ABSTRACT
Prolyl endopeptidase (PREP), also known as prolyl oligopeptidase (POP), is an enzyme that cleaves short peptides (<30 amino acids in length) on the C-terminal side of proline. PREP is highly expressed in multiple carcinomas and is a potential target for cancer therapy. A potent inhibitor of PREP, Y-29794, causes long-lasting inhibition of PREP in mouse tissues. However, there are no reports on Y-29794 effects on cancer cell and tumor proliferation. Using cell line models of aggressive triple-negative breast cancer (TNBC), we show here that Y-29794 inhibited proliferation and induced death in multiple TNBC cell lines. Cell death induced by Y-29794 coincided with inhibition of the IRS1-AKT-mTORC1 survival signaling pathway, although stable depletion of PREP alone was not sufficient to reduce IRS1-AKT-mTORC1 signaling or induce death. These results suggest that Y-29794 elicits its cancer cell killing effect by targeting other mechanisms in addition to PREP. Importantly, Y-29794 inhibited tumor growth when tested in xenograft models of TNBC in mice. Induction of cell death in culture and inhibition of xenograft tumor growth support the potential utility of Y-29794 or its derivatives as a treatment option for TNBC tumors.
Introduction
Prolyl endopeptidase (PREP), also known as prolyl oligopeptidase (POP), is an enzyme that
cleaves short peptides (<30 amino acids in length) on the C-terminal side of proline.Citation1 PREP is ubiquitously expressed with the highest protein expression in the brain, testis, kidney, and thymus.Citation2 PREP was first discovered in 1971 as an oxytocin cleaving enzyme.Citation3 Since then, multiple PREP substrates have been identified and PREP activity implicated in various processes, including signal transduction, protein secretion, learning and memory, and neurodegenerative conditions (reviewed inCitation4).
A potential role for PREP in cancer has also been suggested. For example, PREP expression is commonly elevated in many carcinomas, suggesting it may promote cancer development or growth.Citation5,Citation6 Indeed, Sakaguchi and colleagues reported siRNA-mediated depletion of PREP or treatment with the PREP inhibitor SUAM-14746 inhibited proliferation of human neuroblastoma, gastric, and breast cancer cell lines in vitro.Citation7–9 Further, Jackson and colleagues reported that treatment with a PREP inhibitor J94 suppressed growth of human colon cancer xenograft tumors by >90% in mice.Citation10 Finally, we reported that PREP inhibitor ZPP reduced IRS1-AKT-mTORC1 signaling in pancreatic cancer cell lines and blocked feedback activation of AKT in cells treated with mTORC1 inhibitor rapamycin.Citation11 Together, these results suggest that PREP plays an important role in cancer cell proliferation and survival and that inhibitors of PREP can potentially be developed for cancer treatment.
Y-29794 is a potent, non-peptide inhibitor of PREP that inhibits PREP at nanomolar or sub-nanomolar concentrations.Citation12 Y-29794 can be administered orally and shows long-lasting inhibition of PREP in tissues.Citation12 To date, effects of Y-29794 on cancer cell proliferation and tumor growth have not been elucidated. In this study, we investigated effects of Y-29794 on cell lines of triple-negative breast cancer (TNBC) and corresponding xenograft tumors. Currently there are no targeted therapies for TNBC. Our results indicate that Y-29794 inhibits the IRS1-AKT-mTORC1 survival and growth signaling pathway and blocks proliferation, survival, and in vivo tumor growth of multiple TNBC cell lines.
