ABSTRACT
Intrahepatic cholangiocarcinoma (iCCA) is a highly aggressive malignancy with a poor prognosis. There is no standard treatment beyond first-line chemotherapy and no molecular-targeted drug approved for advanced iCCA. We herein present a case of a 46-y-old Asian iCCA patient with multiple metastases in lung, bone, and liver. The patient progressed rapidly after first- and second-line chemotherapy. According to next-generation sequencing result of somatic Von Hippel–Lindau (VHL) gene mutation, the patient was administered third-line sunitinib and obtained a relatively longer survival of 9 months after taking sunitinib. Additionally, we briefly summarized the current targeted treatment of iCCA. To our knowledge, this is the first report of VHL mutation and sunitinib usage in metastatic iCCA patient. As a highly heterogeneous and aggressive malignancy, we strongly recommend making clinical decisions based on precision medicine concept in advanced iCCA.
Introduction
Cholangiocarcinoma (CCA) is a relatively rare but aggressive malignancy that originates from the epithelium of bile ducts. It is reported that CCA cases account for about 3% of all digestive system malignancies and are divided into three groups according to the anatomical location: intrahepatic CCA (iCCA), perihilar CCA (pCCA), and distal CCA (dCCA).1,Citation2 Most CCA patients are diagnosed with advanced stage at presentation and, consequently, lose the opportunity of radical surgery.Citation3 Unfortunately, to date, the available systemic therapies for patients with advanced CCA are of limited effectiveness. As a result, patients with advanced CCA have a very poor prognosis with median overall survival (OS) less than 12 months.Citation4
Tumorigenesis of CCA is associated with genetic and epigenetic alterations.Citation5 About 40% of the patients have potentially targetable genetic driver alterations, including kinases (FGFR1, FGFR2, FGFR3, PIK3CA, ALK, EGFR, ERBB2, BRAF, and AKT3), other oncogenes (IDH1, IDH2, CCND1, CCND3, and MDM2), and tumor-suppressor genes (BRCA1 and BRCA2).Citation6 In iCCA patients, gene mutations in IDH1, IDH2, FGFR1, FGFR2, FGFR3, EPHA2, and BAP1 predominantly occurred. In recent years, rapid signs of progress in whole-exome and transcriptome sequencing have identified promising molecular targets in CCA. Accordingly, several targeted agents are being evaluated in clinical trials.Citation1,Citation5 Herein, we report a female iCCA patient with multiple metastases harboring somatic Von Hippel–Lindau (VHL) gene mutation and receiving third-line sunitinib, who progressed rapidly after first- and second-line chemotherapy.
Case presentation
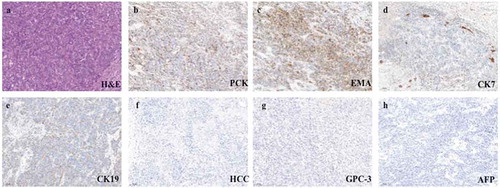
A 46-y-old Asian female patient was admitted to West China Hospital, Sichuan University, in November 2017, complaining of abdominal distension and anorexia for 2 months. Abdominal enhanced magnetic resonance imaging (MRI) showed multiple masses in the left lobe of liver, with the largest diameter of 7.3 cm (). All masses manifested marked rim enhancement at arterial phase and invaded left portal vein. Chest X-ray did not reveal an obvious abnormality. After routine preoperative examination, hepatic left and caudate lobectomy and regional lymphadenectomy were performed on November 30, 2017. Postoperative pathological examination proved poor to moderately differentiated adenocarcinoma from intrahepatic bile duct, invading liver capsular, with immunohistochemistry (IHC) staining of PCK (+), EMA (+), CK7 (partly +), CK19 (partly +), HCC (-), GPC-3 (−), and AFP (−) (). And six lymph nodes involving the left gastric artery, hepatic artery, celiac trunk, and posterior pancreatoduodenal lymph nodes were found to be metastatic. The postoperative primary tumor, regional lymph nodes and distant metastasis (TNM) stage was pT4N1Mx.
