ABSTRACT
Introduction
The hyperthermic intraperitoneal chemotherapy (HIPEC) has been widely applied in clinical practice for peritoneal carcinomatosis (PC). The temperature is one of the important elements affecting the efficacy of HIPEC, and it can become fluctuant by several factors. This study is aimed to explore the role of a stable perfusion temperature in promoting bowel recovery of PC patients due to gastrointestinal malignancies.
Methods
Between January 2012 and July 2013, 59 PC patients undergoing cytoreductive surgery and three-cycle HIPEC were included. Patients having stable perfusion temperature for all cycles were assigned into the study group, with the rest having unstable temperature into the control group. Time of flatus and defecation passage and initiation time of enteral nutrition were compared between both groups to detect the significance in bowel function recovery, with visual analogue scale (VAS) pain intensity and overall survival (OS) compared meanwhile.
Results
In sum, 33 (55.9%) patients obtained stable temperature during HIPEC, and the rest of 26 (44.1%) developed fluctuant perfusion temperature. Average time of flatus (2.3 ± 1.2 vs 3.9 ± 2.2 days, P =.002), defecation passage (5.2 ± 2.1 vs 7.1 ± 2.9 days, P =.004) and enteral nutrition initiation (4.3 ± 1.5 vs 6.7 ± 2.3 days, P <.001) were much shorter in the study group than the control group. Additionally, the VAS score (4.5 ± 2.3 vs 6.3 ± 1.3, P <.001) and 5-year OS rate (17.8% vs 11.1%, P=.135) were both improved, with significance observed in postoperative pain control.
Conclusions
During HIPEC, a precise temperature control could promise an early recovery of bowel function and reduce postoperative pain, without survival significance found based on the current cohort.
Introduction
Digestive malignancies, especially gastric and colorectal cancers, remain leading causes of death worldwide. Once peritoneal carcinomatosis (PC) occurs from advanced gastrointestinal cancers, a poor prognosis must be predicted, often followed by progressive abdominal distension, pain, early satiety, and finally profound cachexia.Citation1 According to previous EVOCAPE I study, the median survival of patients with PC was 3.1 months and 5.2 months for advanced gastric cancer and colorectal cancer, respectively.Citation2 Over the last decades, numerous techniques, such as parietal peritonectomy and intraperitoneal chemotherapy, have been applied to clinical practice.Citation3,Citation4 Recently, several clinical studies have suggested that cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) would be a potential strategy to manage PC from digestive cancers.Citation5–Citation8
Eligible drugs, optimal intraperitoneal perfusion temperature, and sufficient duration constitute the core technique of HIPEC therapy. Although there is no consensus on the optimal temperature, a recognized condition to obtain a thermal homogeneity in the whole abdomen during HIPEC is well accepted.Citation9 Up to the present, only the coliseum technique with a continuous stirring of the viscera by water pump could provide a relatively stable temperature in the in- and out-drains both.Citation10 Nevertheless, such a technique is limited to practice in the theater only with an open abdomen actually. To our knowledge, it is difficult to keep a stable hyperthermia for at least 60 mins, even though such a stirring method has been applied. Several factors, such as body mass index (BMI), numbers of perfusion tubes, flow rate of solutions, and metabolism status, could affect the regional temperature of intraperitoneal chemotherapy. However, few studies have noticed the thermic feature and its associated effects on outcomes.
Uncontrolled hyperthermia may cause some local and systemic side effects to PC patients. However, whether a precise control of perfusion temperature to obtain thermal homogeneity would promise improved outcomes for those patients remains obscured. The aim of the current study is to investigate the effect of stable hyperthermia during HIPEC on postoperative recovery of PC patients from gastrointestinal cancers, with some long-term outcomes also explored meanwhile.
Patients and methods
Patients
This was a single-center, retrospective study on consecutive patients with a confirmed diagnosis of PC due to advanced gastrointestinal cancers. From January 2012 to July 2013, a total of 59 patients had undergone CRS and HIPEC at our center. This study was approved by the Institutional Ethics Committee of our hospital. All included patients provided written informed consents.