Results
Y-29794 inhibits endopeptidase activity in TNBC cell lysates
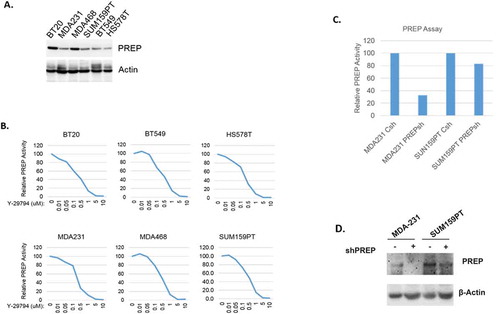
We analyzed endopeptidase activity in lysates from TNBC cells in the absence or presence of Y-29794. We used 6 TNBC cell lines that express PREP protein at varying levels (). Y-29794 inhibited endopeptidase activity in each of the TNBC cell lysates in a dose-dependent manner (). At the 5 and 10 micromolar doses Y-29794 caused complete inhibition of endopeptidase activity in these cell lysates (). Next, we compared endopeptidase activity in control TNBC cells and cells in which PREP was depleted by shRNA. Immunoblots show PREP protein was effectively depleted by the shRNA in MDA231 cells and partially depleted in SUM159PT cells (). Endopeptidase activity was also markedly reduced by PREP shRNA in MDA231 cells and partially reduced in SUM159PT cells (). Together, the results demonstrate that PREP is an active endopeptidase in TNBC cell lysates and that Y-29794 inhibits this endopeptidase activity.
Figure 1. Y-29794 inhibits endopeptidase activity in TNBC cell lysates
Y-29794 inhibits survival and proliferation and alters the cell cycle in TNBC cells
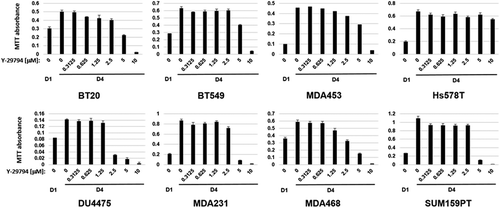
Next, we monitored the effect of increasing Y-29794 doses on TNBC cell proliferation and survival using an MTT assay. As shown in , MTT absorbance increased between 1 and 4 days in untreated cells, demonstrating each of the cell lines were proliferating (). However, proliferation in nearly all the cell lines was reduced by Y-29794 treatment in a dose-dependent manner (the exception to this was Hs578T cells, which were largely resistant to Y-29794 in this assay). Notably, at higher Y-297974 doses of 2.5 to 10 µM the MTT absorbance at day 4 (D4) was reduced below that seen in untreated cells at day 1 (D1). This indicates Y-29794 at these doses was not only inhibiting proliferation but also inducing cell death.
Figure 2. Y-29794 inhibits proliferation and viability of triple-negative breast cancer cell lines
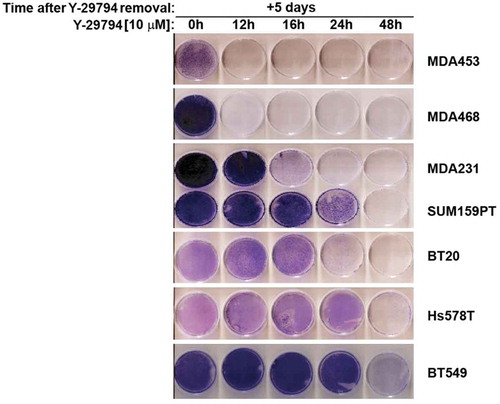
We used a second assay to examine the effect of Y-29794 on TNBC cell survival. For this, TNBC cell lines were plated in 60 mm dishes and either untreated or treated with Y-29794 for increasing amounts of time (12–48 hrs). The cells were then rinsed twice with PBS, refed with drug-free medium (minus Y-29794), and allowed to grow for 5 days. The cells remaining on the plate were then stained with crystal violet (). Untreated cells filled the plate and stained heavily with crystal violet. In contrast, cells treated with Y-29794 showed decreased survival demonstrated by less crystal violet staining. MDA453 and MDA468 cells appeared the most sensitive to Y-29794 in this assay. In these cells, 12 hr treatment with Y-29794 caused complete loss of any stainable cells on the plate. In contrast, Hs578T and BT549 cells were the most resistant to Y-297974 in this assay. In these cells, 48 hr treatment with Y-29794 was needed to cause a loss of stainable cells on the plate. The other cell lines showed intermediate sensitivity to Y-29794 treatment. In total, the results of and demonstrate that Y-29794 reduces proliferation and survival in multiple TNBC cell lines. Some TNBC cell lines appear more sensitive to Y-29794 than others.