Figure 1. Multiple masses in the left lobe of liver revealed by abdominal enhanced MRI in November 2017.
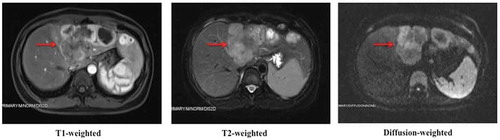
Figure 2. Representative postoperative pathological images of primary tumor (100×; a, hematoxylin and eosin stain; b–h, immunohistochemical stain).
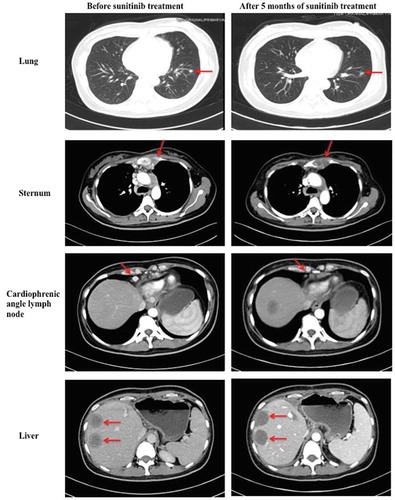
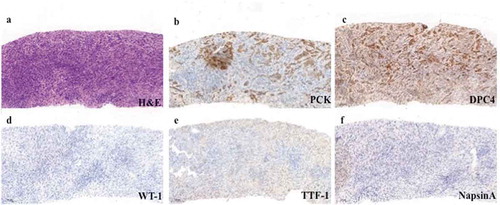
About 1 month after the operation, the patient felt right shoulder pain and activity limitation of the right arm. Enhanced thoracic and abdominal computed tomography (CT) on December 29, 2017, revealed multiple metastases in bilateral lungs, right humerus, left ribs, and sternum. Enlargement of lymph nodes in cardiophrenic angle, hepatogastric ligament, and para-aortic region was also found on CT images. Bone emission computed tomography confirmed multiple bone metastases. Biopsy of sternum mass showed metastatic poorly differentiated adenocarcinoma from intrahepatic bile duct, with IHC staining of PCK (+), CK19 (weak +), DPC4 (+), WT-1 (−), CK5/6 (−), P63 (−), TTF-1 (−), and NapsinA (−) (). Diagnosed with lung and bone metastatic iCCA (pT4N1M1, stage IV), the patient received two cycles of first-line chemotherapy consisting of gemcitabine 1000 mg/m2 on d 1 and 8 and capecitabine 1000 mg/m2 twice a day from d 1 to 14, repeated every 3 weeks (regimen GX). Meanwhile, opioid analgesics and palliative radiotherapy were given to relieve shoulder pain. And zoledronic acid was injected every 3 weeks to prevent pathologic fracture. After two cycles of chemotherapy, CT scan revealed evident enlargement of pulmonary metastases and new lesions in remnant liver. The disease was evaluated as progressed according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1.Citation7 After discussion with the patient and her family, second-line chemotherapy (oxaliplatin 85 mg/m2 on d 1 + irinotecan 150 mg/m2 on d 1 + leucovorin 400 mg/m2 on d 1 + fluorouracil 1200 mg/m2 on d 1 and 2, regimen modified FOLFIRINOX) was administered every 2 weeks. The patient tolerated chemotherapy well and no severe adverse reaction occurred. Unfortunately, in June 2018, after three cycles of modified FOLFIRINOX regimen, imaging examinations showed an increase and enlargement of metastases in lungs, liver, and bones (including spine), indicating progressive disease.
Figure 3. Representative biopsy pathological images of metastatic tumor in sternum (100×; a, hematoxylin and eosin stain; b–f, immunohistochemical stain).
