ABSTRACT
Clinical studies provide strong evidence that obesity and associated adipose tissue (AT) inflammation are risk factors for breast cancer (BrCA); however, mechanistic knowledge of the interaction of obesity, BrCA, and menopausal status has proven to be not only lacking, but contradictory. Obesity-induced inflammation and elevated biosynthesis of estrogens, through aromatase-mediated metabolism of precursors, have been linked with hormone receptor positive (HP) postmenopausal BrCA but not previously associated with premenopausal BrCA risk. Thus, further delineation of the interaction of obesity, inflammation, and aromatase is required for the development of therapeutic treatment options. The purpose of this study was to examine the effect of high fat diet (HFD)-induced inflammation on tumorigenesis in a model of pre and postmenopausal HP BrCA. Female PyMT/MMTV ovary intact and ovariectomized mice were fed low and HFD diets to examine the role of obesity-induced inflammation and hormone production in the development of HP BrCA. Tumor statistics for number, volume, weight, histopathology scoring and gene expression of macrophage and inflammatory mediators were measured in the AT and mammary gland at sacrifice. HFD feedings of ovary intact mice resulted in increased adiposity and tumorigenesis, indicated by increased primary tumor volume, multiplicity, tumor burden, and increased tumor progression represented by histopathological scoring. HFD-induced obesity significantly upregulated aromatase and macrophage marker expression in the AT (F4/80 and CD11c) and mammary gland (Mertk) in a premenopausal model of BrCA. Conversely, HFD feedings had no significant effect on tumorigenesis in a postmenopausal model of BrCA despite large increases in adiposity in ovariectomized mice; however, limitations within the model may have precluded any significant findings. This data suggests that obesity-induced increases in inflammation and hormone production, via aromatase expression, is associated with increases in tumorigenesis in a model of premenopausal HP BrCA in the PyMT/MMTV strain.
Introduction
The etiopathogenesis of BrCA is complex given that it can involve the interaction of genetic and environmental factors. Epidemiological studies provide strong evidence to suggest that obesity is a risk factor for BrCA. However, inconsistencies in the literature imply that the complexity of this relationship remains inadequately understood. In the case of younger women, the link between body mass and BrCA risk is controversial. Some clinical studies report a null or inverse association between obesity and BrCA riskCitation1–Citation4, a few epidemiological studies suggest reduced overall risk of premenopausal BrCA associated with obesityCitation5, while other investigators report that weight gain and central obesity increase the risk of premenopausal BrCACitation6–Citation10. In postmenopausal women the relationship is clearer; the majority of evidence supports a direct link between obesity and the risk for certain clinical subtypes of BrCACitation11–Citation14. For instance, cohort and case control studies indicate that obesity is an independent risk factor for postmenopausal estrogen receptor positive (ER+) and progesterone receptor positive (PR+) BrCACitation13–Citation18 and experimental studies in mice substantiate these claimsCitation14,Citation19–Citation22. Women with the greatest increase in weight have higher risk at older ages and this association appears to be stronger for hormone positive (HP) tumorsCitation23. Given that two out of three women in the United States are overweight or obese, an understanding of the mechanisms that link obesity to BrCA, and the connection with menopausal status, is of critical public health importance.
A number of mechanisms are likely to link obesity to BrCA. However, inflammation is arguably at the forefront. Chronic inflammation is recognized as an important mediator in the clinical and biochemical complications associated with obesity and also breast tumor biology and causation. Inflammation in the adipose tissue (AT), with infiltration by macrophages, is a well-established characteristic of obesity and its presence in the breast creates local conditions that favor breast epithelial cell transformation, cancer cell proliferation and invasion as well as tumor-related neovascularization, that contribute to poor prognosis. Further, obesity-mediated inflammation has been reported to drive estrogen synthesis, which increases the risk of HP BrCA; previous studies have linked the upregulation of AT pro-inflammatory cytokines with the expression of the estrogen synthase cytochrome P450 (aromatase) in murine studiesCitation24 and in both pre and postmenopausal human BrCACitation25,Citation26.
The purpose of this study was to examine mammary tumorigenesis in response to HFD feedings in the PyMT transgenic murine model of tumorigenesis. The PyMT/MMTV is an experimental model that presents morphological similarities with human BrCA. In this model, the expression of the oncoprotein, polyoma virus middle T antigen, is under the control of the mouse mammary tumor virus promoter and is therefore restricted to the mammary epitheliumCitation27. In addition to the tumor progression similarities, the expression of biomarkers in PyMT-induced tumors, to include the loss of ER+and PR+receptors and overexpression of ErbB2/Neu/HER2 as the cancer progresses to malignancy, is also consistent with those associated with poor outcome in human BrCA. While the majority of studies investigating the relationship between HFD-induced obesity and BrCA tumorigenesis have been performed in the FVB/N background, a strain traditionally shown to be HFD-induced obesity resistant, we utilized the C57BL/6 background, a strain that is more susceptible to HFD-induced obesity. Further, by utilizing both intact and ovariectomized mice, we were able to evaluate the effects of HFD feedings in a model of pre and postmenopausal BrCA. Given that inflammation is a likely link between obesity and BrCA, we further examined changes in inflammatory-related parameters in association with the tumorigenic outcomes.
Results
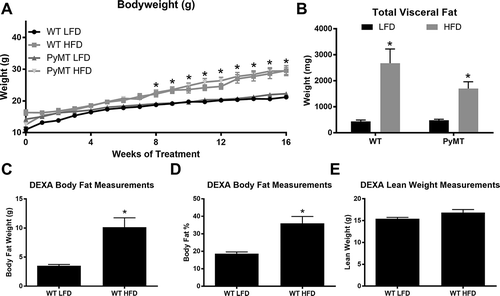
PyMT and wild-type mice fed a HFD have increased body weight gain and larger total visceral fat pad weight than mice fed a LFD
The HFD treatment significantly increased body weight for both the WT and PyMT groups beginning at 8 weeks of diet treatment and remained elevated through the 16-week treatment period (, P < 0.05). A main effect of diet also was observed for the total absolute weight of the visceral fat pads, with the HFD-fed mice having significantly greater total weight (, P < 0.05). Although the PyMT mice had less visceral fat than the WT group there was no statistically significant difference between the HFD-treated groups for bodyweight () and total visceral fat pad weight (). Body composition analysis for the WT mice at week 16 of treatment indicated that the mice fed the HFD had significantly greater body fat weight in grams and body fat percentage (), D, P < 0.05) with no difference for lean mass in grams between diet treatment groups ().
