 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Suicide gene therapy using gene-directed enzyme prodrug therapy (GDEPT) is based on delivering a gene-encoded enzyme to cells that converts a nontoxic prodrug into its toxic metabolite. The bystander effect is thought to compensate for inefficiencies in delivery and expression because the produced toxic metabolite can spread to adjacent non-expressing cells.
The purpose of this study was to assess the significance of bystander effect in GDEPT over the long term in vivo.
We performed experiments using mixtures of yeast cytosine deaminase (yCD) expressing and empty vector (EV) containing cells. First, the bystander effect was assessed in various ratios of colon cancer cell lines RKO with yCD/EV in 2D and 3D culture. Next, tumors raised from RKO with yCD/EV in mice were treated with the prodrug 5-fluorocytosine (5-FC) for 42 days to assess bystander effect in vivo. Cell types constituting relapsed tumors were determined by 5-FC treatment and PCR.
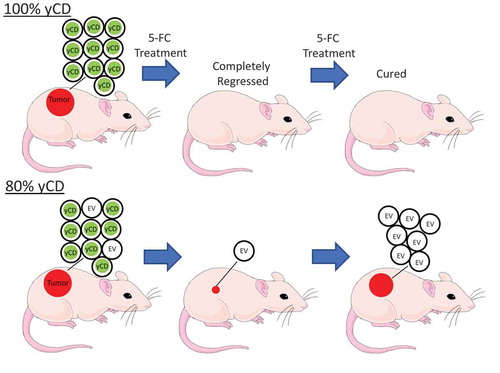
We were able to demonstrate bystander effect in both 2D and 3D. In mice, tumors initially regressed, but they all eventually recurred including those produced from 80% yCD expressing cells. Cells explanted from the recurrent tumors demonstrated that suicide gene expressing cells had been selected against during in vivo treatment with 5-FC.
We conclude that gene therapy of malignant tumors in patients using the yCD/5-FC system will require targeting well over 80% of the malignant cells, and therefore will likely require improved bystander effect or repeated treatment.
Graphical Abstract
Introduction
A variety of gene therapy approaches have attempted to eliminate cells based on their genotype, including gene-based immunotherapy,Citation1 oncolytic viruses,Citation2 anti-gene padlocks,Citation3 CRISPR-Cas,Citation4 and gene-directed enzyme/prodrug therapy (GDEPT).Citation5 One can consider gene therapy of cancer as a three-step problem: first, targeting the malignant cells; second, gene expression in successfully targeted cells; and third, killing them. Investigators have used a variety of methods to deliver gene therapy to malignant cells, including viral delivery,Citation6 nanoparticles,Citation7 immunoliposomes.Citation8 However, delivery efficiency and other issues have prevented routine use of gene therapy with rare exceptions.Citation9,Citation10 Toxicity can be considered a separate issue (i.e. what percent of cells die, assuming that 100% of cells are successfully targeted?).
GDEPT is based on the selective delivery of a foreign enzyme to malignant cells followed by treatment with a well-tolerated prodrug that is activated by the enzyme.Citation5 Historically, the most commonly used GDEPT systems are the HSV-TK (herpes simplex virus-THYMIDINE KINASE)/ganciclovir (GCV) and the CYTOSINE DEAMINASE (CD)/5-fluorocytosine (5-FC). GCV is phosphorylated by HSV-TK and the toxic GCV metabolite induces cell cycle arrest.Citation11 Another GDEPT combination is the enzyme cytosine deaminase (CD) combined with the prodrug 5-fluorocytosine (5-FC).Citation12 Since human cells lack this enzyme, cancer selectivity occurs by delivering exogenous bacterial or yeast CD (yCD) to the malignant cells. CD converts 5-FC into the commonly used chemotherapeutic agent 5-fluorouracil (5-FU), and 5-FU is metabolized either to 5-fluorouridine triphosphate (5-FUTP), which inhibits RNA synthesis, or 5-fluorodeoxyuridine triphosphate (5FdUTP), which inhibits DNA synthesis.
The bystander effect (also known as negative cooperativity) is the killing of adjacent non-enzyme producing cancer cells by enzyme-producing cells, thereby relaxing the requirement to literally deliver the virus to every malignant cell within a tumor. For example, the HSV-TK product GCV triphosphate can spread to adjacent cells, but its spread is dependent on gap junctions that functionally connect neighboring cells.Citation13,Citation14 A theoretical advantage of the CD/5-FC system is that 5-FU can diffuse through the plasma membrane to virus-negative neighboring cancer cells and cause bystander toxicity.Citation15 Reports on the degree of bystander are impressive, many papers claiming that as few as only 2–10% enzyme-expressing cells are sufficient to produce tumor regression.Citation13,Citation14,Citation16,Citation17
In this study, we attempted to demonstrate bystander effect over the long term in vivo. We eliminated the delivery issue by using stably transfected cells, as others have done, and used the reliable yeast CD (yCD)/5-FC system. While we were able to demonstrate bystander effect in vitro and in vivo in the short term, we were unable to demonstrate it in vivo over the long term. Mixed tumors showed initial regression, but subsequently progressed in tumor size with additional time. We tested individual cells explanted from recurrent tumors and demonstrate in vivo selection of non-yCD expressing cells in the mixed tumors. Our data suggest that effective in vivo targeting of cancer using the yCD/5-FC system will require delivery and expression in the vast majority of the malignant cells.
