ABSTRACT
Ras-specific guanine nucleotide-releasing factor 2 (RasGRF2) is a member of the guanine nucleotide exchange factors family which is expressed in a variety of tissues and cancer. However, the role of RasGRF2 in cancer is less reported, especially in colorectal cancer(CRC). Hence, the present study aimed to investigated the function of RasGRF2 and ways in which it affects tumor progression in CRC samples and cell lines. We first measured RasGRF2 mRNA level in 26 paired tumor and nontumor colon tissues after colon cancer surgical resection, and determined RasGRF2 protein level in 97 paired paraffin-embedded colon cancer tissues, and found that levels of RasGRF2 mRNA and protein were increased in colorectal tumor tissues, compared with adjacent non-tumor tissues. We then examined the effects of RasGRF2 knockdown on proliferation, migration and invasion were analyzed in CRC cells (SW480, HCT116 and LS174T). HCT116 cells with RasGRF2 knockdown were injected into the tail vein in nude mice to yield metastatic model, and tumor metastasis was measured as well. We found that knockdown of RasGRF2 in CRC cells reduced their migration and invasion in vitro and metastasis in mice. Furthermore, we explored the underlying molecular mechanism for RasGRF2-mediated CRC migration and invasion. The results showed that knockdown of RasGRF2 in CRC cells impairing the expression of MMP9 and inhibiting the activation of Src/Akt and NF-κB signaling. We conclude that RasGRF2 plays a role in controlling migration and invasion of CRC and modulates the expression of MMP9 through Src/PI 3-kinase and the NF-κB pathways.
Introduction
Colorectal cancer(CRC) is the third most common cancer worldwide, and the leading cause of cancer death in many developed countriesCitation1. Despite some improvement in survival over the past several decades, the overall prognosis for colorectal cancer patients remains poor. This may in part be attributed to the high invasive potential and distant metastasis. Hence, there is an urgent need to understand the molecular mechanisms of invasion and metastasis of CRC and develop new therapeutic strategies to reduce the burden of CRC.
Ras oncoproteins are membrane-localized 21-kDa GTPases that function as molecular switches activating protein kinase cascades in response to a variety of extracellular signalsCitation2. Ras GTPases exist in two conformations, GTP bound (active) and the GDP bound (inactive) statesCitation3. Activation of Ras into its GTP-bound conformation is regulated by proteins known as guanine-nucleotide releasing (or exchange) factors (GRFs or GEFs), which catalyze GDP release. RasGRF1 and RasGRF2 are two members of mammalian Ras GEFs. Ras-GRF1 has been reported to activate Ras in response to raised calcium concentration instead of activated receptor tyrosine kinases and functions downstream of heterotrimeric G proteinsCitation3. RasGRF2 was originally identified by Neil P. FamCitation3. Its gene was mapped to human chromosome 5q13. RasGRF2 is a multi-domain protein that has dual Ras GEF and Rac GEF activitiesCitation4. The Cdc25 homology domain at the C-terminus catalyzes exchange of GDP to GTP on Ras family proteinsCitation5. RasGRF2 is involved in regulating NMDA receptor signaling and synaptic plasticity in neuronal tissuesCitation5 and in the control of T-cell proliferation and lymphomagenesisCitation6. Calvo et al. demonstrated that RasGRF2 also plays a role in modulating tumor cell movement and invasion by inhibiting Cdc42 activation, a key component in actomyosin-contractility-dependent tumor cell movementCitation7.
In recent years, reduced expression of RasGRF2 has been observed in non-small cell lung cancersCitation8, in mammary carcinoma tissuesCitation9 and in benign colorectal adenomaCitation10. The RasGRF2 gene was reported to be aberrantly methylated in the CpG island at the 5′ region in human pancreatic cancer cells, and treatment with decitabine, a DNA methyltransferase inhibitor, restored RasGRF2 gene expressionCitation11. Colony formation of the colorectal cancer cell line HCT116 was reduced by 56% upon restoration of RasGRF2 expression, suggesting a tumor-suppressive role for RasGRF2.
However, our preliminary study revealed an overexpression of RasGRF2 in a couple of colorectal cancer cell lines. Because these observations contradict the reported suppressive role of RasGRF2 in human colon cancer, we carried out more in-depth analyses of the expression of RasGRF2 in colorectal cancer. We used quantitative real time polymerase chain reaction (qPCR), western blotting, and immunohistochemistry (IHC) to assess RasGRF2 expression, and found that it was increased in colon cancer tissues relative to adjacent normal colon tissues. We subsequently assessed the effects of RasGRF2 expression on the proliferation and invasion ability of colon cells by downregulating RasGRF2 mRNA and protein, and tried to elucidate the possible mechanisms underlying the role of RasGRF2 in colon cancer.
