ABSTRACT
Background: Neuroblastoma is the commonest malignancy in neonates. Long non-coding RNA (lncRNA) RNA component of mitochondrial RNA processing endoribonuclease (RMRP) has been reported to be an oncogenic factor in some malignancies. However, its roles and molecular mechanisms in neuroblastoma progression are poor defined.
Methods: The expression of RMRP, microRNA-206 (miR-206), and tachykinin-1 receptor (TACR1) mRNA was measured by RT-qPCR assay. Protein levels of TACR1, phosphorylated extracellular signal-regulated kinases (ERK) 1/2 (p-ERK1/2) and ERK1/2 were detected by western blot assay. Cell proliferation was assessed by CCK-8 and colony formation assays. Cell migratory and invasive capacities were determined using Transwell migration and invasion assays. The interaction between miR-206 and RMRP or TACR1 was verified by luciferase assay. The roles and molecular mechanisms of RMRP knockdown on the growth of neuroblastoma xenografts were examined in vivo.
Results: RMRP was highly expressed in neuroblastoma tissues. RMRP knockdown inhibited proliferation, migration and invasion in neuroblastoma cells. Moreover, TACR1 was a target of miR-206 and RMRP performed as a molecular sponge of miR-206 to sequester miR-206 from TACR1 in neuroblastoma cells. TACR1 overexpression abrogated the inhibitory effect of RMRP downregulation on neuroblastoma cell progression by activating ERK1/2 pathway. Inhibition of TACR1 and ERK1/2 pathway abated RMRP-mediated pro-proliferation effect in neuroblastoma cells. RMRP knockdown hindered neuroblastoma xenograft growth by regulating miR-206/TACR1 axis via inactivating ERK1/2 pathway in vivo.
Conclusion: RMRP knockdown hindered the tumorigenesis and progression of neuroblastoma by regulating miR-206/TACR1 axis via inactivating ERK1/2 pathway, hinting a potential therapeutic target for neuroblastoma.
Introduction
Neuroblastoma is the third commonest extracranial neoplasm in childhood with an estimated 710 new diagnosed cases among children aged 0–14 years old in 2014 in the United StatesCitation1. Neuroblastoma is responsible for approximately 7% of pediatric malignancies and more than 10% cancer-related children mortality.Citation2 Moreover, neonatal neuroblastoma is the most prevalent malignancy in neonate, accounting for more than 20% of neonatal neoplasms.Citation3 Despite great advances have been made in clinical presentation, staging and treatment of neonatal neuroblastoma over the past decades,Citation4 it is imperative to have a deep insight into molecular mechanisms in the development of neuroblastoma to identify potential diagnostic biomarkers or therapeutic targets.
Long noncoding RNAs (lncRNAs), longer than 200 nucleotide (nt) in length, are a class of transcripts without protein coding potential. Moreover, lncRNAs are functionally implicated in a wide repertoire of biological processes such as proliferation, survival and genomic stability.Citation5 Moreover, a wealth of lncRNA have been identified as critical mediators in neuroblastoma progression in recent years.Citation6 For instance, the deficiency of lncRNA neuroblastoma associated transcript-1 (NBAT-1) contributed to proliferation and invasion of neuroblastoma cells.Citation7 RNA component of mitochondrial RNA processing endoribonuclease (RMRP), located on chromosome 9p21-p12, is a long RNA transcript without protein-coding potential.Citation8 Previous studies showed that RMRP functioned as an oncogenic factor in some malignancies such as lung cancer,Citation9 gastric cancerCitation10and glioma.Citation11 However, its roles and molecular mechanisms in neuroblastoma progression have not been explored till now.
In the present study, we demonstrated that RMRP knockdown curbed proliferation, migration and invasion in neuroblastoma cells and hampered tumor growth in mouse xenograft models of neuroblastoma. Mechanism analysis revealed that RMRP exerted its oncogenic effect by regulating microRNA-206/tachykinin-1 receptor (TACR1) via activating extracellular signal-regulated kinases (ERK1/2) in neuroblastoma.
Materials and methods
Clinical specimens and cell culture
Neonatal neuroblastoma and adjacent normal tissues were obtained from 44 cases of neonatal neuroblastoma patients (24 males and 20 females) in the Neonatology Department of First Affiliated Hospital of Zhengzhou University. Clinical stages (I+ II+ III+ IV) of neuroblastoma patients were defined according to the standard of International Neuroblastoma Staging System (INSS). Our study was approved by Ethics Committee in the First Affiliated Hospital of Zhengzhou University. Also, informed consents were obtained from all enrolled patients prior to the initiation of our study. NB-1, SK-N-AS and HEK293T cell lines were all purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). NB-1 cells were cultured in 1:1 mixture of Minimum Essential Medium (MEM, Invitrogen, Carlsbad, CA, USA) and F12 medium (Invitrogen) containing 10% Fetal Bovine Serum (FBS, Invitrogen). SK-N-AS cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM, Invitrogen) supplemented with 0.1 mM Non-Essential Amino Acids (NEAA, Invitrogen) and 10% FBS (Invitrogen). Human embryonic kidney cell line HEK293T was grown in DMEM medium (Invitrogen) containing 10% FBS (Invitrogen). All cells were incubated in a humidified incubator containing 5% CO2 at 37°C.
Reagents and cell transfection
SiRNAs targeting RMRP1 (siRMRP1#1 and siRMRP1#2) and their scramble control (Scrambled), miR-206 mimic (miR-206) and its scramble control (NC), miR-206 inhibitor and its negative control (NC-inhibitor) were all obtained from GenePharma Co. ltd (Suzhou, China). Full length fragments of RMRP or TACR1 coding sequences were amplified by PCR and constructed into pcDNA3.1 vector (Invitrogen) to generate pcDNA3.1-RMRP or pcDNA3.1-TACR1 overexpression plasmid. All these oligonucleotides or plasmids were transfected into NB-1 and SK-N-AS cells by Lipofectamine 2000 reagent (Invitrogen) following the protocols of manufacturer. TACR1 inhibitor Fosaprepitant and ERK1/2 inhibitors PD98059 and Trametinib were purchased from MedChem Express Co. ltd (Monmouth Junction, NJ, USA).