Results
Conversion of 5-FC to 5-FU by yCD expressing cells, and toxicity to other cells
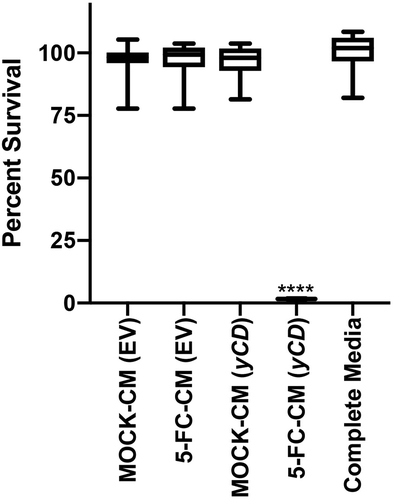
We first confirmed that the previously characterized yCD containing cells were capable of converting 5-FC to 5-FU. We expanded single flow-sorted yCD positive cells and then exposed empty vector or yCD expressing cells to 5-FC at 100 or 1000 μM for 8 or 24 hours and measured 5-FU and 5-FC concentrations in the culture supernatant (). As expected, the empty vector cells were incapable of converting 5-FC to 5-FU, while the yCD expressing cells converted it in a time- and dose-dependent manner. The optimal conversion was with 1000 μM for 24 hours, and a corresponding decrease in the 5-FC concentration was also seen. To demonstrate that this conditioned media would be toxic to other cells, we collected the media and tested it for toxicity (). As expected, the only conditioned media that was toxic was the one collected from yCD expressing cells that had been exposed to 5-FC. The other control conditioned media showed essentially no effect as expected (~100% survival).
Table 1. 5-FU concentration after incubation with EV or yCD cells
Figure 1. yCD +5-FC conditioned media toxicity testing. EV or yCD cells were fed with media with or without 5-FC and conditioned media collected. The cells were treated with the various undiluted conditioned media (12 replicate wells) and viable cells detected using CellTiter-Glo® (ATP quantification) luminescent cell viability assay. Cell toxicity was compared to complete media. ****: p < .0001
5-FC chemosensitivity of mixed cells in 2D cell culture
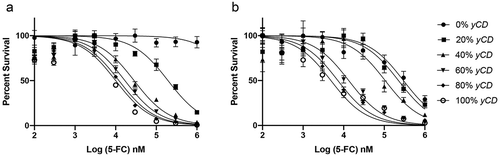
To assess potential bystander toxicity of the yCD/5-FC GDEPT system, we plated cells at varying ratios of yCD expressing and non-expressing (empty vector) cells in 96-well plates. We then treated these cultures with increasing concentrations of 5-FC (from 100 nM to 1 mM), generated killing curves, and calculated IC50s (). Wells composed of 100% empty-vector cells demonstrated resistance to 5-FC at extremely high concentrations (up to 1 mM), whereas cultures containing pure yCD transfected cells demonstrated dose-dependent chemosensitivity to 5-FC as a sigmoid curve (IC50 = Mean ± SD, 9.3 ± 0.7 µM). The killing curve of wells with 80% yCD (IC50 = Mean ± SD, 11.7 ± 2.3 µM) containing cells was essentially superposed on the 100% yCD cells curve (p = .149). The wells with 60% yCD (IC50 = Mean ± SD, 20.2 ± 10.3 µM) closely tracked with 100% yCD and 80% yCD (p = .140), whereas those with 20% yCD were clearly intermediate and distinct from both 100% yCD and 100% EV killing curves. We interpret that the clustering of 100%, 80%, and 60% yCD containing cells on the left side of the graph is consistent with a bystander effect.