Results
Expression of RasGRF2 is upregulated in human CRC tissues and cells
To assess the expression of RasGRF2 in CRC, we first performed real-time PCR in 26 CRC tissues paired with adjacent nontumorous colorectal tissues. Of the 26 paired cases, 16 (61.5%) CRC tissues showed an increased expression of RasGRF2 than paired normal tissues. In addition, 10 (38%) colorectal cancer tissues showed increases of over two-fold at mRNA level ()). Similarly, RasGRF2 was overexpressed at the protein level in cancer tissues compared to matched normal colorectal tissues ()).
Figure 1. Increased expression of RasGRF2 in primary CRC tumors and CRC cell lines. (a) Real-time PCR and (b) western blot analysis of protein levels of RasGRF2 in primary tumors (T), matched adjacent nontumorous colorectal tissues (N). (c) Representative immunohistochemical staining results of RasGRF2 in tissues. (d) Western blot analysis of RasGRF2 expression in several CRC cell lines. GAPDH was used as the internal control for all analysis.
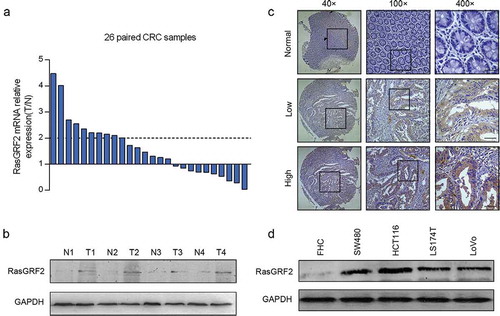
We next analyzed RasGRF2 expression by immunohistochemical staining in 97 paired colorectal cancer tissues and adjacent normal colorectal tissues. Results revealed that 21.6% (21/97) normal colorectal tissues while 46.4% (45/97) colorectal cancer tissues showed positive RasGRF2 expression (P < 0.05). As shown in ), RasGRF2 staining was observed mainly in the cytoplasm of the cancer cells. Significantly stronger RasGRF2 staining was observed in the carcinoma compared to adjacent normal colorectal tissue.
We further examined the expression of RasGRF2 protein in 4 CRC cell lines (SW480, HCT116, LS174T, LoVo) and a normal colorectal cell line (FHC) by western blot analysis. As shown in ), consistent with the results from the primary tissues, a higher level of RasGRF2 protein was observed in the CRC cell lines than in the normal colorectal cell.
Knockdown of RasGRF2 has no effect on the proliferation and apoptosis of CRC cells
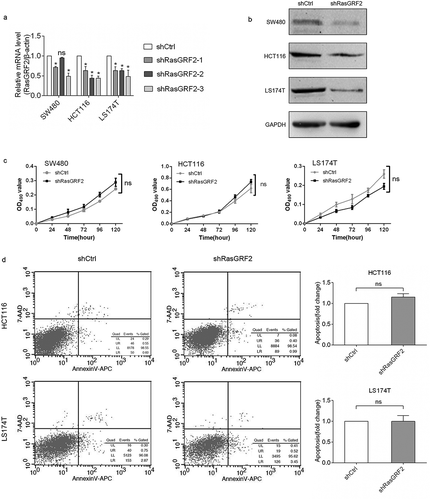
The overexpression of RasGRF2 in CRC tissues and cell lines observed above suggested that RasGRF2 may play a role in sustaining CRC cell proliferation. To test this possibility, we next evaluated the effect of RasGRF2 suppression on CRC cell proliferation and apoptosis. Lentivirus-mediated RNA interference was performed to suppress RasGRF2 expression. After RNA interference, real-time PCR was performed to evaluate the interference efficiency. The results showed that RasGRF2-specific shRNA, especially shRasGRF2-3, successfully silenced the expression of RasGRF2 in SW480, HCT116 and LS174T cells ()). Thus, we selected the shRasGRF2 to interfere with RasGRF2 expression in subsequent experiments. It caused lower RasGRF2 protein expression among all three CRC cell lines tested ()). We then determined the effects of RasGRF2 knockdown on the growth of the CRC cell lines. MTT data showed no significant difference between the knockdown group and the control group ()). The results of flow cytometry also indicated that cells were not under apoptosis ()).
Figure 2. Knockdown of RasGRF2 expression had no effect on cell proliferation and apoptosis of CRC cell lines. (a) Real-time PCR analysis of RasGRF2 mRNA in cells after RNA interference. (b) Western Blot analysis of RasGRF2 protein in cells after RNA interference. (c) Cell proliferation analysis of control and RasGRF2-downexpressing CRC cells, determined by MTT. (d) Apoptotic analysis of control and RasGRF2-downexpressing CRC cells, determined by Annexin V-APC/7-AAD staining and flow cytometry. Values are expressed as the mean± standard deviation.