Figure 3. Y-29794 inhibits viability of triple-negative breast cancer cells
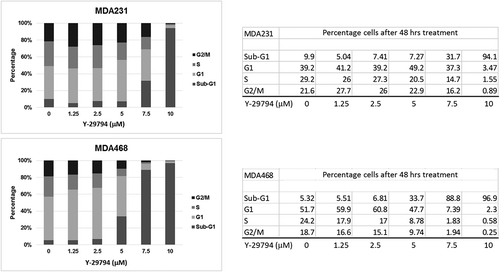
Lastly, we examined the effect of Y-29794 on cell cycle progression in TNBC cells. For this, MDA231 and MDA468 cells were treated with increasing doses of Y-29794 for 48 h. The percentage of cells in G1, S, and G2/M phase were determined by flow cytometry (). We also determined the percentage of cells with sub-G1 DNA content, which is a common indicator of apoptosis. In both MDA468 and MDA231 cells, low doses of Y-29794 caused a modest increase in G1-phase cells and a modest decrease in S and/or G2/M-phase cells. In contrast, higher doses (5, 7.5, 10 mM) caused a progressive increase in cells with sub-G1 DNA content. The results indicate low doses of Y-29794 can arrest or slow cell cycle progression in TNBC cells while high doses can induce cell death.
Y-29794 inhibits the IRS1-AKT-mTORC1 pathway in TNBCs
The IRS1-AKT-mTORC1 pathway promotes cell proliferation, survival, and growth and is frequently upregulated in TNBC. We previously showed that high doses of the PREP inhibitor ZPP could reduce viability of pancreatic cancer cells and this was associated with depletion of IRS1 and inhibition of AKT and mTORC1.Citation11 We therefore assessed the IRS1-AKT-mTORC1 pathway in TNBC cells treated with Y-29794. MDA231, MDA468, and SUM159PT cells were treated with Y-29794 for 16 h and then examined by immunoblotting for different proteins in the pathway. As shown in , IRS1 protein levels were reduced in each of the cell lines treated with increasing Y-29794 doses. The proteasome inhibitor MG132 blocked the decrease in IRS1 levels, supporting the decrease results from enhanced protein degradation (). In contrast, IRS2 protein was not decreased by Y-29794 treatment but appeared shifted to a slightly faster migrating form (). AKT is activated by phosphorylations at serine 473 (S473) and threonine 308 (T308). Levels of activated AKT phosphorylated at S473 and T308 were reduced by Y-29794 in each cell line. Finally, levels of phosphorylated S6K (pS6K), which is indicative of mTORC1 activity, were also reduced in the Y-29794 treated cells. These results demonstrate Y-29794 inhibits the IRS1-AKT-mTORC1 pathway in TNBC cell lines.