Patient selection criteria, as previously published,Citation11 would include all the following: 1) age 20–78 years; 2) Karnofsky performance status (KPS) score >50; 3) life expectancy>3 months; 4) peripheral white blood cell count ≥3,500/mm3 and platelet count ≥80,000/mm3; 5) acceptable liver, renal, cardiovascular, pulmonary, coagulation function, and other major organ functions to endure a major operation; 6) with confirmed histological diagnosis. Of note, the study did not restrict patients with respect to treatment history, tumor TNM staging, or chemotherapy agents.
Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy
After an open abdomen, PC was confirmed and assessed with peritoneal cancer index (PCI) as previously mentioned.Citation12 Afterward, a cytoreductive surgical procedure was performed mainly based on the value of PCI, which ranged from 1 to 39. The level of CRS for gastrointestinal cancer was recorded with the completeness of cancer resection (CCR) score, as previously described.Citation13 Specifically, small nodules on small bowel or mesentery surface were destroyed with beam coagulator. Generally, cisplatin-based hyperthermic therapy was applied to patients with CCR-1 and above, with hyperthermic therapy alone to CCR-0 resected patients.
Catheters, including inflow and outflow tubes, were crossly placed into the abdominal cavity prior to primary abdominal closure. Hence, in our study, a closed technique was utilized to protect the surgical incision and to reduce heat loss during HIPEC. Generally, HIPEC treatment was commonly started at Postoperative day (POD) 1 or 2, with three cycles performed in total within three consecutive days. Note that gut decompression with nasogastric tube or enema was only applied for patient suspicious bowel obstruction. HIPEC was administrated with an automatic hyperthermia perfusion device (BR-TRG-1, Baorui Medical Technology Company, P.R. China), as previously described.Citation13 An enclosed hyperthermia circuit between the device and the abdominal cavity was established with 2–4 perfusion tubes, with 3000 ml 43°C solutions flowing in the loop. The abdominal temperature was checked in real-time with two thermometers placed aside to in- and out-tube, respectively, with 45°C above not allowed. HIPEC lasted for 60 or 90 min each time, with three cycles suggested by default. The duration of 60 and 90 min was applied to patients with CRS-0/1 and CRS-2, respectively. During HIPEC, patients were not sedated but were closely monitored. They were supplied with intravenous non-opioid analgesics, such as acetaminophen and flurbiprofen, to relieve pain. The supine position was used to start the procedure, without any restriction or agitation to the abdomen throughout the perfusion. Thereafter, all tubes were removed gradually in the next 2–3 days.
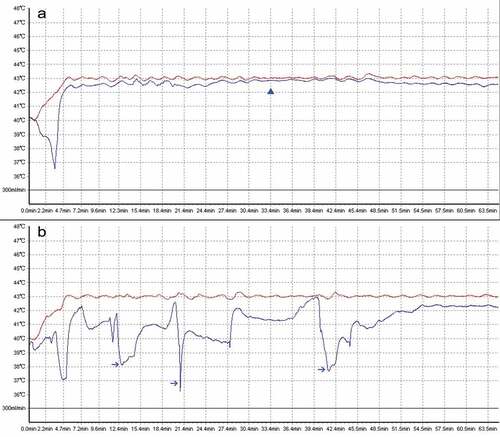
In this study, a stable perfusion temperature was defined as a change of temperature in outflow drain to 43°C not exceeding 0.5°C (43 ± 0.5°C) during the whole process of HIPEC (). Patients with stable perfusion temperature in all three-cycle HIPEC were divided into study group, with the rest into control group.
Figure 1. The demonstration of a thermal homogeneity during HIPEC. A. A typical stable perfusion temperature was obtained. The actual temperature in outflow drain (blue curve) almost equals with the setting temperature in inflow drain (red curve). The blue triangle indicates a stable perfusion temperature; B. A typical unstable perfusion temperature was observed by real-time monitoring out-drain temperature. Those blue arrows indicate extensive fluctuations beyond 1°C to default 43°C.