Knockdown of RasGRF2 inhibited migration and invasion of CRC cells
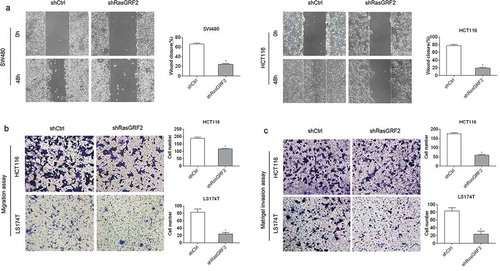
Based on the observation that RasGRF2 was highly expressed in the CRC tissues and cells, and it had no influence on CRC cell proliferation and apoptosis, we further investigated the role of RasGRF2 in CRC cell migration and invasion. The wound healing and Matrigel-free Transwell assays showed that decreased expression of RasGRF2 in HCT116 and SW480 cells resulted in significantly slower wound closure () and ()). Matrigel-based Transwell assays showed that RasGRF2 knockdown significantly inhibited the ability of invasion of CRC cells ()). These results suggest that RasGRF2 plays a key role in promoting CRC cell migration and invasion.
Figure 3. Knockdown of RasGRF2 in CRC cells inhibits cell migration and invasion. (a) Wound healing assays with control-shRNA and shRasGRF2 transfected SW480 and HCT116 cells. Migration of the cells to the wound was visualized at 0, 48h. (b) Matrigel-free Transwell assays revealed the impact of RasGRF2 on migration ability in HCT116 and LS174T. (c) Matrigel-based Transwell assay was performed in HCT116 and LS174T cells with or without downexpression of RasGRF2. This figure shows the representative images and the statistical analysis of three independent experiments. Values are expressed as the mean± standard deviation. *P < 0.05 by Student’s t-test.
RasGRF2 promotes tumor metastasis in vivo
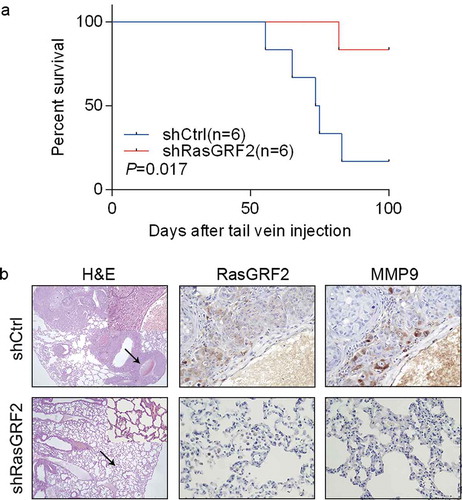
In light of our in vitro finding that RasGRF2 promotes CRC cell migration and invasion, we tested the effect of RasGRF2 knockdown on CRC cell metastasis in vivo. First, we established a lung metastatic mouse model by injecting stably transfected shControl or shRasGRF2 HCT116 cells into the tail vein of 6 mice respectivelyCitation12. As expected, RasGRF2 knockdown in highly invasive HCT116 cell line resulted in longer overall survival ()). Then, we performed hematoxylin/eosin staining on lung sections, results showed that tumor colonies appeared to be better demarcated, with well-circumscribed borders in the RasGRF2 knockdown groups compared with controls ()), consistent with reduced metastatic potential. Collectively, our results suggest that RasGRF2 contributes to CRC metastasis to the lung.
Figure 4. RasGRF2 contributes to CRC cell migration in vivo. (a) Kaplan–Meier survival curves of mice injected with control and RasGRF2-silenced HCT116 cells. n, number of mice use in each group. (b) Tissue sections from tumor-bearing lung of mice inoculated with control and RasGRF2 knockdown cells were stained with hematoxylin and eosin (H&E). IHC staining using antibodies against RasGRF2 and MMP9.
RasGRF2 modulates the expression of MMP9 through Src/PI 3-kinase and the NF-κB pathways
To explore the underlying molecular mechanism for RasGRF2-mediated CRC cells invasion, we investigated whether RasGRF2 affected MMP9 expression in CRC cells. MMPs are key mediators of cell invasionCitation13, and MMP9 is a member of the MMP protein family, and has been shown to play a key role in cancer cell migrationCitation14. As expected, MMP9 expression was inhibited in shRasGRF2 HCT116 cell line (). We also examined the MMP9 expression in lung colonies of the mice. Similarly, compared with the control nodules, lesions formed by RasGRF2-knockdown HCT116 cells contained a markedly reduced staining for MMP9 ()).
Figure 5. RasGRF2-regulated expression of MMP9 is dependent on Src/PI3K/Akt/NF-κB signaling pathway. Western blot analysis of phosphorylation status of various signaling proteins in RasGRF2-downexpressing CRC cell. GAPDH was used as the internal control for total proteins.