Figure 5. Y-29794 inhibits the IRS1/AKT/mTORC1 pathway in triple-negative breast cancer cells
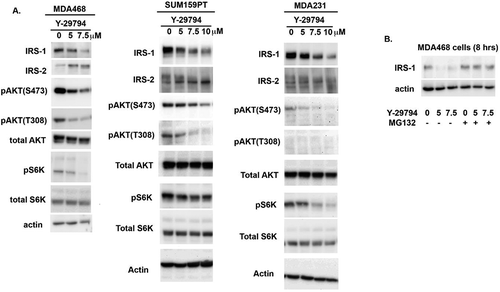
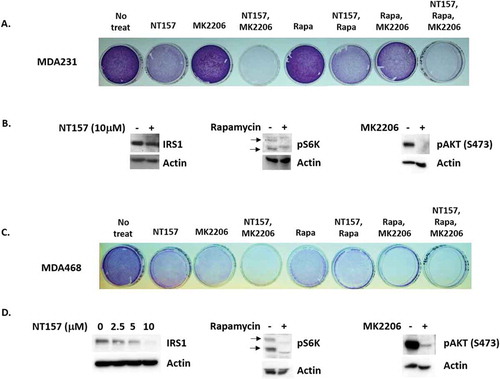
We reasoned that if Y-29794 reduces TNBC cell proliferation and survival by inhibiting IRS1, AKT, and/or mTORC1, then inhibitors of these proteins would have a similar effect. To test this, we treated sub-confluent MDA468 and MDA231 cells with inhibitors of IRS1, AKT, and mTORC1 either alone or in combination. Cells remaining on the plate after 72-h treatment were then stained with crystal violet, as in . To inhibit IRS1 we used the tryphostin molecule NT157 which promotes IRS1 degradation.Citation13 To inhibit AKT we used the allosteric AKT inhibitor MK2206. Rapamycin was used to inhibit mTORC1. As expected, levels of activated AKT (S473 phosphorylated) were reduced by MK2206, levels of IRS1 were reduced NT157, and levels of phosphorylated S6K were reduced by rapamycin (,d). These results confirm that the inhibitors were active against their respective targets. NT157 alone reduced proliferation/survival in MDA231 cells, evidenced by reduced crystal violet staining compared to untreated cells (), while MK2206 or rapamycin alone had minimal effect. In contrast, combined treatment with NT157 plus MK2206, or NT157 plus MK2206 plus rapamycin, caused a near complete loss of survival in MDA231 cells evidenced by a loss of stainable cells on the plate. In MDA468 cells single treatment with NT157, MK2206, or rapamycin reduced proliferation/survival evidenced by a reduced amount of stainable cells on the plate (). Combined treatment with NT157 plus MK2206, rapamycin plus MK2206, or NT157 plus MK2206 plus rapamycin caused a complete loss of survival in these cells (). In sum, the results in show that inhibitors of IRS1, AKT, and mTORC1 reduce proliferation/survival in TNBC cells, similar to Y-29794. These results support the idea that Y-29794 reduces viability in TNBC cells at least in part by inhibiting IRS1-AKT-mTORC1.
Figure 6. Inhibitors of IRS1, AKT, and mTORC1 reduce viability in TNBC cells
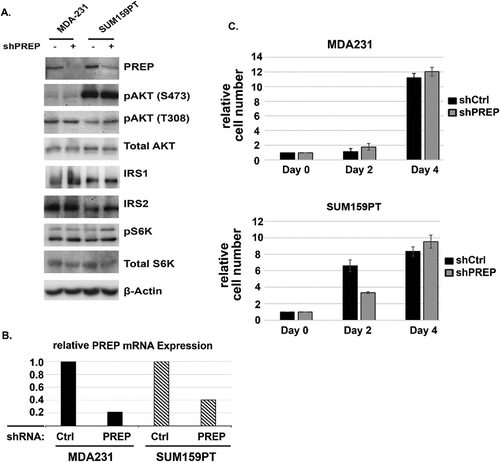
PREP knockdown is insufficient to block the IRS1-AKT-mTORC1 pathway or reduce TNBC cell proliferation
We wished to ask if stable depletion of PREP alone would be sufficient to inhibit TNBC proliferation/survival and inhibit the IRS1-AKT-mTORC1 pathway. To this end, MDA231, MDA468, and SUM159PT cells were infected with a mixture of lentiviruses that encode shRNAs against PREP and also encode puromycin resistance. Control shRNA (non-targeting) lentivirus served as controls. Infected cells were placed in puromycin in order to isolate puromycin resistant populations with stable depletion of PREP. We were unable to isolate MDA468 cells with stable PREP depletion. However, MDA231 and SUM159PT cells with stable depletion of PREP were readily isolated. Immunoblotting and mRNA analysis showed PREP expression was reduced by the shRNA against PREP (,b). However, there was no apparent reduction in the levels or migration of IRS1 and IRS2 in the PREP-depleted MDA231 and SUM159PT cells compared to control cells (). There was also no apparent reduction in pAKT(S473), pAKT(T308), or pS6K levels in the PREP-depleted cells. In addition, the control and PREP knockdown cells appeared to proliferate at a comparable rate (). The results indicate stable knockdown of PREP alone is not sufficient to inhibit the IRS1-AKT-mTORC1 pathway or reduce proliferation/survival in these TNBC cells. Moreover, the results suggest Y-29794 has alternative targets other than PREP through which it inhibits the IRS1-AKT-mTORC1 pathway and proliferation/survival in these cells.