Figure 1. Body weight characteristics for pre-menopausal experiment. WT and PyMT mice were fed either a LFD or HFD for 16 weeks. Body weight was monitored weekly for all groups. Body composition for WT mice was assessed at the conclusion of the study (20 weeks of age); PyMT groups were not analyzed for body composition as the tumors can alter lean mass calculations. Visceral fat pads were removed and weighed at the end of the 16 week diet treatment. a. Body weight in grams. b. Total absolute weight of visceral fat pads. c. Body composition analysis of WT mice, Body fat in grams. d. Body fat percentage. E. Lean weight in grams. *main effect of diet. Data are represented as ±SEM, WT LFD, n = 8 WT HFD, n = 8 PyMT LFD, n = 12 PyMT HFD, n = 15.
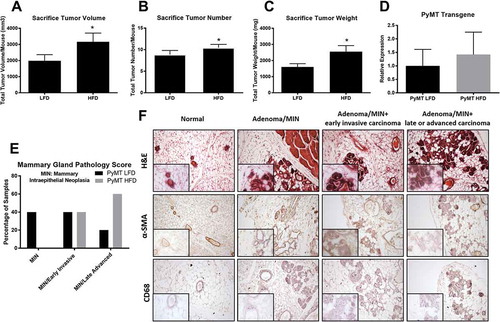
Chronic consumption of a HFD diet increases tumorigenesis and histopathological tumor stage progression in a premenopausal model
Chronic consumption of a HFD significantly increased primary mammary tumor growth and multiplicity with the mean tumor volume/mouse ()) and mean tumor number/mouse () determined at necropsy to be 1989 ± 380 mm3 and 8.85 ± 0.96 for LFD, and 3163 ± 542 mm3 and 10.47 ± 0.76 for HFD mice, respectively (P < 0.05). Similarly, HFD consumption significantly increased tumor burden (i.e. weight) () with the mean/mouse to be 1599.7 ± 206.9mg for LFD and 2554.0 ± 375.4mg for HFD (P < 0.05). Tumorigenesis in this model is classified into 4 distinct stages. Hyperplasia, the earliest change in the mammary gland, is characterized by clusters of densely packed lobules formed on the duct called the hyperplastic lesion. This change occurs around 4–6 weeks of age in this model and considering the age of the mice at sacrifice, it is understandable why we reported no samples in this early stage (). The next progressive stage is MIN (mammary intraepithelial neoplasia)/adenoma and describes a more florid epithelial proliferation still confined to the basement membrane of the duct, with minimal cytological atypia and no evidence of invasion or metastasis. In the current investigation, 40% of the samples classified in the low grade MIN/adenoma stage in the LFD-fed mice while zero mice from the HFD treated animals scored into this grade (). Progressively more advanced and considered the initial stage of malignant transition is the Early Carcinoma classification characterized by great cytological atypia and the identification of early stromal invasion. The tumor cells now appear pleomorphic, showing moderate variation in nuclear morphology, size and shape and the majority of ducts are still morphologically normal, except for focal areas in which there is mild ductal epithelial hyperplasia with a small increase in the number of cell layers. Further, we observe significant dysplasia as indicated by altered alpha-smooth muscle actin (α-SMA) expression in the mammary ducts and infiltration of CD68+immune cells compared to healthy controls (). We report no difference between the percentage of animals from each group that scored into this Early Invasive Carcinogenesis (40% for each group) stage (). Late Carcinoma is classified as poorly differentiated invasive ductal carcinoma. Tissues are composed of solid sheets of epithelial cells with little to no remaining acinar structures visible. The malignant cells in the tumor have marked variation in cellular and nuclear size and shape with vesicular nuclei and prominent nucleoli. We report that 20% of the LFD-treated animals progressed to Late Carcinoma; however, 60% of the HFD treated mice classified into this advanced invasive carcinoma stage (). ()) illustrates a representative H&E, α-SMA, and CD68+stained sample from the progressive grades of dysplasia characterized in this study. Given that tumorigenesis in this murine PyMT model is initially driven by the expression of the polyoma virus middle T oncoprotein, we wanted to establish that the expression of the transgene itself is not affected by diet manipulation, thus resulting in increased tumorigenesis due to differences in transgene expression. In this current experiment, there was no statistically significant effect of diet treatment on expression levels of the transgene ().
Figure 2. Sacrifice tumor and histopathology scoring for pre-menopausal experiment. WT and PyMT mice were fed either a LFD or HFD for 16 weeks. Following 16 weeks of diet feedings mice were euthanized and tumors were removed, measured, counted, and weighed. To confirm that diet did not influence expression of the transgene we measured mRNA expression of the PyMT transgene in the mammary gland. Mammary gland histopathology scoring was performed following H&E staining for LFD and HFD treated groups. a. Sacrifice tumor volume per mouse. b. Sacrifice tumor number per mouse. c. Sacrifice tumor burden (weight) per mouse. d. mRNA expression of the PyMT transgene in the mammary gland. e. Mammary gland histopathology scoring for both treatment groups. f. Representative images from grade of dysplasia used to identify each treatment group. *P < 0.05. Data are represented as ±SEM, PyMT LFD, n = 12 PyMT HFD, n = 15.