Considering rapid progress and no standard third-line treatment approach, we suggested next-generation sequencing (NGS) of operative specimen and plasma. The results of NGS showed Von Hippel–Lindau (VHL) gene mutation (exon 3 p. L158 V) in operative specimen, but not in plasma, which meant our patient harboring somatic rather than germline mutation in VHL gene. There were no meaningful mutations in other genes including IDH 1/2 and FGFR1/2. Given that VHL mutation was reported to be a predictor of sunitinib effect,Citation8,Citation9 we prescribed sunitinib 37.5 mg once a day as third-line treatment on June 5, 2018. The patient obtained stable disease (SD) after 2 months of sunitinib administration. Moreover, about 3 months later, abdominal CT scan revealed shrinkage of cardiophrenic angle lymph node, pulmonary and sternal metastases, and obvious liquefactive necrosis of liver lesions. The last imaging examination on November 12, 2018, indicated similar lesions, and the response was evaluated as SD (). But unfortunately, about 6 months later, in December 2018, suffering from high fever and severe anorexia, and extremely weak, the patient was sent to a local hospital by her family. And she stopped taking sunitinib on December 12, 2018. The patient received best supportive care in the local hospital and finally died on March 23, 2019. Although our patient suffered from multiple metastases and highly aggressive disease, she obtained a relatively longer OS of 15 months since diagnosed with bone and lung metastatic iCCA and survived 9 months after taking third-line sunitinib.
Discussion
Typically, CCA usually spreads through bile ductal and lymphatic systems, making liver and lymph nodes the most common metastatic locations. Bone metastasis is rare in CCA with a very poor prognosis. It is reported that the median OS of CCA with axial bone metastasis is no longer than 4 months.Citation10,Citation11 Systemic chemotherapy has very limited effectiveness in metastatic CCA. The median OS of first-line gemcitabine- or fluoropyrimidine-based chemotherapy, which is recommended by the present National Comprehensive Cancer Network guidelines, is on longer than 1 y. Besides, there is no standard treatment beyond first-line chemotherapy and no molecular-targeted drug approved for this disease.Citation12 In recent years, some oncologists reported targeted therapy based on uncommon targets revealed by NGS.Citation13,Citation14,Citation15 For instance, Bian et al.Citation15 reported a patient with advanced iCCA harboring PIK3CA mutation who achieved maintenance of a partial response for 6.5 months after the mTOR inhibitor everolimus treatment.
Our patient was diagnosed with multiple metastases in lungs and bones only after 1 month of surgery, which was highly aggressive and rarely seen in clinical practice. Both first- and second-line chemotherapy showed poor effect with a progression-free survival (PFS) of no more than 3 months. Considering lethal characteristics and rapid progress of the disease, we prescribed sunitinib as third-line treatment according to the NGS results of somatic mutation in VHL gene. Fortunately, our patient survived a relatively longer time of 9 months since taking sunitinib and obtained an optimal quality of living.
In fact, CCA has remarkable intertumoral and intratumoral heterogeneity, and nearly 40% of the patients have potentially targetable genetic driver alterations.Citation6 The study by Nakamura et al.Citation6 revealed that in iCCA patients, gene mutations in IDH1, IDH2, FGFR1, FGFR2, FGFR3, EPHA2, and BAP1 predominantly occurred, whereas in pCCA/dCCA, ARID1B, ELF3, PBRM1, PRKACA, and PRKACB, mutations were frequently detected. In recent years, precision medicine based on gene sequencing has achieved rapid development and molecularly oriented clinical trials are ongoing. Several small-molecule FGFR inhibitors like NVP-BGJ398 (infigratinib), erdafitinib, derazantinib, and pemigatinib have been assessed in phase I/II trials with encouraging effect and tolerable adverse reactions.Citation16,Citation17,Citation18,Citation19,Citation20,Citation21,Citation22 According to the updated results from a phase II study of infigratinib (NCT02150967), the objective response rate (ORR) was 26.9% (95% confidence interval (CI): 16.8–39.1%) in patients with previously treated advanced CCA containing FGFR2 fusions.Citation22 Recently, researchers have published the final data of a multicenter, phase II study (FIGHT-202) of pemigatinib for second-line treatment in advanced CCA patients. Patients with FGFR2 fusions or rearrangements achieved an ORR of 35.5% (95% CI: 26.5–45.4%), with median PFS of 6.9 months (95% CI: 6.2–9.6 months) and median OS of 21.1 months (95% CI: 14.8 months to not estimable).Citation21 Besides, there are several clinical trials evaluating agents targeting BRAF, IDH1, MET, etc., gene mutations in advanced CCA.Citation23,Citation24,Citation25 However, to the best of our knowledge, there have been no results published of large-scale phase III trials about targeted agents so far.
