ABSTRACT
Colorectal cancer (CRC) is the third most common cancer in the world. More than half of all affected patients develop liver metastases during the course of the disease, and over half experience recurrence despite radical primary surgery. Transketolase-like protein 1 (TKTL1) is a key enzyme in the glucose metabolism of cancer cells, and its expression in tumor tissue was previously shown to indicate a poor prognosis in colorectal cancer. In this study, we investigated the prognostic significance of TKTL1 in 111 patients with surgically resected colorectal liver metastases, with a minimum follow-up time of 10.3 years. TKTL1 expression was examined in tissue samples of both primary tumors and liver metastases, and compared to clinicopathological parameters, disease-free survival, and overall survival. We show that a high expression of TKTL1 in primary tumor tissue associated with poor disease-free survival in patients with synchronous liver metastases (P = .026, Kaplan-Meier log-rank test), but with better disease-free survival in patients with metachronous metastases, although not statistically significantly (P = .073). We found similar tendencies for TKTL1 expression in liver metastases. Thus, TKTL1 could serve as a candidate marker to identify patients who benefit from liver resection or who need more aggressive perioperative chemotherapy.
Introduction
Colorectal cancer (CRC) is the third most common cancer in the world with over 1.8 million new cases diagnosed annually and causing around 881 000 deaths every year.Citation1 More than 50% of patients with CRC will eventually develop liver metastases. Resection of liver metastases in CRC is recommended when R0 resection is technically possible, leaving a minimum of 30% of the liver remnant. Chemotherapy should be combined to resection perioperatively in most cases, whereby even initially non-resectable metastases (that is, borderline resectable cases) may become resectable following chemotherapy. However, about half of all patients develop recurrent disease within 3 years following liver resection.Citation2 The prognosis of patients with stage IIIc to IV disease has improved in recent decades due to more aggressive operative treatment and more precise chemotherapy, where overall survival at 5 years now reaches over 50% following curative-intent liver resection.Citation3,Citation4
Glucose metabolism in cancer cells differs from that in normal cells. Transketolase-like protein 1 (TKTL1), an isoform of transketolase, is one of the key factors in catalyzing the non-oxidative part of the pentose phosphate pathway in cancer cells.Citation5 Previously, we showed that TKTL1 serves as a prognostic marker in colorectal cancer, such that patients with a high tumor expression of TKTL1 had a poor prognosis.Citation6 Here, we aimed to investigate whether TKTL1 serves as a similar marker of poor prognosis among patients with colorectal cancer metastasized to the liver.
Results
Patient characteristics
Between September 1988 and June 2007, colorectal primary tumors were operated on 111 patients, 64 (58%) of whom were men (). The liver resections were performed between August 1997 and July 2007. At the time of the liver resection, 53 (48%) patients were over 65 years old. The primary tumor was located in the rectum in 48 (43%), in the left colon or rectosigmoid junction in 44 (40%), and in the right or transversal colon in 19 (17%) cases. The median time from the operation on the primary tumor to the liver resection was 11.1 months (range 0.7 months–10.3 years).
Of the rectal cancer patients (N = 48), 8 (17%) received neoadjuvant chemoradiotherapy before the operation on the primary tumor (data missing from 10 (21%) patients). Half of all patients (N = 53; 48%) received adjuvant chemotherapy after the operation on the primary tumor (data missing from 26 (23%) patients). Before and after the liver resection, 68 (61%) patients received neoadjuvant chemotherapy (data missing from 4 (4%) patients), and 85 (77%) adjuvant chemotherapy (data missing from 9 (8%) patients) ().
Table 2. Characteristics of the patients’ neoadjuvant and adjuvant treatment in conjunction with liver resection.
Median survival time following liver resection was 5.7 years (2.4 months–19.3 years). Overall survival at 5 years was 60% (67/111) and at 10 years was 37% (41/111). The median follow-up time for living patients was 14.1 years (range 10.3–19.3 years).
During follow-up, 80 patients (72%) experienced recurrence, 17 of whom were already diagnosed within 3 months, 53 within 3 years, and 10 later. The most common site for recurrence was the liver (57%); new metastases were diagnosed in other sites in 43% of cases.