RT-qPCR assay
Total RNAs were extracted from neuroblastoma tissues and cells using Trizol reagent (Invitrogen). For miRNA analysis, RNAs were reversely transcribed into cDNAs using reagents provided by TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems; Foster City, CA, USA) and RT-primers in TaqMan MicroRNA Assays (Applied Biosystems). Then, TaqMan Universal PCR Master Mix and TaqMan MicroRNA Assay primer for miR-206 (has-mir-206) and RNU6B (Applied Biosystems) were used to measure miR-206 expression with RNU6B as an internal control. For expression analysis of RMRP and TACR1, cDNAs were synthesized using M-MLV Reverse Transcriptase (Invitrogen) and random primers, and real time quantitative analysis was performed using SYBR® Premix Ex Taq™ reagent (Takara, Otsu, Japan) and specific quantitative primers for RMRP, TACR1 and GAPDH. GAPDH expression was used to normalize the expression of RMRP and TACR1. Quantitative primer sequences were displayed as below: RMRP, 5ʹ-ACTCCAAAGTCCGCCAAGA-3ʹ (forward) and 5ʹ-TGCGTAACTAGAGGGAGCTGAC-3ʹ (reverse); TACR1, 5ʹ-CTGCTGGTGATTGGCTATGC-3ʹ (forward) and 5ʹ-AGGAGGAAGAAGATGTGGAAGG-3ʹ (reverse); GAPDH, 5ʹ-GGGAGCCAAAAGGGTCAT-3ʹ (forward) and 5ʹ-GAGTCCTTCCACGATACCAA-3ʹ (reverse).
Western blot assay
Total proteins in cells and tissues were extracted using RIPA buffer (Beyotime, Shanghai, China) and quantified using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL, USA). Then, equivalent proteins (50 μg/sample) were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose (NC) membranes (Bio-Rad, Hercules, CA, USA). Next, the membranes were blocked in 5% skimmed milk for 2 h at room temperature and incubated overnight at 4°C with primary antibodies against TACR1 (Abcam, Cambridge, UK), GAPDH (Abcam), phosphorylated-ERK1/2 (Cell Signaling Technology, Danvers, MA, USA), ERK1/2 (Cell Signaling Technology). Subsequently, horseradish peroxidase (HRP)-conjugated secondary antibody was co-incubated with these membranes for additional 1 h at room temperature. At last, specific protein signals were visualized using Pierce™ ECL Western Blotting Substrate (Thermo Fisher Scientific) and quantified using a Quantity One software (Bio-Rad).
CCK-8 assay
Cell proliferative capacity was determined using a Cell Counting Kit-8 (CCK-8) kit (Dojindo Molecular Technologies, Rockville, MD, USA) according to the instructions of manufacturer. Generally, 100 μl of cell suspensions were dispensed into 96-well plates, followed by 24 h of pre-incubation. Then, cells were transfected or treated as described in Figure legends. At the indicated time points after transfection or treatment, 10 μl of CCK-8 solution was added into each well. After incubation for another 3 h, cell absorbance was measured at the wavelength of 450 nm.
Colony formation assay
Transfected NB-1 and SK-N-AS cells were seeded into 60 mm culture dishes and maintained in corresponding complete mediums for approximately 14 days. Next, cells were fixed using absolute ethylalcohol for 15 min, and stained with crystal violet solution (0.1%, Sigma-Aldrich, St. Louis, MO, USA) for additional 10 min. At last, the number of positive colonies with more than 50 cells was counted.
Luciferase activity assay
Partial fragments of RMRP or TACR1 3ʹUTR containing predicted miR-206 binding sites were amplified by PCR and constructed into psiCHECK-2 vector (Promega, Madison, WI, USA) to produce wild type RMRP (RMRP-Wt) or TACR1 (TACR1-Wt) reporter, respectively. Also, mutant type RMRP (RMRP-Mut) or TACR1 (TACR1-Mut) reporter containing mutant miR-206 binding sites was generated using Quik Change® Multi Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) following the instructions of manufacturer. Next, these reporters were respectively transfected into NB-1 and SK-N-AS cells with corresponding oligonucleotides or plasmids. At 48 h post transfection, a dual luciferase reporter assay kit (Promega) was used to detect luciferase activities.
Transwell migration and invasion assays
Cell invasive or migratory ability was assessed using a transwell chamber (BD Biosciences Franklin Lakes, New Jersey, USA) with or without matrigel (BD Bioscience), respectively. Transfected cells (5 × 104 for migration assay and 1 × 105 for invasion assay) were inoculated into the upper chamber and maintained in serum-free medium. Complete medium containing 20% FBS (Invitrogen) was plated into the low chamber. At 48 h after incubation, cells in the upper chambers were removed using a cotton swab. After fixed and stained, cells in the lower surface were imaged using a microscope and cell number was counted in 9 random fields.
Lentiviruses production and infection
Designed RMRP#2 shRNA fragments or its scrambled control shRNA fragments were synthesized and constructed into pLKO.1 plasmid (Addgene, Cambridge, Massachusetts, USA) to generate plasmid containing RMRP knockdown fragment or control plasmid. Then constructed plasmid together with pMD2.G (Addgene) and psPAX2 (Addgene) plasmids was co-transfected into HEK293T cells by Lipofectamine 2000 reagent (Invitrogen). At 72 h after transfection, cell supernatants containing shRNRP#2 or Scrambled lentiviruses were obtained, followed by sieving using 0.45 μm filterable membranes. Then, SK-N-AS cells were infected with harvested shRNRP#2 or Scrambled lentivirus supernatants. After 2 days of infection, these cells were screened using puromycin (Sigma-Aldrich) for at least 7 days to obtain shRNRP#2 or Scrambled stably-transfected SK-N-AS cell line.