VHL gene is a tumor suppressor gene that locates in chromosome 3p25.Citation26 Germline mutations in VHL gene lead to VHL disease, which is an autosomal dominant inherited neoplasia syndrome manifested with multiple benign and malignant tumors, such as retinal and central nervous system (CNS) hemangioblastomas, pheochromocytomas, pancreatic cysts and cystadenomas, pancreatic neuroendocrine tumors, renal cysts, and clear-cell renal cell carcinoma (RCC). The same gene is reported to be inactivated in most cases of sporadic RCC and CNS hemangioblastomas.Citation27,Citation28 Sunitinib, a small-molecule oral TKI, can inhibit multiple targets, such as VEGFR, PDGFR, c-KIT, FLT3, and RET.Citation29 Studies have proved efficacy and safety of sunitinib in the treatment of VHL disease and sporadic RCC.Citation8,Citation9,Citation26,Citation30,Citation31 The study performed by Roma et al.Citation9 showed that first-line sunitinib treatment of VHL disease-related advanced RCC achieved a favorable effect with an overall response rate of 64.3% and a 2-y PFS of 71.4%.
To sum up, the patient in our case suffered from highly aggressive iCCA with multiple metastases in lung, bone (including spine), and liver. Considering rapid progress after two lines of chemotherapy and NGS results of somatic mutation in VHL gene, we prescribed sunitinib as third-line treatment and our patient survived a relatively longer time of 9 months since sunitinib administration. To our knowledge, this is the first report of VHL mutation and sunitinib usage in metastatic iCCA patient. As a highly heterogeneous malignancy, we recommend gene sequencing in advanced iCCA and make clinical decisions according to the precision medicine concept.
No potential conflicts of interest were disclosed
The authors declare that they have no conflict of interest.
Author contributions
Li X and Gou H were the patient’s doctors, reviewed the literature, and wrote the main manuscript. Gao L and Zhang L prepared images and contributed to manuscript drafting. Sun H reviewed the literature and helped with manuscript drafting. All authors reviewed the manuscript and approved its submission.
Informed consent statement
Informed written consent was obtained from the patient for publication of this report and any accompanying images.
- Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma – evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15(2):95–111. doi:10.1038/nrclinonc.2017.157.
- Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–1229. doi:10.1053/j.gastro.2013.10.013.
- Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BSJ, Youssef BAM, Klimstra D, Blumgart LH. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234(4):507–517. doi:10.1097/00000658-200110000-00010.
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, et al. 2010. ABC-0 trial investigators cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 362(14):1273–1281.
- Fouassier L, Marzioni M, Afonso MB, Dooley S, Gaston K, Giannelli G, Rodrigues CMP, Lozano E, Mancarella S, Segatto O, et al. Signalling networks in cholangiocarcinoma: molecular pathogenesis, targeted therapies and drug resistance. Liver Int. 2019;39(Suppl 1):43–62. doi:10.1111/liv.14102.
- Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, Hama N, Hosoda F, Urushidate T, Ohashi S, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47(9):1003–1010. doi:10.1038/ng.3375.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026.