Figure 2. Chemosensitivity in 2D (a) and 3D (b) culture. yCD and EV cells were mixed at various ratios respectively, 100%/0%, 80%/20%, 60%/40%, 40%/60%, 20%/80%, 0%/100%. Cells were treated with at increasing doses of 5-FC for 6 days, and viable cells detected using CellTiter-Glo® (ATP quantification) luminescent cell viability assay. Each sample was tested in 6 replicates and the experiment was repeated on 3 different days (one representative run shown). Error bars represent SE. Note the decrease in survival of 0% yCD in 3D culture at high doses of 5-FC
Chemosensitivity of 5-FC in mixed cultures grow as 3D spheroids
As a better model of in vivo growth, we grew mixed cells as 3D spheroids in hanging drop plates. Following spheroid formation, 5-FC was added at increasing concentrations, and after 6 days, the cells were sacrificed and ATP quantified (). Spheroids composed of 100% yCD containing cells showed toxicity with an IC50 of 4.8 µM (Mean ± SD, 4.8 ± 1.7 µM). Spheroids with 80% yCD (IC50 = Mean ± SD, 8.4 ± 2.0 µM, p = .073 vs. 100% yCD) or 60% yCD (IC50 = Mean ± SD, 11.2 ± 5.9 µM, p = .143 vs. 100% yCD) also clustered at the left side of the graph near 100% yCD, whereas spheroids that were 40% yCD cells were clearly intermediate between 100% yCD and 0% yCD. The 20% yCD graph was almost superimposable on the 0% yCD curve. These findings are qualitatively similar to those in 2D culture. In contrast to 2D results, 100% EV cell spheroids demonstrated some toxicity to 5-FC at the highest doses. The loss of surviving cells suggests that they have acquired deaminase activity through some unknown mechanism, or that the parent drug 5-FC is directly toxic at these doses by a novel mechanism. Insight into this could be obtained by adding uridine, a competitive inhibitor of 5-FU, where if toxicity was reversed would support the hypothesis that deamination was occurring.Citation18
Initial regression of mixed tumors in mice suggests bystander activity
We raised tumors using mixtures of yCD-expressing and non-expressing cells: 100% yCD, 80% yCD/20% EV, 60% yCD/40% EV, 40% yCD/60% EV, 20% yCD/80% EV, and 100% EV in 12 mice for each mixture. Tumors were monitored weekly by measuring them with calipers and calculating tumor volume. When tumors reached a volume of approximately 200 mm3, we assigned the mice to equal groups based on the size of the tumors, so that the mean tumor volume was equivalent in the two groups. One group was treated with 5-FC at 500 mg/kg/day/IP in PBS and the other treated with diluent alone (PBS).
The tumors in the PBS treated control mice (irrespective of yCD/empty vector ratios) all grew rapidly and the mice had to be sacrificed at around 21 days (, Supplemental Figure 1). One hundred percent EV and 100% yCD tumors grew similarly (p = .042 on day 21) and 100% EV treated with 5-FC grew 7.1 times larger on day 21 compared to the initial volume (Supplemental Figure 1f). On the other hand, the raw tumor volume of 80% yCD/20% EV and 60% yCD/40% EV were reduced by 27% and 63% times, respectively, on day 21, compared to the initial volume ().
Figure 3. Response of yCD/empty vector mixed tumors to 5-FC treatment. Tumors were raised from mixtures of yCD containing cells injected in six mice each bilaterally. After initial growth they were assigned to two groups by balancing tumor volume, and treated with diluent (saline) or 5-FC (500 mg/kg/day, IP) as represented by raw tumor volume (a) and relative tumor volume (b). Note initial regression in the 60% and 80% yCD mice, followed by expansion (20% yCD and 40% yCD tumors are presented in Supplemental ). Tumor volumes were compared to 100% yCD bearing cells after Day 35. *:p < .05, **:P < .001. Saline indicates the average of all controls. Error bars represent SE
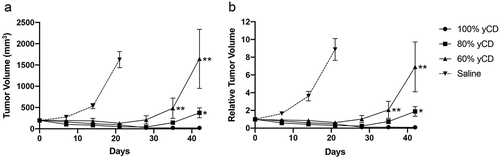
Under the assumption of no bystander effect and zero remaining yCD cells on day 21, the relative tumor volumes of 80% yCD and 60% yCD should be approximately 1.5 (7.6 *0.2, 100% EV relative tumor volume * percent EV cells injected) and 3.1 (7.6 *0.4), respectively (). However, the relative tumor volumes were substantially smaller in 80% yCD (80.3% reduction compared to 1.5) and in 60% yCD (79.5% reduction compared to 3.1) than predicted. Indeed, as 100% yCD regressed, both 80% yCD and 60% yCD tumors similarly regressed until days 21 and 28, respectively, although, the trough of even 80% yCD curve during initial regression did not go completely to zero ().
Additional evidence of bystander effect was shown by the displacement in time of the 20% yCD containing tumors (Supplemental Figure 1e). The degree of displacement exceeds that which would be expected if all of the yCD expressing cells were completely eliminated by the 5-FC and none of the EV cells were eliminated. We consider this evidence of the bystander effect in the short term.