Mice experiment
All animal experiments were approved by Institutional Animal Care and Use Committee of the First Affiliated Hospital of Zhengzhou University. Twelve 6-week-old female athymic nude mice were obtained from Shanghai Laboratory Animal Center of Chinese Academy of Sciences (Shanghai, China) and maintained according to the national standard of the care and use of laboratory animals. Mice were randomly assigned into two groups (Scrambled or shRNRP#2) with 6 mice in each group. Next, shRNRP#2 or Scrambled stably-transfected SK-N-AS cells (5 × 106) were subcutaneously inoculated into the left flank of mice. Then, tumor volumes were monitored every 7 days for total 49 days after injection. At the end of experiments, tumors were resected, imaged and weighted. Moreover, RT-qPCR assay was performed to measure expression of RMRP and miR-206 in xenograft tumors, and western blot assay was carried out to determinate protein levels of TACR1, p-ERK1/2 and ERK1/2 in xenograft tumors.
Statistical analysis
All experiments were repeated at least 3 times with the data displaying as mean ± standard deviation (SD). Student’s t-test or one-way analysis of variance (ANOVA) was applied to analyze significance of difference between or among groups. P < 0.05 was regarded as statistically significant.
Results
RMRP expression was upregulated in neonatal neuroblastoma tissues and positively associated with higher pathological status and poor prognosis for neonatal neuroblastoma patients
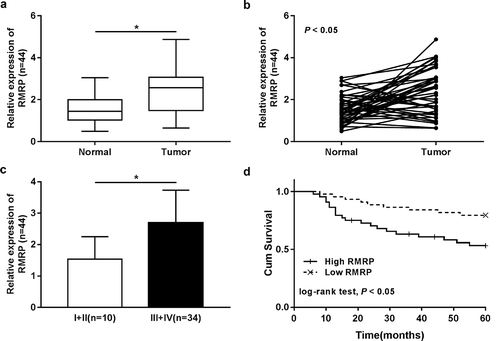
Firstly, expression pattern of RMRP in neonatal neuroblastoma tissues and adjacent normal tissues was determined by RT-qPCR assay. Results showed that RMRP was highly expressed in neonatal neuroblastoma tissues (Tumor, n = 44) compared with adjacent normal tissues (Normal, n = 44) ()). Further analysis revealed that RMRP expression was upregulated in 75% neonatal neuroblastoma tissues (33/44) relative to normal counterparts ()). Additionally, RMRP expression was markedly increased in patients with advanced neonatal neuroblastoma (III+ IV) compared to those in earlier stages (I+ II) ()). To further explore the relationship between RMRP expression and neuroblastoma prognosis, neonatal neuroblastoma patients (n = 44) were divided into high RMRP group and low RMRP group according to the median value of RMRP in neonatal neuroblastoma tissues (high RMRP group: RMRP relative level ≥ median value, low RMRP group: RMRP relative level <median value). Subsequent Kaplan Meier analysis and log-rank test showed that neonatal neuroblastoma patients with higher RMRP expression had a poor prognosis, revealed by deceased cumulative survival rate and shorted cumulative survival time ()). Collectively, these results indicated that RMRP might be associated with the pathogenesis of neonatal neuroblastoma.
Figure 1. RMRP expression was upregulated in neonatal neuroblastoma tissues, and upregulated RMRP was positively associated with aggressive pathological status and poor prognosis of neonatal neuroblastoma patients. (a-c) RT-qPCR assay was performed to measure RMRP expression in neonatal neuroblastoma tissues (Tumor, n = 44) and corresponding adjacent normal tissues (Normal, n = 44) (a and b), neonatal neuroblastoma tissues in different pathological stages (c, n (I+ II) = 10, n (III+ IV) = 34). (d) Kaplan Meier analysis of cumulative survival for neonatal neuroblastoma patients referring to the difference of RMRP level. *P < 0.05.
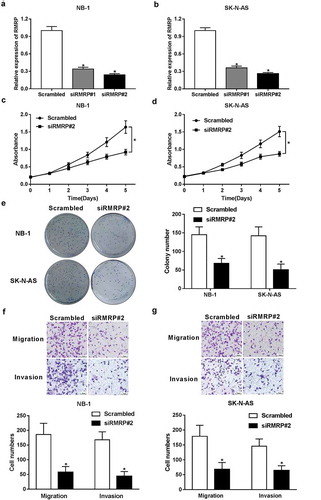
RMRP knockdown inhibited proliferation, migration and invasion in neuroblastoma cells
To further inquire RMRP functions in the development of neuroblastoma, small interference RNAs (siRNAs) specifically targeting RMRP (siRMRP#1 and siRMRP#2) and their corresponding scramble control (Scrambled) were synthesized, followed by the measurement of knockdown efficiency in NB-1 and SK-N-AS cells. As displayed in ,), the introduction of siRMRP#1 and siRMRP#2 resulted in a significant reduction of RMRP level compared with scramble control group in NB-1 and SK-N-AS cells. Additionally, siRMRP#2 was chosen for the subsequent loss-of-function experiments by virtue of its better knockdown efficiency. Then, the effect of RMRP knockdown on neuroblastoma cell proliferation was assessed by CCK-8 assay. Results disclosed that siRMRP#2-induced RMRP downregulation strikingly hampered proliferation of NB-1 and SK-N-AS cells (,)). To further validate this conclusion, the effect of RMRP silence on colony formation capacity of NB-1 and SK-N-AS cells was assessed by colony formation assay. Results revealed that cell colony formation ability was markedly reduced in siRMRP#2-transfected cells relative to Scrambled-transfected cells ()). Transwell migration and invasion assays further manifested that the silence of RMRP hampered migration and invasion of NB-1 and SK-N-AS cells (,)). In a word, these data indicated that RMRP knockdown suppressed proliferation, migration and invasion in neuroblastoma cells.