HFD intake leads to expansion of gonadal fat pad weight and increases aromatase expression and macrophage infiltration into the AT regardless of genotype
A significant main effect of diet was observed in the gonadal fat pad weight of the HFD-treated groups (, P < 0.05); although the PyMT mice had less gonadal fat than the WT group there was no statistically significant difference between the HFD-treated groups. Estrogen synthase (aromatase) mRNA expression was significantly elevated in the gonadal AT of the HFD-fed PyMT mice compared to the LFD-fed PyMT mice (), P < 0.05). Similarly, a significant main effect of diet was observed for macrophage markers F4/80 and CD11c expression in the AT (), P < 0.05).
Figure 3. Markers of inflammation in the gonadal fat and mammary gland in pre-menopausal experiment. WT and PyMT mice were fed either a LFD or HFD for 16 weeks. Gonadal fat pads were removed, weighed and analyzed for aromatase expression and macrophage markers using RT-qPCR. Mammary glands were removed and analyzed for macrophage markers, inflammation and proliferation mediators, and markers of neovascularization using RT-qPCR. a. Total gonadal fat weights for each treatment group. b. Aromatase mRNA gene expression for cancer groups in the gonadal fat. c. mRNA expression of macrophage marker F4/80 and CD11c in gonadal fat. d. mRNA expression of macrophage marker F4/80, CD11c, Mertk in mammary gland. e. Markers of inflammation and proliferation in the mammary gland to include MCP-1, IL-6, TNFα, Ki67. f. Markers of neovascularization in the mammary gland to include Vegfα, MMP2/9. *main effect of diet, #main effect of genotype. Data are represented as ±SEM, WT LFD, n = 8 WT HFD, n = 8 PyMT LFD, n = 12 PyMT HFD, n = 15.
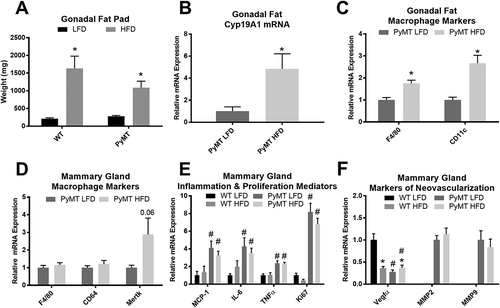
Tumorigenesis increases inflammatory and proliferation markers in the mammary gland
There was no difference in the mRNA expression of macrophage markers (F4/80 and CD64) between the LFD and HFD cancer groups in the mammary gland of PyMT mice. However, HFD-feedings resulted in an upward trend in the expression of the macrophage marker Mer tyrosine kinase (Mertk) in the mammary gland (, p = 0.06). A significant main effect of genotype was observed in the mRNA expression of inflammatory cytokine and chemokine markers (IL-6, TNFα, and MCP-1), and the proliferation marker Ki67 of the mammary gland (, P < 0.05) but there was no effect of diet. A main effect of both diet and genotype was observed for the angiogenic marker Vegfα; albeit, surprisingly this was indicative of a decrease in Vegfα with HFD and cancer. No significant difference in the gene expression of the matrix metalloproteinases (MMP2 and MMP9) was observed between cancer groups in the mammary gland (P < 0.05) ()).
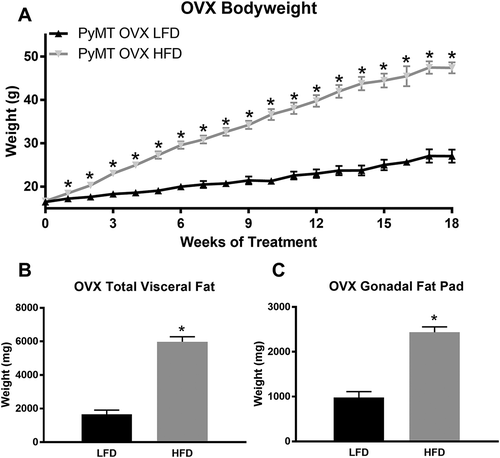
Long-term HFD feedings drastically increases bodyweight gain for ovariectomized mice resulting in significant increases in total visceral fat weights over LFD-fed mice
The HFD treatment significantly (over 2 fold) increased body weight for ovariectomized mice beginning with the first week of diet treatment and remained elevated through the 18-week treatment period (, P < 0.05). A similar main effect of diet was observed for the total absolute weight of the visceral fat pads (3 fold) () and the total gonadal fat pad weight (2 fold) (), with the HFD-fed mice having significantly greater total weight (P < 0.05).
Figure 4. Bodyweight characteristics for post-menopausal experiment. PyMT mice underwent ovariectomy surgery at 5 weeks of age and were allowed one week to recover prior to the initiation of diet treatments. Body weight was measured weekly. Mice were euthanized at 24 weeks of age (18 weeks of diet treatment) and total visceral fat pad weight and total gonadal fat pad weight were determined. a. Body weight in grams. b. Total absolute visceral fat pad weight. c. Total gonadal fat pad weight. *P < 0.05. Data are represented as ±SEM, PyMT OVX LFD, n = 9 PyMT OVX HFD, n = 12.
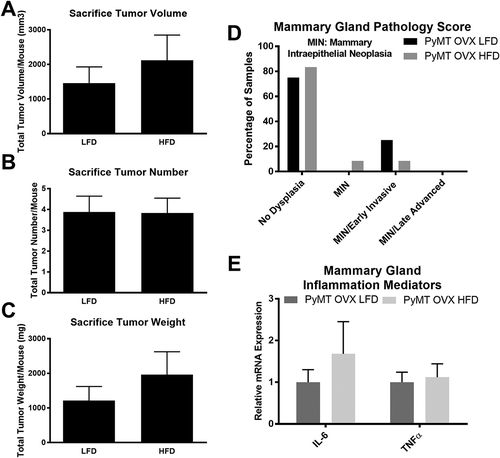
Diet treatments had no effect on tumorigenesis nor inflammatory cytokine expression in the mammary gland of ovariectomized mice
HFD-induced obesity resulted in no significant change in tumor volume (), tumor number (), nor tumor weight () between diet treatment groups in this model. In fact, only a small percentage of mice from each group, 25% and 20% for LFD and HFD-fed mice from each group respectively, reported histopathological mammary gland stage progression into the MIN grade or beyond (). Similarly, HFD treatment had no effect on inflammatory cytokines, IL-6 and TNFα, expression in the mammary gland ().