Recurrence of mixed tumors on long-term treatment
However, all tumors raised from mixtures of yCD expressing cells and empty vector bearing cells subsequently relapsed, despite continued 5-FC treatment. In fact, the length of time to relapse went as a function of the percent of yCD cells in the tumors. For example, the 80% yCD tumors relapsed later when compared to the 60% yCD tumors (). When compared to 100% yCD bearing cells, 80% yCD and 60% yCD tumor volumes became significantly larger (p = .039 and 0.0088, respectively). The tumors were harvested following recurrence 2 months after initial treatment, minced and placed into culture.
The 100% yCD tumors showed complete or nearly complete regression. For three of these tumors in two mice with complete regression, the mice were released from 5-FC and maintained for over 1 year without any regrowth. Two mice with four extremely small tumors were released from 5-FC treatment, during which time their tumors expanded. Following expansion, the mice were again treated with 5-FC and tumors continued to grow in the presence of 5-FC treatment, suggesting that these tumors had become resistant to 5-FC.
Analysis of individual cells from explanted tumors
We attempted to identify the cells in tumors that expanded in mice treated with 5-FC. For 80% yCD and 60% yCD tumors, we explanted two tumors each after 5-FC treatment and two tumors each from the PBS controls. After expansion in flasks, we sorted the cells into single cells in 96-well plates. Following expansion, we selected 22–24 individual clones for each condition and split them into replicate wells containing nothing, G418, 5-FC or 5-FU. Following growth for 2 weeks, the wells were scored for growth by phase microscopy (). In the control wells, 22–24 wells were positive for each of the conditions, and the vast majority of these were G418 resistant, indicating that they had retained the transfected vector. All of the cells were 5-FU sensitive, indicating that none of them had developed resistance to 5-FU. From the PBS treated mice, the majority of the wells were 5-FC sensitive, consistent with the fact that these tumors were comprised of mostly yCD transfected cells. However, in the 5-FC refractory tumors, the vast majority of the wells grew in the presence of 5-FC, indicating that the cells were not yCD positive. Therefore, these results suggest that in mixed tumors refractory to 5-FC in vivo, the yCD bearing cells were mostly eliminated by 5-FC treatment and enough EV cells survived the treatment to subsequently expand. PCR using primers that spanned the yCD insert confirmed the functional results (Supplemental Figure 2). Taken together, we interpret this data as in vivo selection against yCD expressing cells, and survival of enough EV cells (by evading bystander effect) to regenerate the tumor.
Table 2. Single-cell analysis of explanted tumors
Discussion
Using cells with stable expression of yCD, we demonstrated a clear bystander effect in vitro, documented by superimposition of killing curves, and by direct testing of conditioned media from yCD expressing cells exposed to 5-FC. Mouse xenografts showed significant initial regression from bystander activity, but this was followed by recurrence in all tumors raised from mixtures of yCD and EV containing cells. The vast majority of cells in the relapsed tumors were not yCD expressing, which we interpret as in vivo selection against the yCD expressing cells. Because empty vector cells grew out from all of the recurrent tumors, we conclude that they were not completely eliminated by bystander activity. Tumors produced from 100% yCD expressing cells could be cured, including in mice maintained for over a year.
Bystander activity was demonstrated in vivo at early time points. Because the treated tumors were substantially smaller (80.3% for 80% yCD and 79.5% for 60% yCD reduced) than predicted (see results), we conclude that a significant fraction of EV cells were eliminated by bystander effect in the presence of 5-FC and the yCD cells. Furthermore, the displacement in time of the 20% yCD expressing tumors treated with 5-FC compared to PBS controls provides additional support of bystander effect during initial treatment. In addition, the higher the %yCD content of the tumor, the lower the trough during the initial phase of treatment. These findings are consistent with the literature reporting a strong bystander effect, even when only 2–10% of cells express the prodrug-converting enzyme.Citation13,Citation14,Citation16,Citation17
However, on longer-term 5-FC treatment, all tumors produced from mixtures of yCD expressing and non-expressing cells eventually recurred, and the recurrence was delayed as a function of the percent of yCD cells in the tumors (i.e. the 80% yCD tumors relapsed the latest). Cells isolated from these tumors demonstrated selective elimination of the yCD cells and enrichment of EV-containing cells within the relapsing tumors. This was demonstrated both functionally and by genotyping. One caveat in interpreting this data is that our tumors were raised from 5 million cells in total, which took approximately 10 days until randomization and treatment. Accordingly, we would not expect that an empty vector cell would be immediately surrounded by 4 yCD expressing neighboring cells in the 80% yCD tumors, but rather the tumor would be composed of “micro-nodules” of identical cells in 1:4 ratio in these tumors. Accordingly, the treatment of 5-FC might result in the elimination of the yCD expressing nodules, but not permit the effective diffusion of 5-FU into neighboring EV nodules. This could be an ideal model for viral delivery to heterogeneous tumors containing hypoxic regions with relatively low vascularity.