Primary and secondary outcomes
After HIPEC, all patients were closely monitored for the following parameters: unpleasant complaints, vital signs, bowel sound, flatus passage, drainage, and any adverse events. The complete blood count and chemistry panel were routinely examined on the second day after the treatment, with repeat tests performed every 3 days. The primary outcome was the time of bowel function recovery after HIPEC, which was mainly evaluated by a definitive flatus description from the patient. The secondary outcomes included defecation time, postoperative pain (via visual analogue scale, VAS) score, length of postoperative stay, hospital expenditure, incidence of postoperative complications, and three-year overall survival (OS).
Once a flatus passage recorded, an oral feeding was attempted from a trial liquid diet, gradually changed to semi-liquid and soft diet. Chemotherapy was routinely started within 3 weeks after CRS and HIPEC, with a specific regimen selected based on primary histological report. All patients were routinely followed up every 3 months for the first year, every 6 months for the second year and twice a year thereafter. The routine exams in each visit were similar as to our previously published.Citation14 The OS time ranged from the confirmed diagnosis of PC until the last contact after CRC and HIPEC or the date of an informed death.
Statistical analysis
Data were expressed as mean±SD if not indicated otherwise. Clinical parameters between the two groups were compared by chi-square test or Fisher’s exact test for categorized variables, with Student’s t-test or Mann–Whitney U test performed for continuous variables. The Kaplan–Meier method was utilized to explore OS rate and potential prognostic factors, with log-rank test. All data analyses were performed by IBM SPSS software (Ver. 23.0; Chicago, IL) and GraphPad (Ver. 7.0; GraphPad Software Inc, San Diego, California, USA), with a P value of <0.05 considered statistically significant.
Results
Baseline data and surgical intervention
Within the study period, a total of 59 consecutive patients who had undergone CRS and HIPEC treatments were included for the final analysis. Among this cohort, 33 (55.9%) patients had precise control of perfusion temperature during all three cycles of HIPEC, with the rest of 26 (44.1%) patients suffered at least once from unstable hyperthermia. Of note, 29 (49.1%) patients received simplex continuous hyperthermic intraperitoneal perfusion, whereas the rest underwent standard HIPEC with 60 mg/m2 cisplatin. The demographic and surgical characteristics between the two groups are summarized in . Simply, age, gender, body mass index (BMI), ASA score, number of drains, and PCI and CCR scores were similar in both treatment groups (P > .05); however, a stable perfusion temperature was more frequently achieved in PC patients from gastric cancer than those from colorectal cancer (81.8% vs 40.5%, P = .003).
Table 1. Baseline and demographic features of patients with peritoneal carcinomatosis.
Bowel function recovery and early outcomes
As mentioned above, the bowel function recovery after CRS and HIPEC was evaluated mainly by time of flatus, time of defecation, and time to initiate enteral nutrition. In this cohort, the median time of flatus was 3 (range, 2–7) days. It was markedly shortened in the study group compared with the control group (2.3 ± 1.2 vs 3.9 ± 2.2 days, P = .002). Similarly, the median time of defecation and initiation of enteral nutrition were 4 (range, 2–10) days and 3 (range, 2–9) days, respectively. Compared with the control group, both time intervals were significantly shortened (). Besides, surgical drains were removed earlier in the study group than in the control group. The length of postoperative stay was significantly shorter for patients with stable perfusion temperature than for patients with unstable perfusion temperature (10.6 ± 3.2 vs 14.6 ± 5.0 days, P = .002). Although the average expenditure in the study group was lower than in the control group, no significant difference was observed (P = .581). In addition, a logistic regression analysis indicated that demographic characters except tumor location were not associated with the thermal homogeneity during HIPEC.
Table 2. Comparison of outcomes in PC patients after CRS and HIPEC treatments.