- Jonasch E, McCutcheon IE, Waguespack SG, Wen S, Davis DW, Smith LA, Tannir NM, Gombos DS, Fuller GN, Matin SF. Pilot trial of sunitinib therapy in patients with von Hippel–Lindau disease. Ann Oncol. 2011;22(12):2661–2666. doi:10.1093/annonc/mdr011.
- Roma A, Maruzzo M, Basso U, Brunello A, Zamarchi R, Bezzon E, Pomerri F, Zovato S, Opocher G, Zagonel V. First-Line sunitinib in patients with renal cell carcinoma (RCC) in von Hippel–Lindau (VHL) disease: clinical outcome and patterns of radiological response. Fam Cancer. 2015;14(2):309–316. doi:10.1007/s10689-014-9771-y.
- Dowsiriroj P, Paholpak P, Sirichativapee W, Wisanuyotin T, Laupattarakasem P, Sukhonthamarn K, Kosuwon W, Jeeravipoolvarn P. Cholangiocarcinoma with spinal metastasis: single center survival analysis. J Clin Neurosci. 2017;38:43–48. doi:10.1016/j.jocn.2016.12.048.
- Sangsin A, Saiudom D, Pongmanee S, Saengsin J, Leerapun T, Natural History MH. Prognostic factors of cholangiocarcinoma with spinal metastasis: a 10-year single center study. Clin Spine Surg. 2018;31(3):E160–E165. doi:10.1097/BSD.0000000000000625.
- Saeed A, Park R, Al-Jumayli M, Al-Rajabi R, Biologics SW. Immunotherapy, and future directions in the treatment of advanced cholangiocarcinoma. Clin Colorectal Cancer. 2019;18(2):81–90. doi:10.1016/j.clcc.2019.02.005.
- Loaiza-Bonilla A, Clayton E, Furth E, O’Hara M, Morrissette J. Dramatic response to dabrafenib and trametinib combination in a BRAF V600E-mutated cholangiocarcinoma: implementation of a molecular tumour board and next-generation sequencing for personalized medicine. Ecancermedicalscience. 2014;8:479. doi:10.3332/ecancer.2014.479.
- Lavingia V, Fakih M. Impressive response to dual and mek inhibition in patients with braf mutant intrahepatic cholangiocarcinoma-2 case reports and a brief review. J Gastrointest Oncol. 2016;7(6):E98–E102.
- Bian JL, Wang MM, Tong EJ, Sun J, Li M, Miao ZB, Li YL, Zhu BH, Xu JJ. Benefit of everolimus in treatment of an intrahepatic cholangiocarcinoma patient with a PIK3CA mutation. World J Gastroenterol. 2017;23(23):4311–4316. doi:10.3748/wjg.v23.i23.4311.
- Javle MM, Shroff RT, Zhu A, Sadeghi S, Choo S, Borad MJ, Lowery MA, El-Khoueiry A, Macarulla T, Philip AP. A phase 2 study of BGJ398 in patients (pts) with advanced or metastatic FGFR altered cholangiocarcinoma (CCA) who failed or are intolerant to platinum-based chemotherapy. J Clin Oncol. 2016;34(4_suppl):335. doi:10.1200/jco.2016.34.4_suppl.335.
- Javle M, Lowery M, Shroff RT, Weiss KH, Springfeld C, Borad MJ, Ramanathan RK, Goyal L, Sadeghi S, Macarulla T, et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J Clin Oncol. 2018;36(3):276–282.
- Bahleda R, Italiano A, Hierro C, Mita AC, Cervantes A, Chan N, Awad MM, Calvo E, Moreno VGovindan R, et al. Multicenter phase i study of erdafitinib (jnj-42756493), oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced or refractory solid tumors. Clin Cancer Res. 2019;25(16):4888–4897.
- Mazzaferro V, El-Rayes BF, Cotsoglou C, Harris WP, Damjanov N, Masi G, Rimassa L, Personeni N, Braiteh FS, Zagonel V. ARQ 087, an oral pan-fibroblast growth factor receptor (FGFR) inhibitor, in patients (pts) with advanced intrahepatic cholangiocarcinoma (iCCA) with FGFR2 genetic aberrations. J Clin Oncol. 2017;35(15_suppl):4017.