Figure 2. Knockdown of RMRP curbed proliferation, migration and invasion of neuroblastoma cells. (a and b) NB-1 and SK-N-AS cells were transfected with Scrambled control, siRMRP#1 or siRMRP#2, followed by the detection of RMRP level using RT-qPCR assay at 48 h after transfection. (c-g) NB-1 and SK-N-AS cells were transfected with Scrambled control or siRMRP#2. (c and d) At the indicated time points (0, 24, 48, 72 h) after transfection, the effect of RMRP silence on cell proliferation was measured by CCK-8 assay. (e) At 2 weeks post transfection, cell colony number was determined by colony formation assay. (f and g) At 48 h upon transfection, migratory and invasive cell number was detected by Transwell migration and invasion assays, respectively. *P < 0.05.
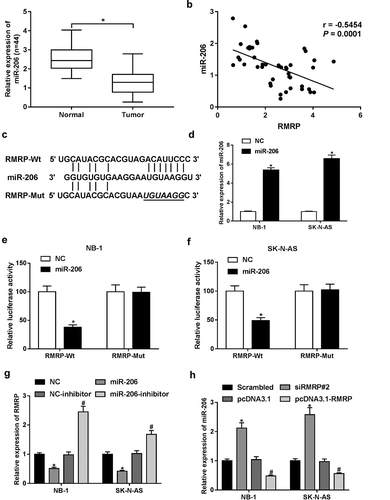
RMRP interacted with miR-206 in neuroblastoma cells
Earlier studies manifested that RMRP could perform as a molecular sponge of miR-206 to exert its oncogenic effects in lung cancer (9) and gastric cancer (10). Moreover, Wang et al. demonstrated that ectopic expression of miR-206 suppressed proliferation and facilitated apoptosis by targeting orthodenticle homeobox 2 in glioma and neuroblastoma cells.Citation12 Hence, we aimed to further investigate whether RMRP has a similar mechanism in neuroblastoma. In the present study, we demonstrated that miR-206 expression was markedly downregulated and negatively associated with RMRP expression in neonatal neuroblastoma tissues (n = 44) (,)), suggesting that miR-206 was implicated in pathogenesis of neonatal neuroblastoma. Ensuing bioinformatics analysis by miRcode online website further uncovered that there existed some complementary sites between miR-206 and RMRP ()). To further explore the function of miR-206 in the development of neuroblastoma, miR-206 mimic and its scramble control (NC) were synthesized. RT-qPCR assay manifested that the introduction of miR-206 mimic led to a notable upregulation of miR-206 level in NB-1 and SK-N-AS cells, hinting that miR-206 mimic could be used for following gain-of-function experiments ()). Then, the effect of miR-206 overexpression on luciferase activity of wild type RMRP reporter (RMRP-Wt) or mutant type RMRP reporter (RMRP-Mut) was determined by a dual-luciferase assay in NB-1 and SK-N-AS cells. As displayed in ,), miR-206 overexpression remarkably inhibited luciferase activity of RMRP-Wt reporter, but had no effect on luciferase activity of RMRP-Mut reporter in NB-1 and SK-N-AS cells. Moreover, miR-206 upregulation markedly suppressed RMRP expression in NB-1 and SK-N-AS cells ()). Conversely, the introduction of miR-206 inhibitor resulted in a notable decrease of RMRP level in NB-1 and SK-N-AS cells ()). Additionally, miR-206 level was dramatically upregulated in RMRP-silenced NB-1 and SK-N-AS cells, but was significantly downregulated in RMRP-overexpressed cells ()). That was to say, RMRP could interact with miR-206 by putative binding sites in neuroblastoma cells.
Figure 3. RMRP acted as a molecular sponge of miR-206 in neuroblastoma cells. (a) Expression pattern of miR-206 in 44 pairs of neonatal neuroblastoma tissues and adjacent normal tissues. (b) Correlation analysis of miR-206 and RMRP in neuroblastoma tissues (n = 44). (c) Putative binding sites between miR-206 and RMRP, and mutant sites in RMRP-Mut reporter. (d) NB-1 and SK-N-AS cells were transfected with miR-206 mimic or its scramble control (NC), followed by the examination of miR-206 level at 48 h after transfection. (e and f) NB-1 and SK-N-AS cells were co-transfected with RMRP-Wt or RMRP-Mut reporter and miR-206 mimic or its scramble control (NC). At 48 h post transfection, relative luciferase activity was detected. (g) NB-1 and SK-N-AS cells were transfected with, miR-206 mimic, miR-206 inhibitor, or their matching controls, followed by the measurement of RMRP level using RT-qPCR assay at 48 h upon transfection. (h) NB-1 and SK-N-AS cells were transfected with scramble control (Scrambled), siRMRP#2, pcDNA3.1 empty vector or pcDNA3.1-RMRP overexpression plasmid. Then, miR-206 level was determined by RT-qPCR assay at 48 h post transfection. *P < 0.05.