TKTL1 expression in tissue samples
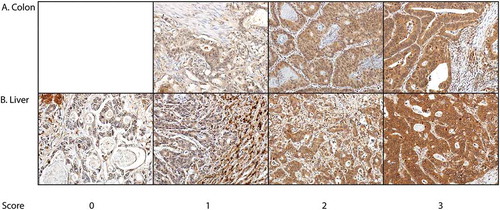
Of the 81 primary tumor samples, none were scored as negative, 8 (10%) as weakly positive, 48 (59%) as moderately positive, and 25 (31%) as strongly positive. Among the 90 liver metastases samples, 5 (6%) were scored as negative, 15 (17%) as weakly positive, 42 (47%) as moderately positive, 27 (30%) as strongly positive, and one sample was excluded from further analysis. We found no significant correlation between the TKTL1 expression of patients’ primary tumor and liver metastases using the Spearman’s rank correlation test (data not shown).
For the statistical analyses, the samples with negative, mild or moderate expressions were grouped as “low” and those with a strong expression as “high”.
Survival analyses
Across the entire cohort, we found no statistically significant relationship between TKTL1 expression in either primary tumors or liver metastases and 1) disease-free survival (DFS) or 2) overall survival (OS) in the Kaplan-Meier log rank or the Cox regression analysis. We also found no association between these when patients were divided into subgroups according to the primary tumor location, that is, left- and right-sided colon and rectal cancer.
Comparison between synchronous and metachronous disease
Patients presenting with synchronous liver metastases and a high TKTL1 expression in primary tumors exhibited shorter DFS compared to patients with a low expression (P = .030, hazard ratio (HR) 2.14, 95% confidence interval (CI) 1.08–4.25; )). A high TKTL1 expression in the liver metastases did not associate with a shorter DFS (P = .081, Kaplan-Meier log-rank test; )). However, only 12% (2/17) of the patients with a high expression in the liver metastases were alive without recurrence 3 years following liver resection compared to 30% (10/33) of those with a low expression. This same association was seen for OS, although this was not statistically significant.
Figure 1. TKTL1 expression in primary CRC tumors in relation to disease-free survival. Patients with (a) synchronous liver metastases (N = 50) and (b) metachronous liver metastases (N = 31).
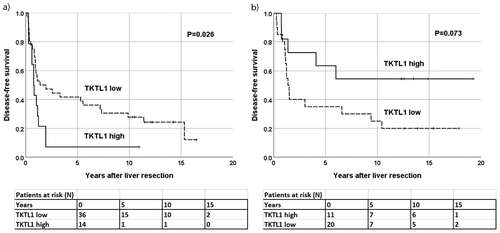
Figure 2. TKTL1 expression in liver metastases of CRC in relation to disease-free survival. Patients with (a) synchronous liver metastases (N = 50) and (b) metachronous liver metastases (N = 39).
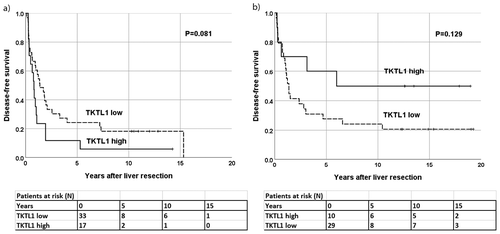
In metachronous disease, patients with a high TKTL1 expression in the primary tumors exhibited a better DFS, although this finding was not statistically significant (P = .084, HR 0.41, 95% CI 0.15–1.13; )). A high TKTL1 expression in the liver metastases did not associate with a better DFS (P = .129, Kaplan-Meier log-rank test; )), although 70% (7/10) of patients with a high expression were alive without recurrence 3 years later compared to 32% (9/28) of those with a low expression. In OS, we found no significant differences between the high and low expression groups (data not shown).
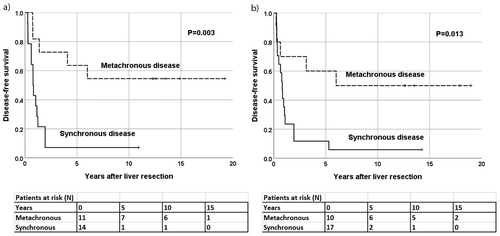
When we analyzed only those patients with a high TKTL1 expression in their primary tumors (N = 25), those with synchronous liver metastases had a significantly worse DFS than those with metachronous liver metastases (P = .005, HR 4.66, 95% CI 1.57–13.8; )). This was also evident in patients with a high TKTL1 expression in the liver metastases (N = 27); DFS was worse among those with synchronous metastases compared to metachronous metastases (P = .019, HR 3.55, 95% CI 1.23–10.2; ). We found no similar differences for subgroups of patients with a low TKTL1 expression in the primary tumors or liver metastases.