- Hollebecque A, Borad M, Sahai V, Catenacci DVT, Murphy A, Vaccaro G, Paulson A, Oh D-Y, Feliz L, Lihou C. A Murphy, interim results of fight-202, a phase II, open-label, multicenter study of (Pemigatinib) INCB054828 in patients with previously treated advanced/metastatic or surgically unresectable cholangiocarcinoma (CCA) with/without fibroblast growth factor (FGF)/FGF receptor (FGFR) genetic alterations. Ann Oncol. 2018;29(8):258.
- Vaccaro G, Melisi D, Al-Rajabi R, Paulson AS, Borad MJ, Gallinson D, Murphy AG, Oh DY, Dotan E, Catenacci DV, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020 Mar 20.
- Javle M, Kelley RK, Roychowdhury S, KH W, GK A-A, Macarulla T, Sadeghi S, Waldschmidt D, AX Z, Goyal L, et al. Updated results from a phase II study of infigratinib (BGJ398), a selective pan-FGFR kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma containing FGFR2 fusions. Ann Oncol. 2018;29(Suppl 8):viii720. doi:10.1093/annonc/mdy424.030.
- Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, Wolf J, Raje NS, Diamond EL, Hollebecque A, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. New Engl J Med. 2015;373(8):726–736. doi:10.1056/NEJMoa1502309.
- Lowery MA, Abou-Alfa GK, Burris HA, Janku F, Shroff RT, Cleary JM, Azad NS, Goyal L, Maher EA, Gore L. Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: Results from the cholangiocarcinoma dose escalation and expansion cohorts. J Clin Oncol. 2017;35(15_suppl):4015.
- Valle JW, Bousmans N, Zhang W, Walgren RA, Vogel A. Cisplatin and gemcitabine plus ramucirumab or merestinib or placebo in first-line treatment for advanced or metastatic biliary tract cancer: A double-blind, randomized phase 2 trial. Ann Oncol. 2016;27(suppl_6):712TiP. doi:10.1093/annonc/mdw371.104.
- Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, Oldfield EH. von Hippel-Lindau disease. Lancet. 2003;361(9374):2059–2067. doi:10.1016/S0140-6736(03)13643-4.
- Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, Latif F, Liu S, Chen F, Duh FM. Mutations of the VHL tumor suppressor gene in renal carcinoma. Nat Genet. 1994;7(1):85–90. doi:10.1038/ng0594-85.
- Lee JY, Dong SM, Park WS, Yoo NJ, Kim CS, Jang JJ, Chi JG, Zbar B, Lubensky IA, Linehan WM, et al. Loss of heterozygosity and somatic mutations of the VHL tumor suppressor gene in sporadic cerebellar hemangioblastomas. Cancer Res. 1998;58(3):504–508.
- Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25(7):884–896. doi:10.1200/JCO.2006.06.3602.
- Jimenez C, Cabanillas ME, Santarpia L, Jonasch E, Kyle KL, Lano EA, Matin SF, Nunez RF, Perrier ND, Phan A, et al. Use of the tyrosine kinase inhibitor sunitinib in a patient with von Hippel-Lindau disease: targeting angiogenic factors in pheochromocytoma and other von Hippel-Lindau disease-related tumors. J Clin Endocrinol Metab. 2009;94(2):386–391. doi:10.1210/jc.2008-1972.
- Young AC, Craven RA, Cohen D, Taylor C, Booth C, Harnden P, Cairns DA, Astuti D, Gregory W, Maher ER, et al. Analysis of VHL gene alterations and their relationship to clinical parameters in sporadic conventional renal cell carcinoma. Clin Cancer Res. 2009;15(24):7582–7592. doi:10.1158/1078-0432.CCR-09-2131.