Figure 7. The effect of PREP knockdown on the IRS1/AKT/mTORC1 pathway and proliferation in triple-negative breast cancer cells
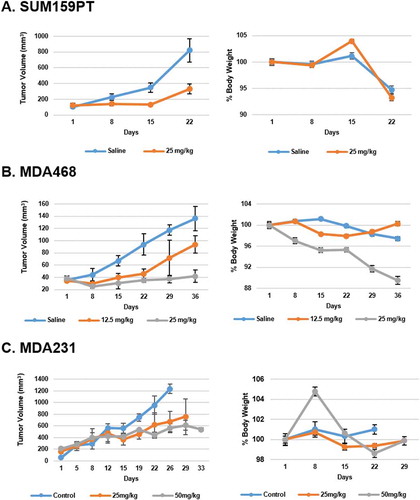
Y-29794 inhibits growth of TNBC xenograft tumors
Lastly, we wished to test the effect of Y-29794 on TNBC tumor growth. MDA468, MDA231, and SUM159PT cells were seeded into the mammary fat pad of NOD-SCID immunocompromised mice for tumor growth. When tumors reached a palpable, measurable size, the mice were treated daily (5 days/week) with either vehicle (saline) or increasing doses of Y-29794 (12.5–50 mg/kg). Treatment was by intraperitoneal injection. Tumor growth was monitored by caliper measure for 3–5 weeks, and overall body weight measured as an indicator of toxicity. As shown in , Y-29794 caused a pronounced reduction in growth of MDA468, MDA231, and SUM159PT tumors while having minimal effect on body weight (body weight reduction during the course of the experiment was 10% or less).
Figure 8. Y-29794 inhibits TNBC xenograft tumor growth
Discussion
PREP is expressed at high levels in multiple carcinomas and is a potential target for cancer therapy.Citation5–10 Multiple PREP inhibitors have been developed and tested in cell lines and, in some cases, in in vivo tumor models. Y-29794 is a potent inhibitor of PREP and is reported to cause long-lasting inhibition of PREP in mouse tissues.Citation12 However, the effect of Y-29794 on cancer cell and tumor proliferation has not been reported. TNBC is an aggressive breast cancer subtype with limited treatment options and poor prognosis. There are currently no effective-targeted therapies for TNBC. In this study, we examined the effect of Y-29794 on multiple TNBC cell lines in vitro and in vivo when grown as xenograft tumors in mice. We found Y-29794 inhibited proliferation and induced death in multiple TNBC cell lines. We found death induced by Y-29794 coincided with depletion/inhibition of the IRS1-AKT-mTORC1 survival signaling pathway. Stable depletion of PREP alone in MDA231 and SUM159PT cells was not sufficient to reduce IRS1-AKT-mTORC1 signaling and induce death. These results suggest Y-29794 targets other proteins in addition to PREP to elicit its cancer killing effect in these cells. Importantly, Y-29794 inhibited growth of MDA468, SUM159PT, and MDA231 xenograft tumors in mice. Taken together, these results support the idea that Y-29794 or its derivatives could be an effective treatment option for TNBC cell tumors.