Figure 5. Sacrifice tumor, histopathology scoring and inflammatory cytokine expression in mammary gland for post-menopausal experiment. PyMT mice underwent ovariectomy surgery at 5 weeks of age and were allowed one week to recover prior to the initiation of diet treatments. Mice were euthanized at 24 weeks of age (18 weeks of diet treatment) and tumors were removed, measured, counted, and weighed. Mammary gland histopathology scoring was performed following H&E staining and markers of inflammation (IL-6 and TNFα) were determined using RT-qPCR. a. Sacrifice tumor volume per mouse. b. Sacrifice tumor number per mouse. c. Sacrifice tumor burden (weight) per mouse. d. Mammary gland histopathology scoring for both treatment groups. e. Markers of inflammation in the mammary gland to include IL-6 and TNFα. *P < 0.05. Data are represented as ±SEM, PyMT OVX LFD, n = 9 PyMT OVX HFD, n = 12.
Discussion
Breast carcinomas continue to be the most commonly occurring cancer in women and the second leading cause of cancer-related deaths. Multiple molecular changes arising as a consequence of increased amounts of body fat are likely to contribute to the rising incidence of BrCA and worse outcomes in the obese population; however, the exact mechanisms driving this relationship have not yet been fully elucidated. We examined mammary tumorigenesis in response to HFD feedings in the PyMT/MMTV transgenic murine model of tumorigenesis, an experimental model that presents morphological similarities with human BrCA. This was done using both ovary-intact and ovariectomized mice in order to determine the influence of menopausal status on this response. Results show that ovary-intact mice fed a HFD not only have large increases in body weight gain and absolute visceral fat pad weight, but also have significant proneoplastic effects on tumorigenesis as demonstrated by increases in primary tumor volume, tumor multiplicity, and total primary tumor burden (i.e. weight). Consistent with the tumor data, histopathology in the mammary gland of ovary-intact mice show an increase in histopathological stage progression with HFD-induced obesity. As expected, HFD treatment of intact mice also led to significant increases in aromatase and macrophage marker (F4/80 and CD11c) mRNA expression in the gonadal AT and an increase in macrophage cell marker Mertk expression in the mammary gland. There also was evidence of an increase in CD68+immune cells with tumor progression. However, we report no significant difference between diet treatment groups for localized inflammatory mediator mRNA expression, suggesting that localized inflammation in the mammary gland may not be responsible for driving the HFD-induced increases in tumorigenesis seen in this model. HFD treatment in ovariectomized PyMT mice results in large increases in adiposity but has no significant effect on tumorigenesis, contrary to what we report in the ovary-intact mice. However, there are several limitations in the study design that may have precluded a positive finding on tumorigenesis. Overall, these data provide evidence that HFD-induced obesity exacerbates tumorigenesis in the HP PyMT/MMTV BrCA model, at least in the intact model (premenopausal).
BrCA is a uniquely heterogeneous disease with different biological and clinical patterns between younger and older women. Thus, menopausal status as a prognostic factor becomes a topic of scrutiny. Differential associations have been identified according to menopausal status as there is clear evidence to support an increased BrCA risk as a function of increasing BMI in postmenopausal womenCitation11–Citation17,Citation28–Citation38; however, studies in premenopausal women are more controversial. Our results are consistent with previous epidemiological studies, such as Chang et al., that have reported that obesity is significantly associated with increasing risk of BrCA in a patient population that were more likely to be premenopausalCitation9. Similarly, Nagrani et al., concluded that central obesity is a risk factor for BrCA irrespective of menopausal statusCitation6. Contradictory to published studies that have determined a positive correlation between obesity and BrCA risk in a postmenopausal model, our data demonstrates no significant effect of HFD-induced obesity on BrCA progression in this murine model. This was a surprising finding considering there is overwhelming data to suggest that obese postmenopausal women are at significantly increased risk of developing HP BrCA. With obesity, ER+and PR+hormone-dependent BrCAs are of importance as estrogen signaling is likely to be a key contributor to obesity-associated BrCA. Importantly, activation of ERα-dependent gene expression as a consequence of adipose inflammation has been observed in murine mammary fat pads and human breast samplesCitation24–Citation26.
The primary site for estrogen biosynthesis in premenopausal women is the ovary but other peripheral sources have increased relative importance in estrogen synthesis. For instance, AT expresses the estrogen synthase cytochrome aromatase p450Citation39,Citation40, which is encoded by the Cyp19 gene, and contributes to estrogen synthesis. In postmenopausal women, obesity and associated inflammation have been shown to contribute to increased BrCA risk as the AT is the primary source of estrogen synthesis due to increased aromatase expressionCitation41–Citation43. In support of this concept, studies link aromatase expression in inflamed AT of obese women with the upregulation of proinflammatory mediators, such as macrophages, as aromatase transcription can be induced by the interaction of inflammatory cytokines with their receptors. Our data is consistent with this concept in that ovary-intact mice fed a HFD have significant increases in aromatase expression in the gonadal AT. We show that increased aromatase expression is associated with increases in certain macrophage markers and increases in not only primary tumor growth but also histopathological grade of the mammary gland in obesity-induced ovary-intact mice. These findings should be interpreted with caution as we were not able to measure protein expression of aromatase given the low concentration of aromatase in tissue. However, we would expect the protein levels to follow gene expression levels. Thus, it is likely that paracrine interactions between macrophages and other cell types operate on an inflammatory axis that results in elevated estrogen biosynthesis and expression; however, further delineation of this axis is required before treatment mechanisms can be established. It is important to note that while we did document an increase in inflammatory cytokines in the mammary gland following tumorigenesis, there was no further increase following HFD treatment. This may be explained by the fact that this analysis was performed on mammary tissue that was largely characterized to either MIN early invasive or MIN late advanced where inflammation may have been maximal in both groups precluding the detection of any differences between dietary treatments. It would have been of interest to examine inflammation at an earlier time-point as differences due to diet treatment may have been evident prior to MIN. Similarly, it would have been of value to measure Vegfα at an earlier time-point given the surprising findings indicative of a decrease in Vegfα with HFD and cancer. It is possible that we may have missed any increase in Vegfa expression that may have occurred at an earlier time-point. Even so, Vegfa protein expression was not measured in the current investigation; therefore, we cannot confirm that protein levels also were decreased with obesity and cancer.