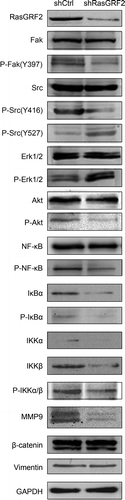
Epithelial-mesenchymal transition (EMT) is considered to a key process during tumor metastasis, so we also examined whether RasGRF2 is involved in the regulation of EMT in CRC. As shown in Supplement , knockdown RasGRF2 had no influence on the expression of EMT-related genes, such as β-catenin and Vimentin.
Previous studies indicated that MMP9 can be regulated by several cancer-related signaling pathwaysCitation15-Citation18, including PI 3-kinase, NF-κB, and the MAP kinase pathways. To determine which of these pathways might be responsible in mediating the effect of RasGRF2 on MMP9 expression in CRC, we investigated how RasGRF2 knockdown affected the activation states of these pathways. As shown in , downregulation of RasGRF2 in CRC cells resulted in decreased phosphorylation of Akt, NF-κB, and several NF-κB regulators, suggesting a suppression of the PI 3-kinase pathway and the NF-κB pathway. Besides, several studies have demonstrated that focal adhesion kinase (FAK) expression and phosphorylation are elevated in invasive human cancersCitation19,Citation20 and cancer cell linesCitation21,Citation22. Meanwhile, PI3K/Akt is one of the pathways that is downstream of FAK-Src complexCitation23. Therefore, we determined the downregulation effect of RasGRF2 on the Fak and Src. Knockdown of RasGRF2 reduced the phosphorylation of FAK at Tyr397 and phosphorylation of Src at Tyr416, but not the phosphorylation of the Src negative regulatory residue Tyr527. In contrast, RasGRF2 knockdown resulted in hyperphosphorylation of Erk1/2, indicating the activation of the MAP kinase pathway. It has been shown that NF-κB is an important transcription factor that regulates genes involved in cancer cell migration and invasionCitation24,Citation25, and its phosphorylation is regulated by many signaling pathways, including Akt pathway. The concurrent decreases in AKT and NF-κB phosphorylation are consistent with this possibility. The results of the present study demonstrated that RasGRF2 knockdown down regulates MMP9 expression through the Src/PI 3-kinase and the NF-κB pathways.
Discussion
It was reported that human RasGRF2 is expressed in a variety of tissuesCitation26 and several reports have shown decreased expression of RasGRF2 in cancer cell linesCitation8-Citation10. Although RasGRF2 is found to be implicated in some kinds of cancer, the biological functions of RasGRF2 have not been fully elucidated. In the current study, our results indicate increased expression of RasGRF2 in CRC tissues as well as in colorectal cells. We found that knockdown of RasGRF2 results in inhibition of migration and invasion in vitro and in vivo, but did not affect the proliferation and apoptosis. Furthermore, we demonstrate that the pro-metastatic activity of RasGRF2 is most likely attributed to RasGRF2-mediated activation of Src/PI 3-kinase pathway and the NF-κB pathway, which could up-regulates the expression of MMP9 in CRC cells.
The discrepancy of RasGRF2 expression between our results and the previous study is probably due to unelucidated mechanism. Several studies have reported aberrant methylation of RasGRF2 in different cancer, such as pancreatic cancersCitation27, non-small cell lung cancerCitation8 and CRCCitation10, which indicated that RasGRF2 expression may be suppressed in these tissues, based on an wide recognition that DNA methylation prevents gene transcriptionCitation28. And this suggested further study of DNA methylation of RasGRF2 should be performed in the future.
Accumulating evidences suggests that RasGRF2 plays a critical role in movement, invasion and lung colonization of many kinds of cancer cellsCitation5,Citation7,Citation29,Citation30. RasGRF2 has been shown to suppress rounded movement of melanoma cellsCitation7, whether RasGRF2 has a role in metastasis of CRC remains unclear. Here we show that RasGRF2 is associated with metastatic potential of CRC cells. Knockdown of endogenous RasGRF2 in CRC cells suppressed cell migration and invasion. Moreover, the in vivo metastatic assays showed that downregulation of RasGRF2 expression significantly decreased lung metastasis. Above of all, our data shows that RasGRF2 promotes CRC invasion and metastasis. An increase in migration and invasion ability is an important character of EMT, which is essential for tumor cells to disseminate to adjacent or distant tissues. β-catenin and vimentin are two key EMT-related markersCitation31. However, in our study, reduced RasGRF2 expression did not affect these two proteins in CRC cells, implying that RasGRF2-mediated CRC cell invasion and metastasis is EMT independent.