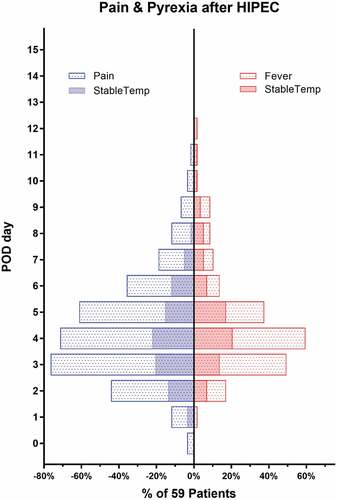
Postoperative complications
After HIPEC, postoperative abdominal pain and low fever were commonly observed, with 76.3% and 64.4% of 59 patients recorded, respectively. Our data showed that most of abdominal pain or pyrexia complaints occurred at postoperative day (POD) 2 or 3 after HIPEC and then vanished almost within 3 days. The daily incidence of pain or pyrexia complaints was relatively lower in the study group than that in the control group either (). The VAC score at POD 3 in the study group was obviously lower than that in the control group (4.5 ± 2.3 vs 6.3 ± 1.3, P < .001). Postoperative complications were observed in 10 patients (6 for pneumonia, 5 for surgical site infection, 3 for adhesive bowel obstruction, and 2 for anastomotic leak). No additional operation was performed for those patients. There was no significant difference in postoperative complication incidence between the two groups (). Of note, HIPEC associated kidney injury was observed in 6 (10.2%) patients in the current study, with 5 cases in the subgroup of cisplatin-based HIPEC. A subgroup analysis () indicated that cisplatin-based HIPEC did not impact on the thermal homogeneity (P = .558), bowel function recovery (P = .242), and length of postoperative stay (P = .675).
Table 3. Postoperative complication comparisons after CRS and HIPEC treatments.
Table 4. Comparison of results in patients with cisplatin used or not during HIPEC procedure.
Long-term outcomes
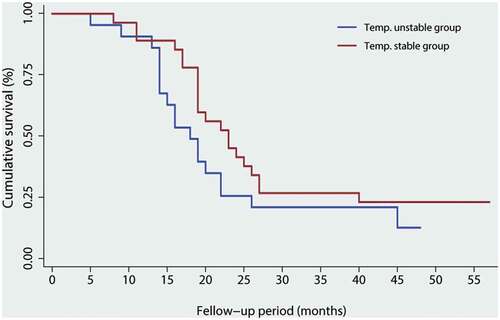
After CRS and HIPEC treatments, disease recurrence was found in 36 (61.0%) patients after a median period of 8.2 months (mean 6.2 months, range 2 to 41 months). Among those, 15 patients and 21 patients had gastric cancer and colorectal cancer, respectively. The median OS for 59 patients was 38 months (range, 5 to 57 months). By using Kaplan-Meier log-rank test (), the estimated 5-year OS in the study group was slightly improved compared with the control group (17.8% vs 11.1%, P = .135). Besides, univariate analyses of 3-year OS rates were performed according to age, gender, BMI, ASA, tumor location, tumor grade, cisplatin HIPEC, thermal homogeneity, and adjuvant chemotherapy. It has shown that older age (P = .014), lower BMI (P = .035), colorectal cancer (P = .027), and higher grade (P = .041) were associated poorer outcomes in such cohort (). However, the thermal homogeneity in the current study was not a determining factor of survival significance (HR, 1.594; 95%CI, 0.975–2.437).
Table 5. Univariate analysis of predictive factors for the overall survival of PC patients from gastrointestinal cancers.
Discussion
In the current study, patients who underwent CRS and HIPEC treatments were retrospectively reviewed. During HIPEC, 55.9% of 59 patients had a stable perfusion temperature and considered as the study group. Compared with those with thermal heterogeneity, postoperative pain was reduced, and time of flatus, enteral nutrition initiation, and postoperative stay were all shortened significantly. Additionally, the OS in the study group was improved compared with the control group; however, there was no significant difference. By univariate analyses, thermal homogeneity was not associated with improved long-term outcomes.