IRS1 and IRS2 are adaptor proteins that mediate signaling downstream of activated cell surface receptors, most notably the insulin receptor (IR), and insulin-like growth factor receptor (IGF-1 R).Citation14 AKT and mTORC1 are activated downstream of IRS1/2 and promote cell survival, proliferation, and growth.Citation15 In our studies Y-29794 caused depletion of IRS1 and inhibition of AKT and mTORC1, and also inhibited TNBC cell proliferation and survival. Combined treatment with small molecule inhibitors of IRS1, AKT, and mTORC1 also reduced survival in at least two TNBC cell lines, MDA231 and MDA468. These results suggest reduced proliferation and cell death in response to Y-29794 results, at least in part, from depletion/inhibition of IRS1 and inhibition of AKT and mTORC1. An important question is how Y-29794 causes depletion of IRS1. Most tyrosine phosphorylations activate IRS1 and IRS2 while serine and threonine phosphorylations, for the most part, inhibit IRS1 and IRS2 and/or promote their degradation. The proteasome inhibitor MG132 rescued IRS1 levels in Y-29794 treated MDA468 cells, indicating the reduction in IRS1 results from increased protein degradation. S6K and mTORC1 kinases are known to phosphorylate IRS1 and promote its degradation as part of a negative feedback mechanism to limit insulin signaling.Citation16,Citation17 However, in the current study Y-29794 inhibited mTORC1/S6K while also causing degradation of IRS1, suggesting the depletion of IRS1 was independent of mTORC1 or S6K. We speculate Y-29794 may trigger IRS1 degradation by activating one or more other kinases that can promote IRS1 serine/threonine phosphorylation. Notably, IRS2 was not depleted in Y-29794 treated cells though it was shifted to a faster migrating form. We speculate the shift in IRS2 to a faster migrating form could result from decreased phosphorylation, including activating tyrosine phosphorylations, and that IRS2 may also be inhibited in Y-29794 treated cells.
MDA468 expressed relatively high levels of PREP protein compared to most other TNBC cell lines and was particularly sensitive to Y-29794 treatment. Despite our attempts, we were unable to isolate MDA468 cells with stable knockdown of PREP. While the reasons for this are unclear, the fact that we were unable to isolate MDA468 cells with stable PREP knockdown raises the possibility that PREP may be essential for survival in certain cell lines, such as MDA468. Y-29794 can inhibit PREP in vitro at sub-nanomolar concentrations.Citation12 In the current study Y-29794 only showed significant TNBC cell killing effect at higher concentrations of 5–10 µM. This raises the possibility that the anti-cancer activity of Y-29794 may result from inhibition of alternative targets other than PREP. In contrast to MDA468, we readily isolated MDA231 and SUM159PT cells with stable knockdown of PREP. However, in contrast to Y-29794, stable depletion of PREP in these cells neither reduced IRS1-AKT-mTORC1 signaling nor reduced proliferation. These findings also suggest Y-29794 has alternative targets other than PREP through which it inhibits IRS1-AKT-mTORC1 and reduces proliferation and survival in these cells. What might these alternative targets be? In our previous study, we found that co-depletion of PREP and the related prolylcarboxypeptidase (PRCP) in pancreatic cancer cells reduced IRS1 levels and caused a greater reduction in pAKT (S473) levels than depletion of either single factor.Citation11 Thus, one possibility is that Y-29794 has some inhibitory effect against PRCP in TNBC cells and it is this inhibitory effect, in combination with PREP inhibition, which reduces IRS1-AKT-mTORC1 and reduces proliferation and survival. PREP is an endopeptidase and member of a broad family of peptidases that includes PRCP and also includes DPP proteins and FAP.Citation18 It is possible Y-29794 has inhibitory activity against one or more of these peptidases that contributes to loss of IRS1-AKT-mTORC1 and inhibition of TNBC cells.
TNBC patients have a poor prognosis, highlighting the need for new treatment options against this deadly disease. We found Y-29794 reduced/inhibited growth of human TNBC xenograft tumors in mice. These findings support the idea that Y-29794 or its derivatives could be an effective treatment option for TNBC cell tumors.