Clinical trials using GDEPT are ongoing and show some promise.Citation19,Citation20 The majority of these trials are with the CD/5-FC system. In March 2019, there were 16 ongoing clinical trials using the CD/5-FC system. Most of these are phase 1 trials, although the Toca 5 trial (NCT02414165) is in phase 2/3 for glioblastoma multiforme and anaplastic astrocytoma. This trial involves resection of the brain tumor followed by 40 injections of a yCD containing retrovirus around the tumor bed combined with oral delivery of an extended release 5-FC drug. This trial began in November 2015 and is scheduled to be completed in March 2023. A phase 2 trial in prostate cancer (NCT00583492) based at Henry Ford and Johns Hopkins Hospitals has reported promising initial results.Citation20 There are almost an equal number (12 trials) using the HSV-tk with gancyclovir or valacyclovir.
There are other advancements and novel applications in the field. There are two potential methods which are currently being investigated, which may circumvent the requirement of almost all cells to be infected for effective yCD/FC therapy. We searched for yCD/FC trials in clinicaltrials.gov, and identified active or completed clinical trials using the yCD/5-FC system. The majority of the phase 2 or higher (6 out of 7) used multiple doses of yCD delivered using viruses or bacteria (Supplemental Table 1). Some of these employed local injections, which might have helped to minimize a systemic immune response. In elegant work by Meliani and colleagues,Citation21 they co-delivered synthetic viral particles encapsulated the drug rapamycin along with AAV vectors and avoided a systemic immune response. Another advance gets around the requirement that the gene-encoding enzyme must be selectively delivered to the majority of the malignant cells and not delivered to normal non-cancer cells. An alternative approach is the use of protein switches. While GDEPT requires selective delivery to cancer cells, with the protein switch approach, the selectivity is conferred by the switch itself. Thus, the switch-encoding gene can be delivered to all cells because the enzymatic activity is only switched on in the presence of a cancer-specific marker. For example, from a library of recombinant chimeric proteins between a HIF-1 binding protein and yCD, Ostermeier and colleagues selected several clones in which cytosine deaminase activity was dependent on the presence of HIF-1.Citation22,Citation23 Another creative application of suicide gene therapy is the introduction of the suicide gene (HSV-TK or inducible Cas9) system into lymphocytes used in donor lymphocyte infusions, in an attempt to prevent graft-versus-host disease, a complication that can be fatal (NCT00423124, NCT00914628, NCT01086735, NCT02477878, and NCT01875237).
In conclusion, the bystander effect was confirmed both in vitro and at early time points in vivo. However, with additional treatment, mixed tumors recurred and the yCD expressing cells were selected against leaving behind only EV containing cells. These data suggest that successful clinical application of suicide gene therapy, at least that using the cytosine deaminase with 5-FC system, will require further improvements in bystander effect and/or repeated viral delivery.
Materials and methods
Cell culture
RKO cellsCitation24 were obtained (ATCC, Manassas, VA) and its identity confirmed by DNA fingerprinting profile using AmpFLSTR® Profiler® PCR Amplification Kit (Life Technologies, Foster City, CA). DNA encoding human codon optimized yeast cytosine deaminase (yCD) was cloned into the pcDNA™3.1 (+) plasmid (Invitrogen, Carlsbad, CA) containing neomycin resistance. The yCD also contains three additional mutations that confer thermostability (A26L, V108I, and I140L as reported by Korkegian and colleagues.Citation25 The creation of RKO cells expressing empty vector (RKO-EV, hereafter EV) and yCD (RKO-yCD, hereafter yCD) was previously reported.Citation22,Citation23 yCD and EV cells were maintained in culture media containing 400 µg/ml G418 sulfate (Corning, Manassas, VA). All experiments were performed using clones from single yCD cells sorted by flow cytometry, plated in 96-well plates, expanded, and confirmed to be sensitive to 5-FC.
Conditioned media experiment
Empty vector cells or yCD cells were plated in a 6-well plate at 1.8 million cells per well to obtain 90–95% confluency after 24 hours. Media was changed to 1 ml of 100 or 1000 μM 5-FC and incubated for 8 or 24 hours. Conditioned Media (CM) were harvested and sterilized by 0.22-μm syringe filtration and were added without dilution to EV cells in a 96-well plate for 6 days (fed with new CM on day 3), and cell viability assessed by the ATP quantification assay as described below. Cell toxicity was evaluated by comparing to complete media with unpaired t-test using GraphPad Prism 8 (GraphPad Software, Inc., La Jolla, CA).