There are several limitations to our study pertaining to the ovariectomized postmenopausal model that warrant cautious interpretations of the findings. We designed this study to replicate a mouse model where we could exclusively look at the influence of AT aromatase expression on mammary tumor initiation and progression independent of endogenous estrogen and progesterone. Therefore, removing the ovaries and developing an obese phenotype prior to tumor initiation was of high importance to our hypothesis. However, tumorigenesis in this model appears to be highly dependent on these endogenous hormones and removing the ovaries at 5 weeks of age likely interfered with tumor initiation in these mice, thus the relatively low tumor number in the ovariectomized versus the ovary intact mice. Another potential limitation is the effect that the removal of the ovaries had on normal mammary gland development. Literature suggests that estrogen is circulating in female mice at 4 weeks of age but progesterone is not bioavailable until 7 weeks of age or even sexual maturation which occurs around 8 weeks of age. Bocchinfuso et al., demonstrated that without functioning estrogen and progesterone, normal mammary gland development does not occurCitation44. Given that we ovariectomized these mice at 5 weeks of age, we cannot establish that decreases in tumor initiation in the ovariectomized mice was due to decreases in bioavailable estrogen or immature mammary gland development. For future studies, it may be beneficial to wait to remove the ovaries until sexual maturation occurs. On the other hand, it should be noted that by this time point this mouse model would have progressed into the MIN histopathological tumor stage; thus, analysis of obesity-induced pathologies would be on tumor progression rather than tumor initiation. Given, the amount of time it takes for increased adiposity to occur in a HFD-induced obesity model, this represents a challenge as this transgenic model produces tumorigenesis in a relatively short time frame. While we were not able to document a significant effect of HFD on tumorigenesis and related inflammatory processes in the ovariectomized postmenopausal model, we believe that this study contributes to the current literature and will help to inform the design of future investigations on the impact of HFD on postmenopausal breast cancer.
In summary, we report that HFD-induced obesity results in increases in mammary tumorigenesis and upregulated aromatase expression in the gonadal AT in the HP PyMT/MMTV murine model of premenopausal BrCA. Although, aromatase expression has been implicated in modulating multiple mechanisms in postmenopausal BrCA, its involvement in tumorigenesis in a premenopausal model has not been established. While it is well known that obesity leads to increased levels of proinflammatory mediators, further research is required to demonstrate the functional link of increased aromatase expression in mammary tumorigenesis. This obesity, inflammation, and aromatase axis provides the basis for developing mechanism-based strategies to reduce the risk of HP BrCA in this growing segment of the population.
Materials & methods
Animals
Male PyMT/MMTV mice on a C57BL/6 background were randomly bred with female wild-type (WT) mice to obtain female mice heterozygous for the PyMT transgene. Two independent experiments were conducted to examine mammary tumorigenesis as it pertains to menopausal status. The first experiment (Exp1) utilized PyMT ovary intact females and female WT littermates were included as a non-disease control. Mice in Exp1 were euthanized at 20 weeks of age (16 weeks of diet treatment). The second experiment (Exp2) included only PyMT ovariectomized females. At 5 weeks of age, mice underwent ovariectomy surgery and were allowed one week to recover prior to the start of diet treatments. Mice in Exp2 were euthanized at 24 weeks of age (18 weeks of diet treatment). All experimental mice were bred and cared for in the animal research facility at the University of South Carolina. They were housed, 3–5/cage, maintained on a 12:12-h light-dark cycle in a low stress environment (22°C, 50% humidity, low noise) and given food and water ad libitum. Principles of laboratory animal care were followed, and the Institutional Animal Care and Usage Committee of the University of South Carolina approved all experiments.
Genotyping protocol
Female mice were used in all experiments and were genotyped for the PyMT transgene using the primer sequences as follows:
PYVT-1 GGAAGCAAGTACTTCACAAGGG,
PYVT-2 GGAAAGTCACTAGGAGCAGGG.
A snip of mouse tail was added to 150ul of DirectPCR tail buffer (Viagen Biotech Inc, Los Angeles, CA) and 2ul of Proteinase K (Viagen Biotech Inc, Los Angeles, CA) and digested at 55°C overnight. The next day samples were incubated at 95°C for an hour then added to a PCR cocktail for amplification. The PCR cocktail contains DNA template, upstream and downstream primers, ddH20, and GoTaq Green Master Mix (Promega Corp, Madison, WI). Samples were run on 2% agarose gel and compared to control samples to determine genotype (520 base pair molecular weight for PyMT/MMTV positive samples).
Ovariectomy surgery
Mice were briefly anesthetized with isoflurane. The dorsal mid-lumbar area was shaved and swabbed with iodine and alcohol. A 2cm dorsal midline skin incision was made halfway between the caudal edge of the ribcage and the base of the tail. The fascia was cleared away using blunt dissection. A single incision of less than 1cm long was made into the muscle wall on both the right and left sides approximately 1cm lateral to the spine. The ovary and the uterine horns located in the fat pad under the dorsal muscle were extracted through the incisions with forceps. Both uterine horns were tied beneath the ovary with a suture (non-absorbable suture 5–0, cat # S-G518R13) and ovaries were removed with single cuts. The uterine horns were placed back into the peritoneal cavity. Muscle incisions were closed with 5–0 absorbable suture (Cat # S-G518R13-U). Wound clips were used to close skin incision. Animals were examined for incision repair or infection for at least 72 hours post-surgery. Wound clips were removed at 7-day post-surgery.