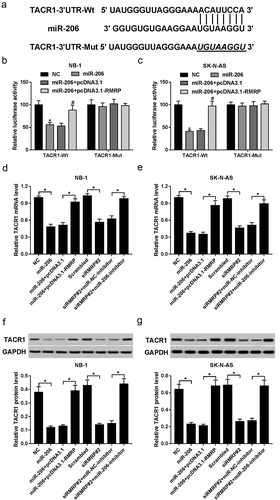
RMRP enhanced TACR1 expression by acting as a competing endogenous RNA (ceRNA) of miR-206 in neuroblastoma cells
Mounting evidence reveals that lncRNAs can act as ceRNAs of miRNAs to regulate expression of miRNAs and target mRNAs.Citation13 Hence, TargetScan online website was used to search for potential targets of miR-206. Among candidate target genes, TACR1 was selected due to its oncogenic effects in numerous malignancies including neuroblastoma ()).Citation14–Citation16 Subsequent luciferase assay further demonstrated that miR-206 overexpression reduced luciferase activity of wide type TACR1 (TACR1-Wt) reporter, while this inhibitory effect of miR-206 was abolished by upregulated RMRP in NB-1 and SK-N-AS cells (,)). However, overexpression of miR-206 alone or along with RMRP had no effect on luciferase activity of mutant type TACR1 (TACR1-Mut) reporter (,)). Moreover, miR-206 inhibited TACR1 expression at mRNA (,)) and protein levels (,)) in NB-1 and SK-N-AS cells. In a word, these results indicated that TACR1 was a target of miR-206. Further analysis revealed that miR-206-mediated suppressive effects on TACR1 mRNA and protein expression was abrogated by RMRP overexpression in NB-1 and SK-N-AS cells (-)). Additionally, siRMRP#2-induced RMRP knockdown resulted in a marked decrease of TACR1 expression at mRNA (,)) and protein (,)) levels in NB-1 and SK-N-AS cells. Furthermore, the deficiency of miR-206 weakened siRMRP#2-mediated inhibitory effect on TACR1 expression in NB-1 and SK-N-AS cells (-)). Taken together, these results suggested that RMRP acted as a ceRNA of miR-206 to sequester miR-206 from TACR1, resulting in the upregulation of TACR1 expression in neuroblastoma cells.
Figure 4. RMRP enhanced TACR1 expression by performing as a ceRNA of miR-206 in neuroblastoma cells. (a) Predicted binding sites between TACR1 3ʹUTR and miR-206, and mutant sites in TACR1-Mut reporter. (b and c) NB-1 and SK-N-AS cells with the transfection of TACR1-WT or TACR1-Mut reporter were also transfected with a scramble control (NC) of miR-206, miR-206 mimic, miR-206 mimic+ pcDNA3.1 or miR-206 mimic+ pcDNA3.1-RMRP, followed by the detection of luciferase activity at 48 h after transfection. (d-g) NB-1 and SK-N-AS cells were transfected with NC, miR-206 mimic, miR-206 mimic+ pcDNA3.1, miR-206+ pcDNA3.1-RMRP, scramble control of siRMRP (Scrambled), siRMRP#2, siRMRP#2+ miR-NC inhibitor, or siRMRP#2+ miR-206 inhibitor. At 48 h upon transfection, TACR1 expression at mRNA (d and e) and protein (f and g) levels was determined by RT-qPCR and western blot assays, respectively. *P < 0.05.
TACR1 overexpression reversed inhibitory effect of RMRP depletion on cell progression by activating ERK signals in neuroblastoma cells
Next, we further explored whether RMRP exerted its oncogenic effects by regulating TACR1 in neuroblastoma cells. As presented in -), TACR1 overexpression abrogated inhibitory effect of RMRP silencing on cell proliferation, migration and invasion of NB-1 and SK-N-AS cells, revealed by restored cell proliferative capacity (,)), increased colony number ()), elevated cell migration number ()) and raised cell invasion number ()) in siRMRP#2-transfected cells following the introduction of pcDNA3.1-TACR1. Earlier studies revealed that human hemokinin-1 (hHK-1) (a ligand of TACR1) could facilitate cell migration and activate Akt, JNK and ERK signal pathways in glioma and melanoma cells,Citation17,Citation18 indicating TACR1 was associated with these signal pathways. Hence, the effects of RMRP and TACR1 on ERK signal pathway were measured by western blot assay. Results revealed that depletion of RMRP markedly reduced p-ERK1/2 level, but had no effect on ERK1/2 level in NB-1 and SK-N-AS cells (,)). In other words, RMRP silence hampered the activation of ERK1/2 pathway in neuroblastoma cells. Moreover, we further demonstrated that TACR1 upregulation abolished siRMRP#2-induced inactivation on ERK1/2 signal in NB-1 and SK-N-AS cells (,)). In summary, these data indicated that RMRP exerted its oncogenic effect by regulating TACR1 via activating ERK1/2 signal in neuroblastoma cells.
Figure 5. TACR1 upregulation abolished RMRP silence-mediated inhibitory effect on cell progression by activating ERK1/2 signal in neuroblastoma cells. (a-f) NB-1 and SK-N-AS cells were transfected with Scrambled control of siRMRP, siRMRP#2, siRMRP#2+ pcDNA3.1 or siRMRP#2+ pcDNA3.1-TACR1, followed by the measurement of cell proliferative capacity (a and b), colony formation number (c), cell migration and invasion number (d), and protein levels of p-ERK1/2 and ERK1/2 (E and F). *P < 0.05.
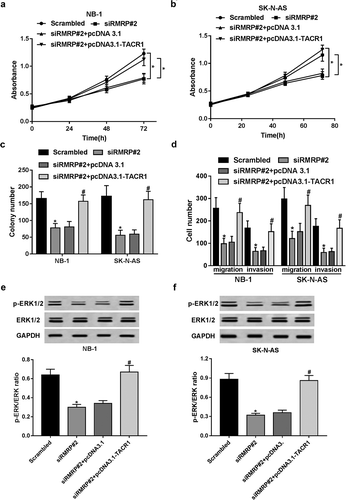
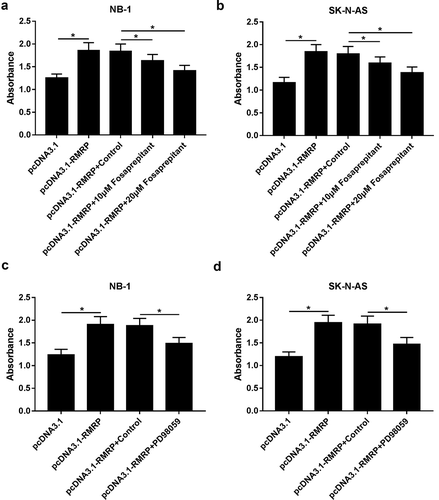
Inhibition of TACR1 and ERK1/2 signal abated RMRP-mediated pro-proliferation effect in neuroblastoma cells
Then, TACR1 inhibitor fosaprepitant and ERK1/2 kinase inhibitor PD98059 were used to further testify our conclusion that oncogenic effect of RMRP was realized by regulating TACR1 via activating ERK1/2 signal in neuroblastoma cells. Our data unveiled that enforced expression of RMRP notably facilitated the proliferation of NB-1 and SK-N-AS cells, while this effect was weakened after the introduction of fosaprepitant (,)). That was to say, the inhibition of TACR1 crippled RMRP-induced pro-proliferation effect in neuroblastoma cells. Additionally, the inactivation of ERK1/2 signal by PD98059 remarkably relieved RMRP-mediated pro-proliferation effect in NB-1 and SK-N-AS cells (,). In summary, these results implied that inhibition of TACR1 and ERK1/2 signal abated RMRP-mediated pro-proliferation effect in neuroblastoma cells.