The expression level of MMPs is implicated to be correlated with the metastatic ability of cancer cellsCitation13. Particularly, increased expression of MMP9 is detected in CRC and it is associated with tumor metastasisCitation32. An earlier study reported knockdown of RasGRF1 or RasGRF2 reduced the expression of MMP3 in fibroblastsCitation33, which revealed that RasGRF2 may affect the expression of MMPs. Similarly, in the current study, we revealed a positive correlation between RasGRF2 and MMP9 expression in colorectal cancer by RasGRF2 knockdown. A previous study suggested that MMP9 was regulated by activating the PI 3-kinase/Akt/NF-κB signaling pathway in Hepatocellular carcinoma cellsCitation16. We found in our study that RasGRF2 silencing suppresses MMP9 expression through the PI 3-kinase and the NF-κB pathways, which may result in attenuated invasion and metastasis in CRC cells. Besides, FAK/Src signaling is known for its important effects on cell migrationCitation34 as well as enhanced MMP9 expressionCitation35. Bolos, et al. noted that FAK interacted with Src to activate PI3K followed by Akt to promote tumorigenicity and metastasisCitation36. In accordance, our data showed that knockdown of RasGRF2 inhibited activation of the Fak/Src signaling pathway.
It is generally believed that the Ras family of GTPases is involved in cell proliferation and apoptosis. And there are two major pathways in oncogenic Ras-driven proliferation: MAPK (Raf/MEK/ERK) and PI3K/Akt/mTOR. While we observed that knockdown of RasGRF2 did not affect proliferation and apoptosis but .results in the upregulation of phospho-Erk level. We suspect that this activation of Erk may be the reason knockdown of RasGRF2 fail to affect cell proliferation and apoptosis. The different characteristics that Erk and Akt exhibit in this study may be due to the cross-inhibition between Ras-ERK and PI3K-Akt pathwaysCitation37. Besides, An earlier study reported that RasGRF2 mediates activation of K-Ras, H-Ras, and to a lesser extent, N-RasCitation33. K-Ras is a central player in intracellular signaling and it may be activated by the EGF receptor or possibly other receptor tyrosine kinases. Mutations of K-Ras result in the loss of its GTPase activity and a constitutive activation of K-Ras signallingCitation38. The cell lines in this paper are all Kras mutated. Therefore, we speculate that Kras mutations are involved in the cell proliferation.
In conclusion, our study shows that RasGRF2 is significantly upregulated in CRC and high RasGRF2 expression is associated with CRC invasion and metastasis. Our results also suggest that RasGRF2 promotes CRC cell invasion by up-regulating MMP9 through activating Src/PI 3-kinase pathway and the NF-κB pathway. Yet, correlations between the incidence of RasGRF2 and other tumour characteristics, such as grading, tumour size and survival rate need to be analyzed by collecting more colon cancer patient tissues. More importantly, the role of RasGRF2 plays in colon cancer needs to be investigated further in future studies.
Materials and methods
Cell lines and culture conditions
The cell lines were purchased from Shanghai Institutes for Biological Sciences (Shanghai, China). FHC, SW480 and LoVo cells were cultured in RPMI 1640 medium (Hyclone, USA), HCT116 cells were cultured in McCoy’s 5A media (Hyclone, USA), while LS174T cells were cultured in Dulbecco’s modified Eagle’s medium (Hyclone, USA). All the medium was supplemented with 10% FBS (Hyclone, USA) and 100 U/ml penicillin-streptomycin mixture (Solarbio, Beijing). The cells were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Lentiviral-mediated RNA interference
A lentiviral vector encoding a short hairpin RNA (shRNA) targeted against RasGRF2 and a negative control lentiviral vector were purchased from Genechem (Shanghai, China). The lentivirus was transfected to SW480, HCT116 and LS174T cells. The sequences of the three shRNAs targeting RasGRF2 are as follows:
RasGRF2-RNAi-1: 5ʹ-TCTAATGGACAAACTTCAA-3ʹ;
RasGRF2-RNAi-2: 5ʹ-AGACATCAAGAAGATTAAA-3ʹ;
RasGRF2-RNAi-3: 5ʹ-TCCTTTCTCCACCAAAGAA-3ʹ.
Stably transfected cell lines were selected using puromycin (4 μg/ml) for 7d.
Human samples
Paired tissues of colorectal cancer and their matched noncancerous samples were obtained from First Affiliated Hospital of Shanxi Medical University and Shanxi Provincial Cancer Hospital. Informed consent was obtained before surgery from each patient. These patients did not receive chemotherapy or radiotherapy before admission. All samples were immediately frozen and stored in liquid nitrogen following surgery.
RNA extraction and real-time PCR
Total RNA was extracted from cells or tissues by TRIzol reagent (Invitrogen, USA) according to the manufacturer’s information. Single-stranded cDNAs were synthesized with a PrimeScriptTM RT regent Kit with gDNA Eraser (Takara, Dalian). qPCRs were carried out with SYBR Green PCR Master Mix (Transgen, Beijing) and StepOneTM & StepOnePlusTM Real-Time PCR System (Applied biosystems, USA). The housekeeping gene β -Actin was applied as an internal control. The following primers were used:
RasGRF2, forward 5ʹ-AACCAAAGAACGAATGCGACC-3ʹ
and reverse 5ʹ-TGGTGAACGTACTCTGACTCTG-3ʹ;
β -Actin, forward 5ʹ-CTCACCATGGATGATGATATCGC-3ʹ
and reverse 5ʹ-AGGAATCCTTCTGACCCATGC-3ʹ.