Diets
For Exp1, PyMT mice and WT non-disease control mice were randomly assigned to either a low fat diet (LFD) or a HFD treatment group beginning at 4 weeks of age. The AIN-76A diet (11.5% kcal as fat) was used for the LFD (Bioserv, Frenchtown, NJ)Citation45,Citation46. AIN-76A is a purified, balanced diet that is phytoestrogen free. Dietary phytoestrogens have been shown to influence anxiety-related behaviors, fat deposition, blood insulin, leptin and thyroid levels as well as lipogenesis and lipolysis in adipocytesCitation47. The D12492 diet (60% kcal as fat) was used for the HFD (Research Diets, New Brunswick, NJ). Mice were fed their respective diets for 16 weeks.
In Exp2, PyMT mice at 6 weeks of age were randomly assigned to the same LFD and HFD-treatment groups used in experiment 1. Mice were fed their respective diets for 18 weeks.
Body weights, food intake, and body composition
Body weight and food intake were monitored weekly for both experiments. Body composition for WT mice was assessed at the conclusion of the study (20 weeks of age) in Exp1. PyMT groups were not analyzed for body composition as the tumors can alter lean mass calculations. Briefly, mice were placed under anesthesia (isoflurane inhalation) and were assessed for lean mass, fat mass, and body fat percentage via dual-energy x-ray absorptiometry (DEXA) (Lunar PIXImus, Madison, WI)Citation46.
Tumor palpations
Tumors were measured beginning at 14 weeks of age (10 weeks of diet treatment) and 14 weeks of age (8 weeks of diet treatment) for each of Exp1 and Exp2, respectively, by the same investigator. PyMT/MMTV mice typically develop palpable mammary tumors between 12 and 16 weeks of ageCitation27 . Upon palpation of a tumor, calipers were used to measure the longest and shortest diameter of the tumor. The number of tumors within each mouse was recorded and the tumor volume was estimated for each tumor using the formula: 0.52 X (largest diameter) X (smallest diameter)Citation2 as previously describedCitation48.
Tissue collection
Following 16 weeks (Exp1) and 18 weeks (Exp2) of dietary treatment, mice were sacrificed for tissue collection. Visible tumors were dissected from mammary glands and measured to determine tumor weight and tumor volume. A portion of remaining thoracic mammary gland tissue was then removed from both the right and left side. Visceral fat pads were removed. These tissues were either snap frozen in liquid nitrogen for gene expression analysis or fixed in 4% formaldehyde for immunohistochemical analysis.
Histology
A portion of the thoracic mammary gland from both Exp1 and Exp2 was excised from each mouse, fixed overnight in 4% formaldehyde, dehydrated with alcohol, and embedded in wax. Paraffin sections were stained with hematoxylin and eosin (H&E). The mammary gland was subsequently evaluated blindly by a pathologist and characterized according to the grade of dysplasia: no hyperplasia, adenoma/mammary intraepithelial neoplasia (MIN), and early and late invasive carcinoma for both experimentsCitation27. Immunohistochemistry was performed to stain for α-SMA (Cell Signaling #19245) to confirm dysplasia grade. Further, monocyte/macrophage infiltration was observed by positive staining of CD68+ (Cell signaling #76437) cells in the mammary gland.
PCR cleanup for sequencing of PyMTtransgene
One gDNA sample and a cDNA sample from each treatment group (PyMT Con 4 and PyMT HFD 2) were used for sequencing experiments of the PyMT transgene. gDNA and cDNA were both included to confirm that the transgenic sequence does not contain plasmid/vector intron insertions. PCR cleanup of samples was performed using EdgeBio Quick Step 2 PCR purification kit following the manufactures protocol (Edge Biosystems, Gaithersburg, MD). Eluates were retained for sequencing.
Sequencing of PyMT transgene
Two Sanger sequencing reactions (one for each primer) were performed for the three samples using Big Dye chemistry (Applied Biosystems, Foster City, CA) following manufacturer’s recommendations unless otherwise noted. Specifically, each 20 µL reaction contained 1ul of Big Dye version 3.1, 0.5ul of primer (10 mM of either PYVT1 or PYVT2) and 5ul of PCR cleaned product. Chain termination PCR was performed for 50 cycles. Products were purified of excess primers and dNTPS using Edge Bio Performa Gel Filtration Cartridge (Edge Biosystems, Gaithersburg, MD) and detected using an Applied Biosystem 3730 DNA Analyzer (Applied Biosystems, Foster City, CA). Sequences were visualized and assembled using the Sequencher Version 5.2.4 (http://www.genecodes.com).
Real-time quantitative PCR
Gonadal AT and mammary gland was homogenized under liquid nitrogen using a polytron, and total RNA was extracted using chloroform and the E.Z.N.A. Total RNA Kit II (Omega Bio-tek, Norcross, GA)Citation49. In Exp1, quantification of gonadal AT mRNA gene expression for macrophage markers (F4/80 and CD11c) and the adrenal enzyme estrogen synthase (aromatase, Cyp19A1), mammary gland mRNA expression of macrophage markers (F4/80, CD11c and Mertk), inflammatory markers (MCP-1, IL-6, and TNFα), markers of angiogenesis (Vegfα, MMP2 and MMP9), and proliferation (Ki67) were performed as previously described (Applied Biosystems, Foster City, CA)Citation50. Quantification of mammary gland expression of the PyMT transgene was performed using a custom designed assay with forward primer: GGGCGGGTCTGAGTCCAT, reverse primer: AAATGAGCCCTCTGCAAATCC, and fluorescent probe: GGGAGGGTCTGATTCTTCG. Primers designed using the program Primer Express 3.0.1 (Applied Biosystems, Foster City, CA). In Exp2, quantification of gonadal AT mRNA gene expression for Cyp19A1 (aromatase) and mammary gland mRNA expression of inflammatory cytokines (IL-6 and TNFα) were performed as previously describedCitation50. Murine 18s rRNA was used as the housekeeping gene to normalize all the data obtained. Quantitative reverse transcriptase polymerase chain reaction analysis was carried out as per the manufacturer’s instructions and all primers used were TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA).