Figure 3. Patients with a high TKTL1 expression in (a) primary CRC tumors (N = 25) and (b) liver metastases (N = 27). Synchronous and metachronous disease in relation to disease-free survival.
When comparing the subgroups of patients who received adjuvant chemotherapy with those who did not and further dividing them into groups based on TKTL1 score, we found no statistically significant differences in overall survival. In patients who had synchronous disease and received adjuvant chemotherapy after liver resection, a high TKTL1 expression in primary tumors or in liver metastases associated with a worse DFS than low TKTL1 expression (P = .015 and P = .027, respectively). On the contrary, in patients who had metachronous disease and received adjuvant chemotherapy, a high TKTL1 expression in liver metastases associated with a better DFS (P = .034).
Discussion
In our patient cohort, a high TKTL1 expression served as a marker of poor prognosis in patients with synchronous liver metastases of colorectal cancer. By contrast, in patients with metachronous liver metastases, a high expression in both the primary tumor and liver metastases indicated a better prognosis. In other cancers, a high TKTL1 expression was shown to indicate a poor prognosis.Citation7–Citation11 Thus, our results provide a new dimension to this marker.
Previous studies demonstrated that metastatic colorectal cancer patients with synchronous liver metastases have a worse prognosis than patients with metachronous metastases.Citation12 This was apparent in our study as well: only 29% (19/66) of all patients with synchronous liver metastases were alive without recurrence 3 years after liver resection, whereas that percentage increased to 44% (20/45) for patients with metachronous disease. However, OS at 10 years after liver resection was 33% (22/66) among patients with synchronous metastases and 42% (19/45) among those with metachronous metastases, indicating that patients with recurrent disease can also live several years thanks to current surgical and oncological treatments.
In our previous study of TKTL1 in colorectal cancer, we showed that patients with a high TKTL1 expression in the primary tumors exhibited a worse prognosis compared to those with a low expression.Citation6 Most patients, however, had local or locally advanced disease, and only 190 (23%) had stage IV CRC at the time of diagnosis. In the current study, all patients had advanced disease.
When we analyzed DFS in patients with a low TKTL1 expression, we observed no obvious difference between patients with synchronous and metachronous disease. Instead, when the TKTL1 expression was high, the difference became apparent. When the TKTL1 score was analyzed from the primary tumors, DFS at 3 years reached only 7% (1/14) for patients with a high TKTL1 score and synchronous disease; among those with a high TKTL1 score and metachronous disease, DFS reached 73% (8/11). The same difference was seen for TKTL1 expression in liver metastases. These findings suggest that synchronous and metachronous mCRC are different types of disease, possibly explaining the reverse prognostic significance of TKTL1.
In a previous study by Diaz-Moralli et al., the expression of TKTL1 in primary colorectal tumors was studied in both local and metastatic disease. They noticed that in CRC stages I through III TKTL1 expression increased progressively, but in stage IV there was a strong decrease in expression.Citation13 A similar pattern was also found in the incidence of mutated RAS in CRC. Thus, Diaz-Moralli et al. argued that RAS mutations might lead to the activation of TKTL1 expression, possibly permitting carcinogenic cells to consume glucose in the absence of oxygen, produce lactate, and acidify their microenvironment, thereby increasing the invasiveness of cancer. Based on those findings, it seems that the expression pattern of TKTL1 changes when colorectal cancer becomes metastatic. This supports our findings concerning the differences between synchronous and metachronous disease. When patients are diagnosed with a primary colorectal tumor and liver metastases at the same time or within 6 months, a high TKTL1 expression serves as a negative prognostic marker. Yet, if liver metastases appear later, this signals a better prognosis. As such, we speculate that a high TKTL1 expression increases CRC’s invasiveness and the metastatic ability. When patients have a high expression in their primary tumors but no synchronous metastases, however, there may be some other factor that strongly diminishes the cancer’s metastatic ability.
The strengths of our study lie in the unique material consisting of tissue samples of both the primary colorectal tumors and corresponding liver metastases and a long follow-up time. Yet, the patient material is quite small, and the subgroups of patients with synchronous and metachronous disease are even smaller. Information concerning the possible neoadjuvant and adjuvant therapies was not available for all patients, and the subgroups of patients with different treatments or no treatment were so small that we were not able to estimate the effect of possible chemotherapy on either TKTL1 expression or survival reliably. However, in the subgroups of patients with synchronous or metachronous disease, nearly all patients had received neoadjuvant and/or adjuvant chemotherapy. In addition, we did not have information concerning the patients’ RAS and BRAF mutation status or the mismatch repair (MMR) protein status of the tumors, since these were not routinely analyzed in our clinic prior to 2007.Citation14 Future research should examine TKTL1 expression and the RAS mutational status in a larger material of colorectal tumor samples.