The current combined treatments for PC from gastrointestinal cancer involve the cytoreductive surgery to reduce tumor burden first, followed by intraperitoneal application of chemotherapy with thermic isotonic solutions for at least 60 min.Citation15 The goal of this regional therapy was an eradication of microscopic residual disease within abdomen and pelvis. The widespread application of these treatments for gastrointestinal peritoneal metastases has been generously recognized by numerous studies.Citation16 For the last two decades, much more attentions have been paid to drug selection, optimal temperature, and duration; however, technical details during each HIPEC, such as stability of flow rate and perfusion temperature, heat circulation dynamics, and distribution, are commonly neglected, with their roles on treatment outcomes rarely found. Some interesting studies on heat changes during HIPEC showed that hyperthermia actually had a limited penetration depth on the abdominal wall, with various temperature distributions amongst the various abdominal sites meanwhile.Citation17,Citation18
As previously reported, malignant cells would be selectively destroyed by continuous hyperthermia in the range of 41°C to 43°C, and the microcirculation in tumor niches would attenuate, even complete shutdown, in response to stable hyperthermia.Citation19,Citation20 Besides, there is also evidence that uniformed heat might affect cellular metabolism and change cytotoxic parameters of perfused drug in HIPEC.Citation21 A recent experiment indicated that HIPEC therapy might not only kill tumor cells but also enhance anticancer immune response via exposure of heat shock protein 90.Citation22
On the other hand, as a “double-edged sword,” some side effects of HIPEC therapy would occur if intraperitoneal hyperthermia was not well controlled. These side effects include: 1) Heat-associated injuries; 2) Edema of the intestinal wall, which further leads to ileus, bowel obstruction, bowel perforation, even fistula; 3) abdominal symptoms, such as persistent abdominal distension, pain, even generalized peritonitis; 4) delayed abdominal wound healing, even wound infection if overheated solutions immersed into the abdominal wall. In order to reduce its incidence, as always emphasized, a real-time monitor of vital signs and perfusion parameters should be seriously implemented in clinical practice. Recent evidence strongly suggested a sophisticated multidisciplinary team including experienced surgeons and medical oncologists should be established for HIPEC to better control hyperthermia and drug selection individually.
To our best knowledge, some technical details during HIPEC should be noticed to detect and correct thermal homogeneity problem. First, omentum should be excised as much as possible to reduce the incidence of disease recurrence and out-flow drain stuck by floated omentum. Second, prophylactic pain control should be applied to keep the abdomen maximally distended during HIPEC. Besides, to our knowledge, a hemi-recumbent position should be advocated during HIPEC to better use of gravity for solution drainage. Third, the flow rate of perfusion should be high enough to achieve relatively thermal homogeneity. An animal study using swine model indicated that higher flow rate could cause more rapid heating of peritoneum and greater peritoneal temperature gradients. In the clinical study, it was associated with improved peritoneal heating with relatively lower visceral temperature meanwhile.Citation23 Finally, the catheters used for perfusion and drainage should be wide enough in diameter and placed cross bi-direction into the abdomen. To our knowledge, two in- and out-flow drains would be the optimal numbers in default configuration.
The limitations of this study stemming from its retrospective design should be addressed. As a single-center study, a potential selection bias would be unavoidable, and the relatively small sample size would compromise the power of our findings. Besides, the treatment strategy was quite variable due to the surgeon’s preference and the patient’s personal choice. As a result, long-term survival significance based on the current categorization may be confounded. Of note, the current applied three-cycle HIPEC is not widely used compared with one-cycle fashion. Hence, it would be insufficient to generalize the findings to a larger HIPEC cohort. Finally, in clinical practice, unstable perfusion temperature whenever noticed should be corrected instantly via any ways to protect the benefits of patients. This reality would produce an ethical obstacle to start a prospective study. As a result, other similar studies with the retrospective design either may be integrated together to perform a meta-analysis if available.
