nakada M, Murakami T, Yano S. High efficacy of third generation EGFR inhibitor AZD9291 in a leptomeningeal carcinomatosis model with EGFR-mutant lung cancer cells. Oncotarget. 2016; 7(4):3847–3856. doi:10.18632/oncotarget.6758.
- Remon J, Planchard D. AZD9291 in EGFR-mutant advanced non-small-cell lung cancer patients. Future Oncol. 2015; 11(22):3069–3081. doi:10.2217/fon.15.250.
- Yang JC-H, Kim D-W, Kim S-W, Cho BC, Lee J-S, Ye X, Yin X, Yang Z, Jiang H, Ahn M-J. Osimertinib activity in patients (pts) with leptomeningeal (LM) disease from non-small cell lung cancer (NSCLC): updated results from BLOOM, a phase I study. J Clin Oncol. 2016;34(15_suppl):9002.
- Sone T, Kasahara K, Shirasaki H, Amino Y, Nishi K, Kurokawa K, Kita T, Araya T, Yoneda T, Tanimura K, et al. A retrospective analysis of the efficacy of immune checkpoint inhibitors (ICIs) to advanced non-small cell cancer (NSCLC) patients (pts) with central nerve system (CNS) metastasis. J Clin Oncol. 2018; 36: abstr e21042.
- Herrlinger U, Wiendl H, Renninger M, Forschler H, Dichgans J, Weller M. Vascular endothelial growth factor (VEGF) in leptomeningeal metastasis: diagnostic and prognostic value. Br J Cancer. 2004; 91(2):219–224. doi:10.1038/sj.bjc.6601953.
- Kato T, Seto T, Nishio M, Goto K, Yamamoto N, Okamoto I, Tao L, Yu W, Khaznadar T, Tajima K, et al. Erlotinib Plus Bevacizumab Phase ll Study in Patients with Advanced Non-small-Cell Lung Cancer (JO25567): Updated Safety Results. Drug Saf. 2018; 41(2):229–237. doi:10.1007/s40264-017-0596-0.