Measurement of 5-fluorocytosine and 5-fluorouracil concentrations in cell culture media
5-Fluorocytosine (5-FC) and 5-fluorouracil (5-FU) were extracted from 100 µL of cell culture media with 1 mL of acetonitrile containing the internal standards 5-chlorouracil (5CU) for 5-FU and 5-aza-2ʹ-deoxycytidine-15N4 for 5-FC. All analytes were purchased from Toronto Research Chemicals (Toronto, Ontario, Canada). After centrifugation, the supernatant was evaporated then reconstituted with 200 µL of acetonitrile (LCMS grate, EMD Millipore Co., Billerica, MA):water (Milli-Q water, Millipore, Milford, MA) (1:1, v/v). Chromatographic separation of the analytes achieved with a Atlantis C18 column (100 mm x 2.1 mm, 3 μm; Waters Corporation, Milford, MA) and isocratic elution with a 10 mm ammonium acetate (J.T. Baker, Phillipsburg, NJ):acetonitrile with 0.1% formic acid (Analytical/LCMS grade, Sigma Aldrich, St Louis, MO) (1:1, v/v) mobile phase at a flow rate of 0.25 mL/min over a 2 min total analytical run time. An AB Sciex 5500 triple quadrupole mass spectrometer (SCIEX, Framingham, MA) operated in positive electrospray ionization mode was used for the detection of 5-FC and 5-aza-2ʹ-deoxycytidine15N4. The mass spectrometer operated in negative electrospray ionization mode for 5-FU and 5CU. The mass spectrometer was programmed to monitor the following multiple reaction monitoring transitions: 128.9→41.9 for 5-FU, 144.8 →41.9 for 5CU, 130.0 →58.0 for 5-FC and 249.0→117.0 for 5-aza-2ʹdeoxycytidine15N4. The calibration curve was computed using area ratio peak of the analyte to the internal standard by using a 1/xCitation2 weighting function over the range of 10–2,000 ng/mL with dilutions of up to 1:100.
In vitro mixing assays in 2D cell culture
Sensitivity of the cells to 5-fluorocytosine (5-FC) (Sigma-Aldrich®, St. Louis, MO) was evaluated by the ATP-based CellTiter-Glo® Luminescent Cell Viability Assay (Promega, Madison, WI). yCD and EV were mixed at ratios of 10:0, 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9, 0:10, respectively. The cells were plated in 96-well plates, at 1,500 cells per well, in 50 µl of culture media. After 48 hours, 50 µl of culture media was treated with 5-FC at the final concentration of 0, 0.1 µM, 0.3 µM, 1 µM, 3 µM, 10 µM, 30 µM, 100 µM, 300 µM, and 1 mM. Mixed cultures were fed after 3 days, sacrificed after 6 days, relative cell proliferation was measured on Wallac Victor 1420 multilabel counter (PerkinElmer, Waltham, MA). The survival rate was calculated using the equation Tsample/Tcontrol × 100, where Tsample is ATP quantification of the sample wells with 5-FC or 5-FU and T control is ATP quantification without 5-FC or 5-FU. The half maximal inhibitory concentration (IC50) values were determined using GraphPad Prism 8 (GraphPad Software, Inc). Cell toxicity (IC50s) was compared to 100% yCD bearing cells with unpaired t-test using GraphPad Prism 8 (GraphPad Software, Inc).
In vitro mixing assays in 3D cell culture
yCD and EV cells were mixed at various ratios of 10:0, 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9, 0:10, respectively. The cells were plated in Perfecta3D® 384-Well Hanging Drop Plate (3D Biomatrix, Ann Arbor, MI, USA), at 300 cells per well, in 20 µl of complete media. After 2 days, 5 µl of selection media was added to obtain the final concentration of 2.5% Matrigel® Matrix (Corning, Corning, NY) and 5-FC concentrations of 0, 0.1 µM, 0.3 µM, 1 µM, 3 µM, 10 µM, 30 µM, 100 µM, 300 µM, and 1 mM. The cells were fed at 3 days. After a total of 6 days, spheroids were lysed using spheroid lysis buffer (Scivax, Kanagawa, Japan) and incubated at room temperature for 10 minutes. Lysed cells were transferred to white 386-well plates (Sigma-Aldrich, St. Louis, MO) and ATP was quantified by CellTIter-Glo® Luminescent Cell Viability Assay (Promega, Madison, WI) on a Wallac Victor 1420 multilabel counter. IC50 values were determined as described above.