In summary, the findings demonstrate that HIPEC is a safe regional therapy for PC patients from gastrointestinal malignancies. A stable perfusion temperature during HIPEC could reduce postoperative pain, and importantly promote the recovery of bowel function for those cohorts.
Disclosure of potential conflicts of interest
The authors report no conflict of interest.
Authors’ contributions
Conceived and designed the experiments: HY, YY. Performed the study: YJ, YY, CL, CS. Analyzed the data: YY, CC, HY, SW. Contributed reagents/materials/analysis tools: PJ, CL, CC, YY, HY. Drafted the paper: All authors. Designed the software used in the analysis: YY, CL, SW. Revision and Response: YY. All authors read and approved the final manuscript.
Meeting presentation
Abstract has been presented at the ESMO World Congress on Gastrointestinal Cancer 2017 and published in Annals of Oncology, 28, Issue suppl_3, 2017, mdx261.170 (https://doi.org/10.1093/annonc/mdx261.170).
Acknowledgments
We thank Mrs. Donglian Chen and Mrs. Qiongyun Zhao for her dedicated works in telephone follow-up and dataset management. We also thank Mrs. Shenyan Wu for language editing and sentence polishing of the manuscript.
Availability of data and materials
The datasets used during the current study available from the corresponding author on reasonable request.
Additional information
Funding
References
- Glockzin G, Ghali N, Lang SA, Agha A, Schlitt HJ, Piso P. 2007. Peritoneal carcinomatosis. Surgical treatment, including hyperthermal intraperitoneal chemotherapy. Chirurg. 78(12):1100,1102–1106, 1108–1110. DOI:10.1007/s00104-007-1419-0.
- Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL, et al. 2000. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 88(2):358–363. DOI:10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o
- Sugarbaker PH. 2012. Parietal peritonectomy. Ann Surg Oncol. 19(4):1250. DOI:10.1245/s10434-012-2229-2.
- Gilly FN, Carry PY, Sayag AC, Brachet A, Panteix G, Salle B, Bienvenu J, Burgard G, Guibert B, Banssillon V, et al. 1994. Regional chemotherapy (with mitomycin C) and intra-operative hyperthermia for digestive cancers with peritoneal carcinomatosis.. Hepatogastroenterology. 41(2):124–129.
- Dunn D. 2010. Surgical treatment of patients with peritoneal surface malignancy: cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. J Wound, Ostomy Continence Nurs. 37(4):379–385. DOI:10.1097/WON.0b013e3181e399fe.
- Wu X-J, Yuan P, Li Z-Y, Bu Z-D, Zhang L-H, Wu A-W, Zong X-L, Li S-X, Shan F, Ji X, et al. 2013. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves the survival of gastric cancer patients with ovarian metastasis and peritoneal dissemination. Tumour Biol. 34(1):463–469. DOI:10.1007/s13277-012-0571-4
- Goere D, Malka D, Tzanis D, Gava V, Boige V, Eveno C, Maggiori L, Dumont F, Ducreux M, Elias D, et al. 2013. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg. 257(6):1065–1071. DOI:10.1097/SLA.0b013e31827e9289
- Chia CS, You B, Decullier E, Vaudoyer D, Lorimier G, Abboud K, Bereder J-M, Arvieux C, Boschetti G, Glehen O, et al. 2016. Patients with peritoneal carcinomatosis from gastric cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is cure a possibility? Ann Surg Oncol. 23(6):1971–1979. DOI:10.1245/s10434-015-5081-3
- Kusamura S, Dominique E, Baratti D, Younan R, Deraco M. 2008. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 98(4):247–252. DOI:10.1002/jso.21051.
- Elias D, Raynard B, Boige V, Laplanche A, Estphan G, Malka D, Pocard M. 2005. Impact of the extent and duration of cytoreductive surgery on postoperative hematological toxicity after intraperitoneal chemohyperthermia for peritoneal carcinomatosis. J Surg Oncol. 90(4):220–225. DOI:10.1002/jso.20253.