Western blot analysis
Total proteins were isolated from cell or tissues using a lysis buffer (Beyotime, Shanghai). Proteins were separated by 10% SDS-PAGE and then transferred to nitrocellulose membrane. The nitrocellulose membrane was blocked with milk and incubated with primary antibodies overnight. The detailed information about the antibodies is shown in S1 Table. The immune complexes were finally visualized using the ECL system (Thermo Fisher Scientific, USA).
Tissue microarray
CRC tissue microarray (TMA) was generated from formalin-fixed, paraffin-embedded tissues of 96 patients collected from First Affiliated Hospital of Shanxi Medical University and Shanxi Provincial Cancer Hospital to detect RasGRF2 protein expression through immunohistochemical analysis. The diagnoses were conducted by at least two pathologists who were blind to the patients’ information.
MTT
Cells were seeded at a density of 1 × 103 per well in a 96-well plate and allowed to grow for 1–5 days, respectively. After the incubation with 5mg/mL MTT in PBS for 4 h, cells were treated with 150 µl of dimethylsulphoxide to dissolve the formazan crystals. The optical density was determined using a microplate reader at the wavelength of 490 nm. The readings were normalized by abstracting the value of a blank control.
Flow cytometry analysis of cell apoptosis
Cells were stained with Annexin V-APC/7-AAD Apoptosis Detection Kit (KeyGEN BioTECH, Jiangsu) following the manufacturer’s instruction. Data were obtained using a BD Biosciences FACS Calibur flow cytometer and analyzed using FlowJo software.
Wound healing assays
Wound healing analysis was performed to measure cell migration. Cells were plated in the 6 well plates and incubated until they reached nearly complete confluence. Subsequently, a scratch was made to the cell layer with the pipette tip and this point was considered as 0 h. Then, cells were cultured in 5% FBS medium at 5% CO2 and 37°C for an additional 48 hours. The wound was detected by photomicrographs.
Cell invasion assays
Cells were seeded onto a matrix gel made with serum-free media in a transwell migration chamber (Corning, USA) with 8 µm pore size membrane on the bottom. The chamber was inserted in a well of a 24-well plate containing the corresponding media with 10% (v/v) FBS. After 48 hours, the invaded cells in the lower membrane were stained with crystal violet and counted under a microscope (Olympus, Japan).
Experimental metastasis model
BALB/c nude male mice (4–5 weeks old) were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Hunan, China). The animals were randomly divided into two groups. HCT116 cells with stable transfection of shRNA or control were harvested and washed with PBS and re-suspended at 2 × 107 cells/ml in a serum-free medium. Suspended cells were injected into the tail veins of mice (0.2 ml/mice). The weight of mice was recorded for 7 weeks followed by sacrifice at the end of observation. The lungs were removed and lung metastasis was measured by hematoxylin/eosin staining. All experiments were performed according to the Animal Care and Use Committee guidelines of Shanxi Medical University.
Histology and immunohistochemistry
Sections from formalin-fixed, paraffin-embedded tissues (4μm) were immunostained with NovoLink polymer detection system (Novocastra Laboratories) according to manufacturer’s instructions after antigen retrieval with 10 mM citrate buffer (pH 6.0) for 5 min at 120°C in autoclave. The sections were incubated with rabbit polyclonal antibodies to RasGRF2 (Abcam, ab121577; dilution 1:200) or rabbit polyclonal antibodies against MMP9 (Cell Signaling, no. 13667; dilution 1:300) at 37°C for an hour. Hematoxylin/eosin-stained lung sections of metastatic lesions were detected as well.
Disclosure/Conflicts of interest
No potential conflict of interest was reported by the authors.
Supplemental Material
Download Zip (89.5 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–117. doi:10.3322/caac.21220.
- Huang S, Ren X, Wang L, Zhang L, Wu X. Lung-cancer chemoprevention by induction of synthetic lethality in mutant KRAS premalignant cells in vitro and in vivo. Cancer Prev Res (Phila). 2011;4(5):666–673. doi:10.1158/1940-6207.CAPR-10-0235.
- Fam NP, Fan WT, Wang Z, Zhang LJ, Chen H, Moran MF. Cloning and characterization of Ras-GRF2, a novel guanine nucleotide exchange factor for Ras. Mol Cell Biol. 1997;17(3):1396–1406.
- Fernandez-Medarde A, Santos E. The RasGrf family of mammalian guanine nucleotide exchange factors. Biochim Biophys Acta. 2011;1815(2):170–188. doi:10.1016/j.bbcan.2010.11.001.