Xenograft tumors in vivo
Under IACUC approval, 4- to 5-week-old athymic nude female mice (Harlan, Frederick, MD) were randomly divided into six groups. Twelve mice for each group were injected with yCD/empty vector cell mixes at the following ratios: 100% yCD, 80% yCD/20% EV, 60% yCD/40% EV, 40%yCD/60% EV, 20% yCD/80% EV, and 100% EV. Five million cells were mixed in 200 µl of a solution containing equal parts/volume of PBS and Matrigel®Matrix and subcutaneously injected into both flanks of the nude mice. Tumor volume was measured with calipers according to formula Volume = Length × Width2/2 and mice were weighed once per week. When tumors reached approximately 200 mm3, we assigned the mice to equal groups based on the size of the tumors, so that the mean tumor volume was equivalent in the two groups. Treatment consisted of 500 mg/kg 5-FC or PBS injected intraperitoneally (i.p.) every day continuously for 42 days. Results were evaluated as both raw tumor volume and relative tumor volume, where tumor volume at Day 0 was taken as 1. Mice were sacrificed prematurely if tumors grew to a size approaching the protocol size limit, but otherwise measurements were stopped at 42 days. At the 2-month time point, tumor xenotransplants were excised for molecular and functional analyses. Tumor volumes were compared to 100% yCD bearing tumors after regression (i.e. Days 35 and 42) with two-tailed Mann–Whitney test using GraphPad Prism 8 (GraphPad Software, Inc).
Explanted tumors
Mice were euthanized and tumors excised using sterile technique. They were minced, digested with 375 units/ml collagenase IV (Invitrogen) and 250 units/ml hyaluronidase (Sigma) in DMEM/20% FBS, and plated in 3 T25 flasks. Following successful growth, the cells from each tumor were sorted into single cells in a 96-well plate. Following expansion in the 96-well plate for 2 weeks, the wells were subcultured and 22–24 wells were replica plated into control (no treatment), 5-FC (100 μM), 5-FU (10 μM), and G418 (0.8 mg/ml) treatment. Following growth for 2 weeks, wells were scored as positive or negative by phase microscopy.
PCR
PCR was performed using HiFi platinum supermix (Thermo Fisher, Waltham, MA) with 10 μM forward (T7, 5ʹ-TAATACGACTCACTATAGGG-3ʹ) and reverse (BGH, 5ʹ-TAGAAGGCACAGTCGAGG-3ʹ) primers and cycling parameters: 94°C x 3 min, followed by 94°C x 30 s, 58°C x 30 s, 68°C x 30 s for 30 cycles, followed by 4°C hold. PCR products were then run on 8% polyacrylamide TBE gel, ethidium bromide-stained and photographed. Controls included water and EV and yCD cells. We used 100 bp DNA ladder (Thermo Fisher Scientific, Cat#15628-019), Low Molecular Weight DNA Ladder (New England BioLabs, Cat# N3233L).
Statistical analysis
All experiments were performed in six biological replicates unless otherwise noted. Results are expressed as mean ± SD or SE. Mean difference was compared with unpaired t-test using GraphPad Prism 8. P < .05 was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download Zip (872.9 KB)Acknowledgments
We acknowledge Drs. Anirban Maitra, Chaoxin Hu, R. Clay Wright, Alexis Norris, Akira Horii, and Vasan Yegnasubramanian for helpful discussions.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Rosenberg SA, Aebersold P, Cornetta K, Kasid A, Morgan RA, Moen R, Karson EM, Lotze MT, Yang JC, Topalian SL, et al. Gene transfer into humans–immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323:570–578. doi:10.1056/NEJM199008303230904.
- Wu ZJ, Tang FR, Ma ZW, Peng XC, Xiang Y, Zhang Y, Kang J, Ji J, Liu XQ, Wang XW, et al. Oncolytic viruses for tumor precision imaging and radiotherapy. Hum Gene Ther. 2018;29:204–222. doi:10.1089/hum.2017.189.
- Shi C, Parker AR, Hua L, Morrell CN, Lee SC, Bandaru V, Dumler JS, Wu TC, Eshleman JR. Anti-gene padlocks eliminate Escherichia coli based on their genotype. J Antimicrob Chemother. 2008;61:262–272. doi:10.1093/jac/dkm482.
- Chen ZH, Yu YP, Zuo ZH, Nelson JB, Michalopoulos GK, Monga S, Liu S, Tseng G, Luo JH. Targeting genomic rearrangements in tumor cells through Cas9-mediated insertion of a suicide gene. Nat Biotechnol. 2017;35:543–550. doi:10.1038/nbt.3843.
- Zhang J, Kale V, Chen M. Gene-directed enzyme prodrug therapy. Aaps J. 2015;17:102–110. doi:10.1208/s12248-014-9675-7.
- Smith JK, Agbandje-McKenna M. Creating an arsenal of Adeno-associated virus (AAV) gene delivery stealth vehicles. PLoS Pathog. 2018;14:e1006929. doi:10.1371/journal.ppat.1006929.
- Jiang Q, Zhao S, Liu J, Song L, Wang ZG, Ding B. Rationally designed DNA-based nanocarriers. Adv Drug Deliv Rev. 2019. doi:10.1016/j.addr.2019.02.003.