- Liu Y, Ishibashi H, Takeshita K, Mizumoto A, Hirano M, Sako S, Takegawa S, Takao N, Ichinose M, Yonemura Y, et al. 2016. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal dissemination from small bowel malignancy: results from a single specialized center. Ann Surg Oncol. 23(5):1625–1631. DOI:10.1245/s10434-015-5056-4
- Harmon RL, Sugarbaker PH. 2005. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int Seminars Surg Oncol. 2(1):3. DOI:10.1186/1477-7800-2-3.
- Ye J, Ren Y, Wei Z, Peng J, Chen C, Song W, Tan M, He Y, Yuan Y. 2018. Nephrotoxicity and long-term survival investigations for patients with peritoneal carcinomatosis using hyperthermic intraperitoneal chemotherapy with cisplatin: A retrospective cohort study. Surg Oncol. 27(3):456–461. DOI:10.1016/j.suronc.2018.05.025.
- Song W, Yuan Y, Wang L, He W, Zhang X, Chen C, Zhang C, Cai S, He Y. 2014. The prognostic value of lymph nodes dissection number on survival of patients with lymph node-negative gastric cancer. Gastroenterol Res Pract. 2014: 603194. DOI:10.1155/2014/603194.
- Sugarbaker PH. 2016. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: progress toward a new standard of care. Cancer Treat Rev. 48: 42–49. DOI:10.1016/j.ctrv.2016.06.007.
- Gonzalez-Moreno S, Gonzalez-Bayon LA, Ortega-Perez G. 2010. Hyperthermic intraperitoneal chemotherapy: rationale and technique. World J Gastrointest Oncol. 2(2):68–75. DOI:10.4251/wjgo.v2.i2.68.
- van Ruth S, Verwaal VJ, Hart AA, van Slooten GW, Zoetmulder FA. Heat penetration in locally applied hyperthermia in the abdomen during intra-operative hyperthermic intraperitoneal chemotherapy. Anticancer Res. 2003;23(2B):1501–1508.
- Rettenmaier MA, Mendivil AA, Gray CM, Chapman AP, Stone MK, Tinnerman EJ, Goldstein BH. 2015. Intra-abdominal temperature distribution during consolidation hyperthermic intraperitoneal chemotherapy with carboplatin in the treatment of advanced stage ovarian carcinoma. Int J Hyperthermia. 31(4):396–402. DOI:10.3109/02656736.2015.1007399.
- Sticca RP, Dach BW. 2003. Rationale for hyperthermia with intraoperative intraperitoneal chemotherapy agents. Surg Oncol Clin N Am. 12(3):689–701. DOI:10.1016/s1055-3207(03)00029-2.
- de Bree E, Tsiftsis DD. 2007. Principles of perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis. Recent Results Cancer Res. 169: 39–51. DOI:10.1007/978-3-540-30760-0_4.
- Benoit L, Duvillard C, Rat P, Chauffert B. 1999. The effect of intra-abdominal temperature on the tissue and tumor diffusion of intraperitoneal cisplatin in a model of peritoneal carcinomatosis in rats. Chirurgie. 124(4):375–379. DOI:10.1016/s0001-4001(00)80009-4.
- Zunino B, Rubio-Patino C, Villa E, Meynet O, Proics E, Cornille A, Pommier S, Mondragon L, Chiche J, Bereder J-M, et al. 2016. Hyperthermic intraperitoneal chemotherapy leads to an anticancer immune response via exposure of cell surface heat shock protein 90. Oncogene. 35(2):261–268. DOI:10.1038/onc.2015.82
- Furman MJ, Picotte RJ, Wante MJ, Rajeshkumar BR, Whalen GF, Lambert LA. 2014. Higher flow rates improve heating during hyperthermic intraperitoneal chemoperfusion. J Surg Oncol. 110(8):970–975. DOI:10.1002/jso.23776.