Figure 6. Inhibition of TACR1 and ERK1/2 signal abated RMRP-mediated pro-proliferation effect in neuroblastoma cells. (a and b) NB-1 and SK-N-AS cells were transfected with pcDNA3.1 or pcDNA3.1-RMRP for 24 h, and then cultured for another 48 h in the presence or absence of DMSO (Control) or Fosaprepitant (10 μM or 20 μM), followed by the determination of cell proliferative capacity using CCK-8 assay. (C and D) NB-1 and SK-N-AS cells were transfected with pcDNA3.1 or pcDNA3.1-RMRP for 24 h, and then cultured for additional 48 h in the presence or absence of DMSO (Control) or PD98059 (10 μM), followed by the measurement of cell proliferative ability using CCK-8 assay. *P < 0.05.
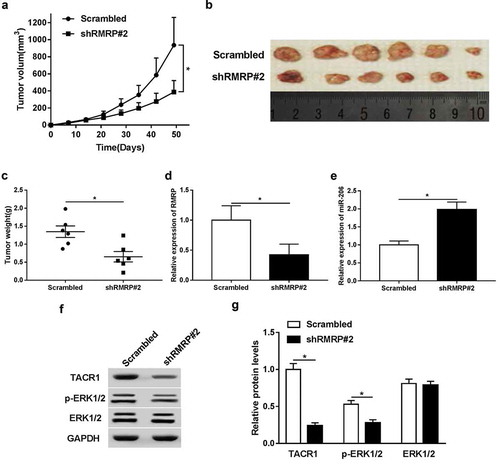
The depletion of RMRP suppressed neuroblastoma xenograft growth by regulating miR-206/TACR1 axis via inactivating ERK1/2 signal in vivo
Then the effect of RMRP knockdown on tumorigenesis of neuroblastoma was further investigated in mouse xenograft models. Results showed that RMRP knockdown curbed xenograft growth, revealed by the reduction of tumor volume and tumor weight in RMRP-silenced mouse xenografts (-)). Moreover, we further demonstrated that the levels of RMRP ()), TACR1 (,)) and p-ERK1/2 (,)) were strikingly reduced and miR-206 level ()) was markedly increased in RMRP-depleted neuroblastoma xenograft tumors. And, RMRP knockdown or not had no effect on ERK1/2 overall protein abundance. Collectively, these data indicated that RMRP knockdown inhibited neuroblastoma xenograft growth by regulating miR-206/TACR1 axis via inactivating ERK1/2 signal in vivo.
Figure 7. The depletion of RMRP hindered neuroblastoma xenograft tumor growth by regulating miR-206/TACR1 axis via inactivating ERK1/2 signal in vivo. shRNRP#2 or Scrambled stably-transfected SK-N-AS cells were injected into null mice to construct mouse xenograft models of neuroblastoma with or without RMRP knockdown, respectively. (a) Tumor volumes were monitored every 7 days for total 49 days after injection. (b and c) At the end of experiments, excised tumors were imaged (b) and weighted (c). (d and e) Expression of RMRP and miR-206 in resected tumors was determined by RT-qPCR assay. (f and g) Protein levels of TACR1, p-ERK1/2 and ERK1/2 were detected using western blot assay. *P < 0.05.
Discussion
Neuroblastoma is a serious threat for infants and young children, and lncRNAs have been demonstrated to be closely linked with the pathogenesis of neuroblastoma.Citation6 Previous studies revealed that RMRP level was markedly increased in tumor tissues of lung cancerCitation9 and glioma,Citation11 and RMRP knockdown hampered tumor progression such as proliferation, migration and invasion by regulating various targets in these malignancies.Citation9–Citation11 In the present study, we demonstrated that RMRP expression was upregulated in neuroblastoma tissues, indicating RMRP was implicated in tumorigenesis of neuroblastoma. Moreover, increased RMRP was positively associated with advanced disease status and poor prognosis. Functional analyses manifested that RMRP knockdown suppressed proliferation, migration and invasion in neuroblastoma cells.
Prior findings also revealed that RMRP could act as a molecular sponge of miR-206 to exert its oncogenic effects in lung cancerCitation9 and gastric cancer.Citation10 MiR-206, a well-studied miRNA, has been reported to be implicated in pathogenesis of plentiful diseases including various types of malignancies, heart failure, and Alzheimer’s disease.Citation19 Moreover, miR-206 expression was downregulated and miR-206 performed as a tumor suppressor in multiple malignancies such as clear cell renal cell cancer (ccRCC),Citation20 lung cancerCitation21 and hepatocellular cancer.Citation22 For example, Xiao et al. demonstrated that miR-206 expression was downregulated in ccRCC and miR-206 suppressed ccRCC cell proliferation in vitro and inhibited ccRCC tumor growth in vivo.Citation20 Pan et al. also showed that miR-206 exerted pro-apoptosis, anti-proliferation, anti-angiogenesis and anti-metastasis effects in lung cancer.Citation21 Additionally, the enforced expression of miR-206 suppressed proliferation and promoted apoptosis by targeting orthodenticle homeobox 2 in glioma and neuroblastoma cells.Citation12 Hence, in the present study, miR-206 expression in neuroblastoma tissues was determined by RT-qPCR assay. Results showed that miR-206 expression was notably decreased and negatively associated with RMRP expression in neuroblastoma tissues, suggesting that RMRP might exert its oncogenic effect by downregulating miR-206 in neuroblastoma. To further validate this conjecture, bioinformatics analysis was performed using miRcode online website with the outcome showing that there existed some complementary sites between RMRP and miR-206. Subsequent luciferase assay further manifested that RMRP could interact with miR-206 in neuroblastoma cells. Additionally, ectopic expression of miR-206 suppressed RMRP expression, and RMRP overexpression also resulted in a notable decrease of miR-206 level in neuroblastoma cells.