The weakness in immunohistochemistry as a study method is that the scoring is always subjective, and the staining scale from zero to three is gradual. To maximize objectivity and to validate the scoring, we used two researchers who independently scored the staining pattern of the tissue samples, and we scored three samples from each tumor.
In conclusion, it seems that a high TKTL1 expression in the primary colorectal tumor in patients with synchronous liver metastases signifies a poor prognosis to such an extent that it might be reasonable to consider aggressive chemotherapy instead of liver resection. In metachronous disease, it remains unclear if a high expression alone indicates a better prognosis or if it reflects other invasiveness-reducing characteristics that we have yet to identify.
Materials and methods
Patients
This study included 111 patients who had both primary colorectal tumors and liver metastases operated on at Helsinki University Hospital between September 1988 and July 2007. The primary colorectal tumors were operated on between 1988 and 2007, and the liver metastases between 1997 and 2007. Tissue samples of both the primary tumors and liver metastases were obtained from 60 patients, samples of the primary tumor only from 21 patients, and samples of the liver metastases only from 30 patients.
The Ethics Committee of the Helsinki University Hospital approved the study protocol (Dnro HUS 226/E6/06, extension TMK02 §66 17.4.2013). Collection and analysis of tissue samples were approved by the National Supervisory Authority for Welfare and Health (Valvira Dnro 10041/06.01.03.01/2012). Clinical data were retrieved from patient records.
Preparation of tissue microarray samples and immunohistochemistry
Formalin-fixed and paraffin-embedded tissue samples were obtained from the Department of Pathology of the Helsinki University Hospital. For each patient, one tumor sample of the primary CRC tumor and another from the liver metastases were selected. Representative tumor and healthy tissue areas were marked on hematoxylin and eosin slides with assistance from an experienced pathologist (JH). Six punches (1 mm in diameter) were taken from each sample’s tumor area, when possible from the invasion-front areas. The tissue microarray (TMA) blocks were constructed using a TMA Grand Master 3D instrument (Histech Ltd Budapest, Hungary). Two series of blocks were constructed, each containing three tumor samples from each patient’s 1) colorectal tumor blocks and 2) liver metastasis blocks.
From the TMA blocks 4-µm-thick sections were cut, fixed on to slides, and dried for 12 to 24 h at 37°C. Sections were subsequently deparaffinized in xylene and rehydrated through graded ethanol and distilled water. For antigen retrieval, the slides were treated with Tris-HCl (pH 8.5) in a PreTreatment module (LabVision Corp.) for 20 min at 98°C. Staining of sections took place in an Autostainer 480 (LabVision) with an anti-human TKTL1 antibody (Rida Pentocheck IHC, Clone JFC12T10, R-Biopharm AG) diluted to 1:200 with Dako REAL Antibody Diluent S2022 (Dako). The primary antibody was kept on glass overnight (O/N) followed by a 30-min incubation with the secondary peroxide-conjugated rabbit/mouse ENV (K5007) Dako REAL Envision/HRP antibody (Dako). Finally, the slides were visualized with Dako REAL DAB + Chromogen kept on glass for 10 min. Between each step of the staining procedure, slides were washed with PBS 0.04% Tween20. The slides were counterstained with Meyer’s hematoxylin, washed in tap water for 10 min, and finally mounted in an aqueous mounting medium (Aquamount, BHD). We used a gastric and a colon cancer specimen known to be positive for TKTL1 as the positive control in each staining.
Scoring
The immunohistochemically stained tumor samples were scored independently by KA and JH using no clinical data or information. In liver tissue, TKTL1 staining was visible in epithelial cells, but also in the stroma and inflammatory cells. The cytoplasmic positivity of the epithelial tumor cells was scored on a four-grade scale, where the absence of staining was scored as 0, mild staining as 1, moderate staining as 2, and strong staining as 3 (). The highest score was selected to represent the tumor. Samples with no tumor tissue or with too few cells for adequate judging were excluded. Samples receiving different scores from the two researchers were reevaluated until consensus was reached.
Statistical analyses
We used SPSS Statistics 25 (SPSS Inc., Chicago, IL, USA) for all statistical analyses and to create the figures. We considered P < .05 as statistically significant, and a statistical trend was defined as P < .1. DFS and OS were calculated from the date of each patient’s first liver resection. DFS was defined as the time to any recurrence of CRC or death from any cause. OS was calculated until death from any cause. Follow-up data were available for all patients, and we set the data cutoff date as October 31, 2017.