Materials and methods
Y-29794 synthesis and use Y-29794-oxalate was custom synthesized at Exclusive Chemistry Ltd (www.exchemistry.com, Obninsky, Russia) with a 98% purity. Y29794-tosylate was synthesized at MedKoo Biosciences (Chapel Hill, NC) with a purity of 99%. Y-29794 oxylate and Y-29794 tosylate were dissolved in DMSO for cell line studies. For tumor studies in mice, Y-29794 oxylate was dissolved in saline, and Y-29794 tosylate was dissolved in Cremaphor EL:Ethanol (1:1), and then diluted with saline.
Chemicals NT157 was purchased from Selleckchem and dissolved in DMSO at final concentration of 10 mM. MK2206 was purchased from Selleckchem and dissolved in DMSO at a final concentration of 10 mM. Rapamycin was purchased from Selleckchem and dissolved in DMSO at final concentration of 10 mM.
MTT assay Cells were seeded in a 96-well plate at a density of 2000 cells/well. Cells were treated with Y-29794-oxylate (0.3125, 0.625, 1.25, 2.5, 5, 10 µM) for 4 days. Cells in the control group were treated with DMSO. After 4 days, medium was removed and 100 µL MTT solution was added at a final concentration of 1 mg/ml and the plates incubated at 37°C for 4 h. After incubation, cells were lysed with the addition of 100 µL of 0.01 M HCl/10%SDS solution and incubated overnight at 37°C. Absorbance was read at 570 nm using a spectrophotometer.
Determining Prolyl Endopeptidase Enzymatic Activity Intracellular PREP activity is determined using the fluorogenic substrate Z-Gly-Pro-AMC as described (cite the JBC paper). Briefly, cells grown in a 100 mm dish were washed twice with phosphate-buffered saline (PBS), layered with 0.5 ml assay buffer (50 mm HEPES pH 7.5; 200 mm NaCl; 1 mm EDTA; 1 mm dithiothreitol (DTT)) and lysed by three cycles of thawing and freezing. The lysates were removed from the culture dishes by a cell scraper and centrifuged at 12,000 × g for 10 min. Hundred micrograms of the clear supernatant in a 100-μl volume was transferred into a well of a 96-well plate (black color) in triplicate and used immediately for PREP activity measurement. To measure the PREP activity, Z-Gly-Pro-AMC was added into the wells for a final concentration of 10 μm. The substrate turnover was monitored continuously for 30 min at 360 nm excitation and 460 nm emission using a BioTek Mx microplate reader.
Immunoblots Cell lysates were prepared in lysis buffer (50 mM Tris, pH 7.5), 150 mM NaCl, 5 mM EDTA, 0.5% NP40) and complete mini-protease inhibitor cocktail tablet (Roche, 1 tablet per 10 mls lysis buffer). The following antibodies were used (catalog number, lot number, and dilution used): rabbit anti-IRS1 (A301-158A, lot no. A301-158A-1, 1:500) and rabbit IRS2 (A301-432, lot no. A301-432A-1, 1:500) were from Bethyl Laboratories; rabbit anti-phoshpho-p70S6K1 (T389) (9234, lot no. 12, 1:000), rabbit anti-p70S6K (2708, lot no. 7, 1:1000), rabbit anti-phospho-AKT (T308) (2965, 1:1000), rabbit anti-phospho-AKT (S473) (4060, lot no. 23, 1:1000), rabbit anti-AKT (4691, 1:1000) were from Cell Signaling; goat anti-PREP (AF4308, lot no. CBCS0108041, 1:500) was from R&D Systems; mouse anti-µ-Actin (sc-47778, lot no. K2712, 1:500) was from Santa Cruz). Primary antibodies were detected with goat anti-mouse (65–6520, lot no. QG215721, 1:10,000) (Life Technologies) and goat anti-rabbit (7074, lot no. 27, 1:2000) (Cell Signaling) secondary antibodies coupled to horseradish peroxidase, using Immobilon Western Chemiluminescence HRP substrate (EMD Millipore). Bands were visualized using the BioSpectrum Imaging System (UVP).