It is well known that miRNAs play essential roles in the development of cancers by regulating expression of tumor suppressors or oncogenesCitation23 Hence, bioinformatics analysis was performed by TargetScan online website to search for potential targets of miR-206. Among candidate targets, TACR1 was selected by virtue of its oncogenic effect in multiple tumors including neuroblastoma.Citation14–Citation16
TACR1, also known as neurokinin-1 receptor (NK-1R), is a G-protein-coupled receptor and belongs to neurokinin receptor family.Citation24,Citation25.TACR1 was involved in regulating various physiological and biological processes such as proliferation and inflammation.Citation26 Mounting data suggested that TACR1 was overexpressed in multiple tumors, and TACR1 antagonists exerted anti-tumor effects in numerous malignancies including neuroblastoma, glioma, and colon cancer.Citation14-Citation16 For example, the blockade of TACR1 by its antagonist L-733060 inhibited proliferation and facilitated apoptosis in gliomas and neuroblastoma cells.Citation27–Citation29 The inhibition of TACR1 by its antagonist Fosaprepitant suppressed proliferation and facilitated apoptosis in neuroblastoma cell lines, and hindered tumor growth in a xenograft model of neuroblastoma.Citation30 Due to anti-tumor effects of TACR1 antagonists in neuroblastoma, some researches further proposed that TACR1 might be a promising therapeutic target for neuroblastoma.Citation31
Our study substantiated that TACR1 was a target of miR-206. Moreover, RMRP could act as a ceRNA of miR-206 to sequester miR-206 from TACR1, resulting in a marked downregulation of miR-206 level and a notable upregulation of TACR1 level in neuroblastoma cells. Additionally, increased TACR1 could abolish the inhibitory effect of RMRP knockdown on proliferation, migration and invasion of neuroblastoma cells. In other words, RMRP exerted its oncogenic effect by regulating miR-206/TACR1 axis in neuroblastoma cells.
Zheng et al. pointed out that miR-206 overexpression inhibited the activation of Akt and ERK signals in gastric cancer cells.Citation32 Williams et al. also demonstrated that substance P (SP, a ligand of TACR1)-induced TACR1 activation stimulated ERK phosphorylation and ERK signal was involved in mediating TACR1-induced NF-κB activation in lung cancer cells.Citation33 Also, human hemokinin-1 (hHK-1), a ligand of TACR1, could facilitate cell migration and activate Akt, JNK and ERK signal pathways in glioma and melanoma cells.Citation17,Citation18 Consequently, we further explored whether RMRP could regulate ERK signal in neuroblastoma cells. Results showed that RMRP knockdown hindered activation of ERK1/2 signal, while this effect was reversed by TACR1 upregulation in neuroblastoma cells. Moreover, we further demonstrated that introduction of TACR1 antagonist (Fosaprepitant) and ERK1/2 inhibitor (PD98059) remarkably weakened RMRP-mediated pro-proliferation effect in neuroblastoma cells. Also, our study demonstrated that clinically relevant third generation MEK1/2 inhibitor Trametinib suppressed cell proliferation and abrogated RMRP-mediated pro-proliferation effect in neuroblastoma cells (Supplementary Figure 1), further suggesting that RMRP facilitated neuroblastoma cell proliferation by activating MEK/ERK pathway. In vivo experiments further revealed that the depletion of RMRP suppressed tumor growth by regulating miR-206/TACR1 axis via inactivating ERK1/2 pathway in mouse xenograft models of neuroblastoma.
Collectively, in the present study, we demonstrated that the silence of RMRP impeded the development and progression of neuroblastoma by sequestering miR-206 from TACR1 via inactivating ERK1/2 signal in vitro and in vivo, providing a deep insight into molecular mechanisms underlying the etiology of neuroblastoma and some potential therapeutic targets for neuroblastoma.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download Zip (253.9 KB)Acknowledgments
None
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83–103. doi:10.3322/caac.21219.
- Irwin MS, Park JR. Neuroblastoma: paradigm for precision medicine. Pediatr Clin North Am. 2015;62:225–256. doi:10.1016/j.pcl.2014.09.01.
- Fisher JP, Tweddle DA. Neonatal neuroblastoma. Semin Fetal Neonatal Med. 2012;17:207–215. doi:10.1016/j.siny.2012.0.002.
- Interiano RB, Davidoff AM. Current management of neonatal neuroblastoma. Curr Pediatr Rev. 2015;11:179–187.
- Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–1261. doi:10.1038/nm.3981.
- Pandey GK, Kanduri C. Long noncoding RNAs and neuroblastoma. Oncotarget. 2015;6:18265–18275. doi:10.18632/oncotarget.421.
- Pandey GK, Mitra S, Subhash S, Hertwig F, Kanduri M, Mishra K, Fransson S, Ganeshram A, Mondal T, Bandaru S, et al. The risk-associated long noncoding RNA NBAT-1 controls neuroblastoma progression by regulating cell proliferation and neuronal differentiation. Cancer Cell. 2014;26:722–737. doi:10.1016/j.ccell.2014.09.014.
- Hermanns P, Reicherter K, Lee B. RMRP (RNA component of mitochondrial RNA processing endoribonuclease). Atlas Genetics Cytogenetics Oncol Haematol. 2008;12.
- Meng Q, Ren M, Li Y, Song X. LncRNA-RMRP acts as an oncogene in lung cancer. PLoS ONE. 2016;11:e0164845. doi:10.1371/journal.pone.016484.