Statistical analysis
All data were analyzed using commercial software (GraphPad Software, Prism 7, La Jolla, CA, USA). For Exp1, total body weight was analyzed using a two-way analysis of variance at each time point. Total visceral fat weight, total gonadal fat weight, mRNA analysis of gonadal fat F4/80, mammary gland inflammation and proliferation mediators and VEGFα were analyzed using a two-way analysis of variance. All DEXA information, sacrifice tumor data, mRNA analysis of gonadal fat CD11c, CD64, Cy19A1, mammary gland mRNA analysis of MMP2/9 were analyzed using a two tailed t-test. Bonferroni correction was used for all post-hoc analyses. In Exp2, all data was analyzed using a two tailed t-test. Statistical significance was set with an α value of P ≤ 0.05. Data are represented as mean ±SEM.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Berstad P, Coates RJ, Bernstein L, Folger SG, Malone KE, Marchbanks PA, Weiss LK, Liff JM, McDonald JA, Strom BL, et al. A case-control study of body mass index and breast cancer risk in white and African-American women. Cancer Epidemiol Biomarkers Prev. 2010;19:1532–1544. doi:10.1158/1055-9965.EPI-10-0025.
- Cheraghi Z, Poorolajal J, Hashem T, Esmailnasab N, Doosti Irani A. Effect of body mass index on breast cancer during premenopausal and postmenopausal periods: a meta-analysis. PLoS One. 2012;7:e51446. doi:10.1371/journal.pone.0051446.
- Anderson GL, Neuhouser ML. Obesity and the risk for premenopausal and postmenopausal breast cancer. Cancer Prev Res (Phila). 2012;5:515–521. doi:10.1158/1940-6207.CAPR-12-0091.
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi:10.1016/S0140-6736(08)60269-X.
- Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15:556–565. doi:10.1634/theoncologist.2009-0285.
- Nagrani R, Mhatre S, Rajaraman P, Soerjomataram I, Boffetta P, Gupta S, Parmar V, Badwe R, Dikshit R. Central obesity increases risk of breast cancer irrespective of menopausal and hormonal receptor status in women of South Asian Ethnicity. Eur J Cancer. 2016;66:153–161. doi:10.1016/j.ejca.2016.07.022.
- Schapira DV, Kumar NB, Lyman GH, Cox CE. Obesity and body fat distribution and breast cancer prognosis. Cancer. 1991;67:523–528.
- Schapira DV, Kumar NB, Lyman GH. Obesity, body fat distribution, and sex hormones in breast cancer patients. Cancer. 1991;67:2215–2218.
- Chang S, Buzdar AU, Hursting SD. Inflammatory breast cancer and body mass index. J Clin Oncol. 1998;16:3731–3735. doi:10.1200/JCO.1998.16.12.3731.
- Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1686–1691. doi:10.1158/1055-9965.EPI-05-0042.
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi:10.1038/nrc1408.
- Patterson RE, Cadmus LA, Emond JA, Pierce JP. Physical activity, diet, adiposity and female breast cancer prognosis: a review of the epidemiologic literature. Maturitas. 2010;66:5–15. doi:10.1016/j.maturitas.2010.01.004.
- Brown KA, Simpson ER. Obesity and breast cancer: progress to understanding the relationship. Cancer Res. 2010;70:4–7. doi:10.1158/0008-5472.CAN-09-2257.
- Cleary MP, Grossmann ME, Ray A. Effect of obesity on breast cancer development. Vet Pathol. 2010;47:202–213. doi:10.1177/0300985809357753.
- Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–635.
- Hellmann SS, Thygesen LC, Tolstrup JS, Gronbaek M. Modifiable risk factors and survival in women diagnosed with primary breast cancer: results from a prospective cohort study. Eur J Cancer Prev. 2010;19:366–373. doi:10.1097/CEJ.0b013e32833b4828.
- Stark A, Stahl MS, Kirchner HL, Krum S, Prichard J, Evans J. Body mass index at the time of diagnosis and the risk of advanced stages and poorly differentiated cancers of the breast: findings from a case-series study. Int J Obes (Lond). 2010;34:1381–1386.
- Simpson ER, Brown KA. Minireview: obesity and breast cancer: a tale of inflammation and dysregulated metabolism. Mol Endocrinol. 2013;27:715–725. doi:10.1210/me.2013-1011.
- Nunez NP, Perkins SN, Smith NC, Berrigan D, Berendes DM, Varticovski L, Barrett JC, Hursting SD. Obesity accelerates mouse mammary tumor growth in the absence of ovarian hormones. Nutr Cancer. 2008;60:534–541. doi:10.1080/01635580801966195.
- Nkhata KJ, Ray A, Dogan S, Grande JP, Cleary MP. Mammary tumor development from T47-D human breast cancer cells in obese ovariectomized mice with and without estradiol supplements. Breast Cancer Res Treat. 2009;114:71–83. doi:10.1007/s10549-008-9991-7.
- Ray A, Nkhata KJ, Grande JP, Cleary MP. Diet-induced obesity and mammary tumor development in relation to estrogen receptor status. Cancer Lett. 2007;253:291–300. doi:10.1016/j.canlet.2007.02.005.
- Cleary MP, Hu X, Grossmann ME, Juneja SC, Dogan S, Grande JP, Maihle NJ. Prevention of mammary tumorigenesis by intermittent caloric restriction: does caloric intake during refeeding modulate the response? Exp Biol Med (Maywood). 2007;232:70–80.