- Ma X, Espana-Serrano L, Kim WJ, Thayele Purayil H, Nie Z, Daaka Y. betaArrestin1 regulates the guanine nucleotide exchange factor RasGRF2 expression and the small GTPase Rac-mediated formation of membrane protrusion and cell motility. J Biol Chem. 2014;289(19):13638–13650. doi:10.1074/jbc.M113.511360.
- Jimeno D, Gomez C, Calzada N, de la Villa P, Lillo C, Santos E. RASGRF2 controls nuclear migration in postnatal retinal cone photoreceptors. J Cell Sci. 2016;129(4):729–742. doi:10.1242/jcs.180919.
- Calvo F, Sanz-Moreno V, Agudo-Ibanez L, Wallberg F, Sahai E, Marshall CJ, Crespo P. RasGRF suppresses Cdc42-mediated tumour cell movement, cytoskeletal dynamics and transformation. Nat Cell Biol. 2011;13(7):819–826. doi:10.1038/ncb2271.
- Chen H, Suzuki M, Nakamura Y, Ohira M, Ando S, Iida T, Nakajima T, Nakagawara A, Kimura H. Aberrant methylation of RASGRF2 and RASSF1A in human non-small cell lung cancer. Oncol Rep. 2006;15(5):1281–1285.
- Qiu C, Yu M, Shan L, Snyderwine EG. Allelic imbalance and altered expression of genes in chromosome 2q11-2q16 from rat mammary gland carcinomas induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Oncogene. 2003;22(8):1253–1260. doi:10.1038/sj.onc.1206233.
- Jacinto FV, Ballestar E, Ropero S, Esteller M. Discovery of epigenetically silenced genes by methylated DNA immunoprecipitation in colon cancer cells. Cancer Res. 2007;67(24):11481–11486. doi:10.1158/0008-5472.CAN-07-2687.
- Hagihara A, Miyamoto K, Furuta J, Hiraoka N, Wakazono K, Seki S, Fukushima S, Tsao MS, Sugimura T, Ushijima T. Identification of 27 5ʹ CpG islands aberrantly methylated and 13 genes silenced in human pancreatic cancers. Oncogene. 2004;23(53):8705–8710. doi:10.1038/sj.onc.1207783.
- Shimaoka H, Takeno S, Maki K, Sasaki T, Hasegawa S, Yamashita Y. A cytokine signal inhibitor for rheumatoid arthritis enhances cancer metastasis via depletion of NK cells in an experimental lung metastasis mouse model of colon cancer. Oncol Lett. 2017;14(3):3019–3027. doi:10.3892/ol.2017.6473.
- Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89(17):1260–1270.
- van Kempen LC, Coussens LM. MMP9 potentiates pulmonary metastasis formation. Cancer Cell. 2002;2(4):251–252.
- Byun HJ, Hong IK, Kim E, Jin YJ, Jeoung DI, Hahn JH, Kim YM, Park SH, Lee H. A splice variant of CD99 increases motility and MMP-9 expression of human breast cancer cells through the AKT-, ERK-, and JNK-dependent AP-1 activation signaling pathways. J Biol Chem. 2006;281(46):34833–34847. doi:10.1074/jbc.M605483200.
- Cheng JC, Chou CH, Kuo ML, Hsieh CY. Radiation-enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction pathway. Oncogene. 2006;25(53):7009–7018. doi:10.1038/sj.onc.1209706.
- Lee CW, Lin CC, Lin WN, Liang KC, Luo SF, Wu CB, Wang SW, Yang CM. TNF-alpha induces MMP-9 expression via activation of Src/EGFR, PDGFR/PI3K/Akt cascade and promotion of NF-kappaB/p300 binding in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292(3):L799–812. doi:10.1152/ajplung.00311.2006.
- Luo Y, Liang F, Zhang ZY. PRL1 promotes cell migration and invasion by increasing MMP2 and MMP9 expression through Src and ERK1/2 pathways. Biochemistry. 2009;48(8):1838–1846. doi:10.1021/bi8020789.
- Kanteti R, Mirzapoiazova T, Riehm JJ, Dhanasingh I, Mambetsariev B, Wang J, Kulkarni P, Kaushik G, Seshacharyulu P, Ponnusamy MP, et al. Focal adhesion kinase a potential therapeutic target for pancreatic cancer and malignant pleural mesothelioma. Cancer Biol Ther. 2018;19(4):316–327. doi:10.1080/15384047.2017.1416937.
- Alexopoulou AN, Ho-Yen CM, Papalazarou V, Elia G, Jones JL, Hodivala-Dilke K. Tumour-associated endothelial-FAK correlated with molecular sub-type and prognostic factors in invasive breast cancer. BMC Cancer. 2014;14:.237. doi:10.1186/1471-2407-14-237.
- Hauck CR, Sieg DJ, Hsia DA, Loftus JC, Gaarde WA, Monia BP, Schlaepfer DD. Inhibition of focal adhesion kinase expression or activity disrupts epidermal growth factor-stimulated signaling promoting the migration of invasive human carcinoma cells. Cancer Res. 2001;61(19):7079–7090.