- Zhuo H, Zheng B, Liu J, Huang Y, Wang H, Zheng D, Mao N, Meng J, Zhou S, Zhong L, et al. Efficient targeted tumor imaging and secreted endostatin gene delivery by anti-CD105 immunoliposomes. J Exp Clin Cancer Res. 2018;37:42. doi:10.1186/s13046-018-0712-8.
- no authors listed. Voretigene neparvovec-rzyl (Luxturna) for inherited retinal dystrophy. Med Lett Drugs Ther. 2018;60:53–55.
- Pol J, Kroemer G, Galluzzi L. First oncolytic virus approved for melanoma immunotherapy. Oncoimmunology. 2016;5:e1115641. doi:10.1080/2162402X.2015.1115641.
- Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986;46:5276–5281.
- Mullen CA, Kilstrup M, Blaese RM. Transfer of the bacterial gene for cytosine deaminase to mammalian cells confers lethal sensitivity to 5-fluorocytosine: a negative selection system. Proc Natl Acad Sci U S A. 1992;89:33–37. doi:10.1073/pnas.89.1.33.
- Freeman SM, Abboud CN, Whartenby KA, Packman CH, Koeplin DS, Moolten FL, Abraham GN. The “bystander effect”: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993;53:5274–5283.
- Dilber MS, Abedi MR, Christensson B, Bjorkstrand B, Kidder GM, Naus CC, Gahrton G, Smith CI. Gap junctions promote the bystander effect of herpes simplex virus thymidine kinase in vivo. Cancer Res. 1997;57:1523–1528.
- Dong Y, Wen P, Manome Y, Parr M, Hirshowitz A, Chen L, Hirschowitz EA, Crystal R, Weichselbaum R, Kufe DW, et al. In vivo replication-deficient adenovirus vector-mediated transduction of the cytosine deaminase gene sensitizes glioma cells to 5-fluorocytosine. Hum Gene Ther. 1996;7:713–720. doi:10.1089/hum.1996.7.6-713.
- Trinh QT, Austin EA, Murray DM, Knick VC, Huber BE. Enzyme/prodrug gene therapy: comparison of cytosine deaminase/5-fluorocytosine versus thymidine kinase/ganciclovir enzyme/prodrug systems in a human colorectal carcinoma cell line. Cancer Res. 1995;55:4808–4812.
- Huber BE, Austin EA, Richards CA, Davis ST, Good SS. Metabolism of 5-fluorocytosine to 5-fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proc Natl Acad Sci U S A. 1994;91:8302–8306. doi:10.1073/pnas.91.17.8302.
- Nadal JC, van Groeningen CJ, Pinedo HM, Peters GJ. Schedule-dependency of in vivo modulation of 5-fluorouracil by leucovorin and uridine in murine colon carcinoma. Invest New Drugs. 1989;7:163–172. doi:10.1007/BF00170853.
- Cloughesy TF, Landolfi J, Hogan DJ, Bloomfield S, Carter B, Chen CC, Elder JB, Kalkanis SN, Kesari S, Lai A, et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci Transl Med. 2016;8:341ra75. doi:10.1126/scitranslmed.aad9784.
- Freytag SO, Stricker H, Lu M, Elshaikh M, Aref I, Pradhan D, Levin K, Kim JH, Peabody J, Siddiqui F, et al. Prospective randomized phase 2 trial of intensity modulated radiation therapy with or without oncolytic adenovirus-mediated cytotoxic gene therapy in intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2014;89:268–276. doi:10.1016/j.ijrobp.2014.02.034.
- Meliani A, Boisgerault F, Hardet R, Marmier S, Collaud F, Ronzitti G, Leborgne C, Costa Verdera H, Simon Sola M, Charles S, et al. Antigen-selective modulation of AAV immunogenicity with tolerogenic rapamycin nanoparticles enables successful vector re-administration. Nat Commun. 2018;9:4098. doi:10.1038/s41467-018-06621-3.
- Wright CM, Wright RC, Eshleman JR, Ostermeier M. A protein therapeutic modality founded on molecular regulation. Proc Natl Acad Sci U S A. 2011;108:16206–16211. doi:10.1073/pnas.1102803108.
- Wright RC, Khakhar A, Eshleman JR, Ostermeier M. Advancements in the development of HIF-1alpha-activated protein switches for use in enzyme prodrug therapy. PloS One. 2014;9:e114032. doi:10.1371/journal.pone.0114032.
- Boyd D, Florent G, Kim P, Brattain M. Determination of the levels of urokinase and its receptor in human colon carcinoma cell lines. Cancer Res. 1988;48:3112–3116.
- Korkegian A, Black ME, Baker D, Stoddard BL. Computational thermostabilization of an enzyme. Science. 2005;308:857–860. doi:10.1126/science.1107387.