- Shao Y, Ye M, Li Q, Sun W, Ye G, Zhang X, Yang Y, Xiao B, Guo J. LncRNA-RMRP promotes carcinogenesis by acting as a miR-206 sponge and is used as a novel biomarker for gastric cancer. Oncotarget. 2016;7:37812–37824. doi:10.18632/oncotarget.9336.
- Feng W, Li L, Xu X, Jiao Y, Du W. Up-regulation of the long non-coding RNA RMRP contributes to glioma progression and promotes glioma cell proliferation and invasion. Arch Med Sci. 2017;13:1315–1321. doi:10.114/aoms.2017.66747.
- Wang R, Hu Y, Song G, Hao CJ, Cui Y, Xia HF, Ma X. MiR-206 regulates neural cells proliferation and apoptosis via Otx2. Cell Physiol Biochem. 2012;29:381–390. doi:10.119/000338493.
- Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi:10.1016/j.cell.2011.07.014.
- Munoz M, Covenas R. Neurokinin-1 receptor: a new promising target in the treatment of cancer. Discov Med. 10;2010:305–313.
- Munoz M, Covenas R. Involvement of substance P and the NK-1 receptor in cancer progression. Peptides. 2013;48:1–9. doi:10.1016/j.peptides.2013.07.024.
- Munoz M, Rosso M, Covenas R. The NK-1 receptor: a new target in cancer therapy. Curr Drug Targets. 12;2011:909–921.
- Mou L, Kang Y, Zhou Y, Zeng Q, Song H, Wang R. Neurokinin-1 receptor directly mediates glioma cell migration by up-regulation of matrix metalloproteinase-2 (MMP-2) and membrane type 1-matrix metalloproteinase (MT1-MMP). J Biol Chem. 2013;288:306–318. doi:10.1074/jbc.M112.389783.
- Zhang Y, Li X, Li J, Hu H, Miao X, Song X, Yang W, Zeng Q, Mou L, Wang R. Human hemokinin-1 promotes migration of melanoma cells and increases MMP-2 and MT1-MMP expression by activating tumor cell NK1 receptors. Peptides. 2016;83:8–15. doi:10.1016/j.peptides.2016.07.004.
- Novak J, Kruzliak P, Bienertova-Vasku J, Slaby O, Novak M. MicroRNA-206: a promising theranostic marker. Theranostics. 2014;4:119–133. doi:10.710/thno.72.
- Xiao H, Xiao W, Cao J, Li H, Guan W, Guo X, Chen K, Zheng T, Ye Z, Wang J, et al. miR-206 functions as a novel cell cycle regulator and tumor suppressor in clear-cell renal cell carcinoma. Cancer Lett. 2016;374:107–116. doi:10.1016/j.canlet.2016.01.032.
- Pan JY, Sun CC, Bi ZY, Chen ZL, Li SJ, Li QQ, Wang YX, Bi YY, Li DJ. miR-206/133b cluster: A weapon against lung cancer? Mol Ther Nucleic Acids. 2017;8:442–449. doi:10.1016/j.omtn.2017.06.002.
- Pang C, Huang G, Luo K, Dong Y, He F, Du G, Xiao M, Cai W. miR-206 inhibits the growth of hepatocellular carcinoma cells via targeting CDK9. Cancer Med. 2017;6:2398–2409. doi:10.1002/cam4.1188.
- Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi:10.1038/nrg2634.
- Regoli D, Drapeau G, Dion S, D’Orleans-Juste P. Receptors for substance P and related neurokinins. Pharmacology. 1989;38:1–15. doi:10.1159/000138512.
- Fong TM, Anderson SA, Yu H, Huang RR, Strader CD. Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor. Mol Pharmacol. 41;1992:24–30.
- Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev. 2014;94:265–301. doi:10.112/physrev.00031.2013.
- Munoz M, Rosso M, Perez A, Covenas R, Rosso R, Zamarriego C, Piruat JI. The NK1 receptor is involved in the antitumoural action of L-733,060 and in the mitogenic action of substance P on neuroblastoma and glioma cell lines. Neuropeptides. 2005;39:427–432. doi:10.1016/j.npep.200.03.004.
- Akazawa T, Kwatra SG, Goldsmith LE, Richardson MD, Cox EA, Sampson JH, Kwatra MM. A constitutively active form of neurokinin 1 receptor and neurokinin 1 receptor-mediated apoptosis in glioblastomas. J Neurochem. 2009;109:1079–1086. doi:10.1111/j.1471-419.2009.06032.x.
- Munoz M, Perez A, Covenas R, Rosso M, Castro E. Antitumoural action of L-733,060 on neuroblastoma and glioma cell lines. Arch Ital Biol. 142;2004:105–112.
- Henssen AG, Odersky A, Szymansky A, Seiler M, Althoff K, Beckers A, Speleman F, Schafers S, De Preter K, Astrahanseff K, et al. Targeting tachykinin receptors in neuroblastoma. Oncotarget. 2017;8:430–443. doi:10.18632/oncotarget.13440.
- Berger M, Von Schweinitz D. Therapeutic innovations for targeting childhood neuroblastoma: implications of the Neurokinin-1 receptor system. Anticancer Res. 2017;37:5911–5918. doi:10.21873/anticanres.12037.
- Zheng Z, Yan D, Chen X, Huang H, Chen K, Li G, Zhou L, Zheng D, Tu L, Dong XD. MicroRNA-206: effective inhibition of gastric cancer progression through the c-Met pathway. PLoS ONE. 2015;10:e0128751. doi:10.1371/journal.pone.012871.
- Williams R, Zou X, Hoyle GW. Tachykinin-1 receptor stimulates proinflammatory gene expression in lung epithelial cells through activation of NF-kappaB via a G(q)-dependent pathway. Am J Physiol Lung Cell Mol Physiol. 2007;292:430–437. doi:10.112/ajplung.0047.200.