- Schneider GB, Kurago Z, Zaharias R, Gruman LM, Schaller MD, Hendrix MJ. Elevated focal adhesion kinase expression facilitates oral tumor cell invasion. Cancer. 2002;95(12):2508–2515. doi:10.1002/cncr.10992.
- Lin KL, Chien CM, Hsieh CY, Tsai PC, Chang LS, Lin SR. Antimetastatic potential of cardiotoxin III involves inactivation of PI3K/Akt and p38 MAPK signaling pathways in human breast cancer MDA-MB-231 cells. Life Sci. 2012;90(1–2):54–65. doi:10.1016/j.lfs.2011.10.020.
- Ko BS, Chang TC, Chen CH, Liu CC, Kuo CC, Hsu C, Shen YC, Shen TL, Golubovskaya VM, Chang CC, et al. Bortezomib suppresses focal adhesion kinase expression via interrupting nuclear factor-kappa B. Life Sci. 2010; 86(5–6): 199–206.doi:10.1016/j.lfs.2009.12.003.
- Golubovskaya V, Kaur A, Cance W. Cloning and characterization of the promoter region of human focal adhesion kinase gene: nuclear factor kappa B and p53 binding sites. Biochim Biophys Acta. 2004;1678(2–3):111–125. doi:10.1016/j.bbaexp.2004.03.002.
- Anborgh PH, Qian X, Papageorge AG, Vass WC, DeClue JE, Lowy DR. Ras-specific exchange factor GRF: oligomerization through its Dbl homology domain and calcium-dependent activation of Raf. Mol Cell Biol. 1999;19(7):4611–4622.
- Furuta J, Nobeyama Y, Umebayashi Y, Otsuka F, Kikuchi K, Ushijima T. Silencing of Peroxiredoxin 2 and aberrant methylation of 33 CpG islands in putative promoter regions in human malignant melanomas. Cancer Res. 2006;66(12):6080–6086. doi:10.1158/0008-5472.CAN-06-0157.
- Zlotorynski E. Epigenetics: DNA methylation prevents intragenic transcription. Nat Rev Mol Cell Biol. 2017;18(4):212–213. doi:10.1038/nrm.2017.25.
- Hernandez-Garcia R, Iruela-Arispe ML, Reyes-Cruz G, Vazquez-Prado J. Endothelial RhoGEFs: A systematic analysis of their expression profiles in VEGF-stimulated and tumor endothelial cells. Vascul Pharmacol. 2015;74:.60–72. doi:10.1016/j.vph.2015.10.003.
- Ruiz S, Santos E, Bustelo XR. The use of knockout mice reveals a synergistic role of the Vav1 and Rasgrf2 gene deficiencies in lymphomagenesis and metastasis. PLoS One. 2009;4(12):e8229. doi:10.1371/journal.pone.0008229.
- Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119(6):1429–1437. doi:10.1172/JCI36183.
- Alkiewicz K, Koziel P, Bednarczyk M, Blazelonis A, Mazurek U, Muc-Wierzgon M. Expression of Migration-Related Genes in Human Colorectal Cancer and Activity of a Disintegrin and Metalloproteinase 17. Biomed Res Int. 2016;2016:.8208904. doi:10.1155/2016/8208904.
- Wang Q, Siminovitch KA, Downey GP, McCulloch CA. Ras-guanine-nucleotide-releasing factors 1 and 2 interact with PLCgamma at focal adhesions to enable IL-1-induced Ca(2+) signalling, ERK activation and MMP-3 expression. Biochem J. 2013;449(3):771–782. doi:10.1042/BJ20121170.
- Hamaguchi M, Yamagata S, Thant AA, Xiao H, Iwata H, Mazaki T, Hanafusa H. Augmentation of metalloproteinase (gelatinase) activity secreted from Rous sarcoma virus-infected cells correlates with transforming activity of src. Oncogene. 1995;10(6):1037–1043.
- Bolos V, Gasent JM, Lopez-Tarruella S, Grande E. The dual kinase complex FAK-Src as a promising therapeutic target in cancer. Onco Targets Ther. 2010;3:.83–97. doi:10.2147/ott.s6909.
- Hsia DA, Mitra SK, Hauck CR, Streblow DN, Nelson JA, Ilic D, Huang S, Li E, Nemerow GR, Leng J, et al. Differential regulation of cell motility and invasion by FAK. J Cell Biol. 2003;160(5):753–767. doi:10.1083/jcb.200212114.
- Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. 2011;36(6):320–328. doi:10.1371/journal.pone.0008229.
- Ahmed D, Eide PW, Eilertsen IA, Danielsen SA, Eknaes M, Hektoen M, Lind GE, Lothe RA. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis. 2013;2:.e71. doi:10.1038/oncsis.2013.35.