- Krishnan K, Bassett JK, Macinnis RJ, English DR, Hopper JL, McLean C, Giles GG, Baglietto L. Associations between weight in early adulthood, change in weight and breast cancer risk in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2013;22:1409–1416.
- Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, Zhou XK, Blaho VA, Hla T, Yang P. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila). 2011;4:329–346. doi:10.1158/1940-6207.CAPR-10-0381.
- Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, Du B, Brogi E, Crawford CB, Kopelovich L, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila). 2011;4:1021–1029. doi:10.1158/1940-6207.CAPR-11-0110.
- Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, Kopelovich L, Hudis CA, Dannenberg AJ. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2:356–365. doi:10.1158/2159-8290.CD-11-0241.
- Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, Pollard JW. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–2126. doi:10.1016/S0002-9440(10)63568-7.
- Amaral P, Miguel R, Mehdad A, Cruz C, Monteiro Grillo I, Camilo M, Ravasco P. Body fat and poor diet in breast cancer women. Nutr Hosp. 2010;25:456–461.
- Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15:556–565. doi:10.1634/theoncologist.2009-0285.
- Dirat B, Bochet L, Escourrou G, Valet P, Muller C. Unraveling the obesity and breast cancer links: a role for cancer-associated adipocytes? Endocr Dev. 2010;19:45–52.
- Nagaiah G, Hazard HW, Abraham J. Role of obesity and exercise in breast cancer survivors. Oncology (Williston Park). 2010;24:342–346.
- Baer HJ, Tworoger SS, Hankinson SE, Willett WC. Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol. 2010;171:1183–1194. doi:10.1093/aje/kwq045.
- Maccio A, Madeddu C, Gramignano G, Mulas C, Floris C, MassaD, Astara G, Chessa P, Mantovani G. Correlation of body mass index and leptin with tumor size and stage of disease in hormone-dependent postmenopausal breast cancer: preliminary results and therapeutic implications. J Mol Med. 2010;88:677–686. doi:10.1007/s00109-010-0611-8.
- Chen X, Lu W, Zheng W, Gu K, Chen Z, Zheng Y, Shu XO. Obesity and weight change in relation to breast cancer survival. Breast Cancer Res Treat. 2010;122:823–833. doi:10.1007/s10549-009-0708-3.
- Malin A, Matthews CE, Shu XO, Cai H, Dai Q, Jin F, Gao YT, Zheng W. Energy balance and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:1496–1501. doi:10.1158/1055-9965.EPI-04-0880.
- Thomson CA, Thompson PA. Dietary patterns, risk and prognosis of breast cancer. Future Oncol. 2009;5:1257–1269. doi:10.2217/fon.09.86.
- Brown KA, Simpson ER. Obesity and breast cancer: mechanisms and therapeutic implications. Front Biosci (Elite Ed). 2012;4:2515–2524.
- Gross A, Newschaffer CJ, Hoffman Bolton JA, Rifai N, Visvanathan K. Adipocytokines, inflammation, and breast cancer risk in postmenopausal women: A prospective study. Cancer Epidemiol Biomarkers Prev. 2013;22:1319–1324.
- Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M. Aromatase–a brief overview. Annu Rev Physiol. 2002;64:93–127. doi:10.1146/annurev.physiol.64.081601.142703.
- Simpson ER, Brown KA. Obesity and breast cancer: role of inflammation and aromatase. J Mol Endocrinol. 2013;51:T51–9. doi:10.1530/JME-13-0217.
- Bowers LW, Brenner AJ, Hursting SD, Tekmal RR, deGraffenried LA. Obesity-associated systemic interleukin-6 promotes pre-adipocyte aromatase expression via increased breast cancer cell prostaglandin E2 production. Breast Cancer Res Treat. 2015;149:49–57. doi:10.1007/s10549-014-3223-0.
- McTiernan A, Wu L, Chen C, Chlebowski R, Mossavar-Rahmani Y, Modugno F, Perri MG, Stanczyk FZ, Van Horn L, Wang CY. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity (Silver Spring). 2006;14:1662–1677. doi:10.1038/oby.2006.191.
- Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocr Rev. 2009;30:343–375. doi:10.1210/er.2008-0016.
- Bocchinfuso WP, Korach KS. Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J Mammary Gland Biol Neoplasia. 1997;2:323–334.
- Grotto D, Zied E. The Standard American Diet and its relationship to the health status of Americans. Nutr Clin Pract. 2010;25:603–612. doi:10.1177/0884533610386234.
- Enos RT, Davis JM, Velazquez KT, McClellan JL, Day SD, Carnevale KA, Murphy EA. Influence of dietary saturated fat content on adiposity, macrophage behavior, inflammation, and metabolism: composition matters. J Lipid Res. 2013;54:152–163. doi:10.1194/jlr.M030700.
- Warden CH, Fisler JS. Comparisons of diets used in animal models of high-fat feeding. Cell Metab. 2008;7:277. doi:10.1016/j.cmet.2008.03.014.
- Steiner J, Davis J, McClellan J, Enos R, Carson J, Fayad R, Nagarkatti M, Nagarkatti P, Altomare D, Creek K, et al. Dose-dependent benefits of quercetin on tumorigenesis in the C3(1)/SV40Tag transgenic mouse model of breast cancer. Cancer Biol Ther. 2014;15:1456–1467. doi:10.4161/15384047.2014.955444.
- EZNA Total RNA Kit II Manual. 2015. Accessed 2016 Oct 27 http://omegabiotek.com/store/wp-content/uploads/2013/09/R6934-Total-RNA-Kit-II-Combo-Online1.pdf
- Enos RT, Velazquez KT, McClellan JL, Cranford TL, Walla MD, Murphy EA. Lowering the dietary omega-6: omega-3 does not hinder nonalcoholic fatty-liver disease development in a murine model. Nutr Res. 2015;35:449–459. doi:10.1016/j.nutres.2015.04.003.