Survival curves were created using the Kaplan-Meier method, and the differences between groups were assessed using the log-rank test. The survival analyses were also carried out using the Cox proportional hazards model. For these analyses, tumor samples with negative, mild or moderate TKTL1 expressions were grouped as “low” and those with a strong expression as “high”. The correlations between the immunohistochemical expression and clinicopathological variables were calculated using the Spearman’s correlation test.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Päivi Peltokangas for her excellent technical assistance and Harri Mustonen for his advice on the statistical analyses. This study was financially supported by the State Research Funding (RP, CH, and HI), the Finnish Cancer Foundation (RP), Suomen Onkologiayhdistys (RP), Finska Läkaresällskapet (KA, CB, and CH), the K. Albin Johansson Foundation (KA and CB), the Sigrid Jusélius Foundation (CH), and Medicinska Understödsföreningen Liv och Hälsa (CH).
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;49:509.
- Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi:10.1093/annonc/mdw235.
- House MG, Ito H, Gönen M, Fong Y, Allen PJ, DeMatteo RP, Brennan MF, Blumgart LH, Jarnagin WR, D’Angelica MI. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744–752. doi:10.1016/j.jamcollsurg.2009.12.040.
- Peltonen R, Österlund P, Lempinen M, Nordin A, Stenman U-H, Isoniemi H. Postoperative CEA is a better prognostic marker than CA19-9, hCGβ or TATI after resection of colorectal liver metastases. Tumor Biol. 2018;40(1):1010428317752944. doi:10.1177/1010428317752944.
- Xu X, Hausen Zur A, Coy JF, Löchelt M. Transketolase-like protein 1 (TKTL1) is required for rapid cell growth and full viability of human tumor cells. Int J Cancer. 2009;124:1330–1337. doi:10.1002/ijc.24078.
- Ahopelto K, Böckelman C, Hagström J, Koskensalo S, Haglund C. Transketolase-like protein 1 expression predicts poor prognosis in colorectal cancer. Cancer Biol Ther. 2016;17:163–168. doi:10.1080/15384047.2015.1121347.
- Kayser G, Sienel W, Kubitz B, Mattern D, Stickeler E, Passlick B, Werner M, Hausen Zur A. Poor outcome in primary non-small cell lung cancers is predicted by transketolase TKTL1 expression. Pathology. 2011;43:719–724. doi:10.1097/PAT.0b013e32834c352b.
- Chen H, Yue J-X, Yang S-H, Ding H, Zhao R-W, Zhang S. Overexpression of transketolase-like gene 1 is associated with cell proliferation in uterine cervix cancer. J Exp Clin Cancer Res. 2009;28:43. doi:10.1186/1756-9966-28-43.
- Schwaab J, Horisberger K, Ströbel P, Bohn B, Gencer D, Kähler G, Kienle P, Post S, Wenz F, Hofmann W-K, et al. Expression of Transketolase like gene 1 (TKTL1) predicts disease-free survival in patients with locally advanced rectal cancer receiving neoadjuvant chemoradiotherapy. BMC Cancer. 2011;11:363. doi:10.1186/1471-2407-11-363.
- Lange CA, Tisch-Rottensteiner J, Böhringer D, Martin G, Schwartzkopff J, Auw-Haedrich C. Enhanced TKTL1 expression in malignant tumors of the ocular adnexa predicts clinical outcome. Ophthalmology. 2012;119(9):1924–1929. doi:10.1016/j.ophtha.2012.03.037.
- Grimm M, Munz A, Teriete P, Nadtotschi T, Reinert S. GLUT-1+/TKTL1+ coexpression predicts poor outcome in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:743–753. doi:10.1016/j.oooo.2014.02.007.
- Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L, Sobrero A, et al. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev. 2015;41:729–741. doi:10.1016/j.ctrv.2015.06.006.
- Diaz-Moralli S, Tarrado-Castellarnau M, Alenda C, Castells A, Cascante M. Transketolase-like 1 expression is modulated during colorectal cancer progression and metastasis formation. PLoS ONE. 2011;6(9):e25323. doi:10.1371/journal.pone.0025323.
- Gong J, Cho M, Fakih M. RAS and BRAF in metastatic colorectal cancer management. J Gastrointest Oncol. 2016;7:687–704. doi:10.21037/jgo.2016.06.12.