PREP knockdown MDA231 and SUM159PT cells were plated at ~50% confluence in a 12 well dish. The following day polybrene was added to the medium at a final concentration of 5 µg/ml, and then 10 µl of control shRNA lentivirus (catalog number sc 108080, lot E0918, purchased from Santa Cruz) or PREP shRNA lentivirus (catalog number sc 76244, lot D1519, purchased from Santa Cruz) was added. The next day the medium was removed and the cells were rinsed with PBS and refed. Three days later the cells were trypsinized and replated in 3 60 mm dishes. Four days later the cells were refed with medium containing 1 µg/ml puromycin for SUM159PT cells and 0.75 µg/ml puromycin for MDA231 cells. Three days later the cells were transferred to 10 cm dishes for freezing and further analysis. SUM159PT and MDA231 cells without viral infection died within ~4 days when grown in these puromycin doses and served as controls.
Tumor growth measurements MDA468, SUM159PT, and MDA231 cells were seeded in the mammary fat pad of NOD-SCID mice for tumor growth studies. 1 × 106 cells were used for the initial seedings. When tumors reached a palpable and measurable size, the mice were divided into groups for treatment with either vehicle or Y-29794 (12.5, 25, or 50 mg/kg doses). Vehicle was saline for the oxylated form of Y-29794 that was used to treat MDA468 and SUM159PT tumors, and cremaphor for the tosylated form of Y-29794 that was used to treat MDA231 cell tumors. MDA468 and SUM159PT had an n = 8 for all groups (vehicle and Y-29794 treated). For MDA231 had an n = 3 for the control group, and an n = 4 for the Y-29794 treatment groups.
Additional information
Funding
References
- Szeltner Z, Polgar L. Structure, function and biological relevance of prolyl oligopeptidase. Current Prot Pept Sci. 2008;9:96–107.
- Myohanen TT, Pyykko E, Mannisto PT, Carpen O. Distribution of prolyl oligopeptidase in human peripheral tissues and in ovarian and colorectal tumors. J Histochem Cytochem. 2012;60:706–715.
- Walter R, Shlank H, Glass JD, Schwartz IL, Kerenyi TD. Leucylglycinamide released from oxytocin by human uterine enzyme. Science. 1971;173:827–829.
- Myohanen TT, Garcia-Horsman JA, Tenorio-Laranga J, Mannisto PT. Issues about the physiological functions of prolyl oligopeptidase based on its discordant spatial association with substrates and inconsistencies among mRNA, protein levels, and enzymatic activity. J Histochem Cytochem. 2009;57:831–848.
- Goossens F, De Meester I, Vanhoof G, Scharpe S. Distribution of prolyl oligopeptidase in human peripheral tissues and body fluids. Eur J Clin Chem Clin Biochem. 1996;34:17–22.
- Larrinaga G, Perez I, Blanco L, Lopez JI, Andres L, Etxezarraga C, Santaolalla F, Zabala A, Varona A, Irazusta J. Increased prolyl endopeptidase activity in human neoplasia. Regul Pept. 2010;163:102–106.
- Tanaka S, Suzuki K, Sakaguchi M. The prolyl oligopeptidase inhibitor SUAM-14746 attenuates the proliferation of human breast cancer cell lines in vitro. Breast Cancer. 2017;24:658–666.
- Suzuki K, Sakaguchi M, Tanaka S, Yoshimoto T, Takaoka M. Prolyl oligopeptidase inhibition-induced growth arrest of human gastric cancer cells. Biochem Biophys Res Comm. 2014;443:91–96.
- Sakaguchi M, Matsuda T, Matsumura E, Yoshimoto T, Takaoka M. Prolyl oligopeptidase participates in cell cycle progression in a human neuroblastoma cell line. Biochem Biophys Res Comm. 2011;409:693–698.
- Jackson KW, Christiansen VJ, Yadav VR, Silasi-Mansat R, Lupu F, Awasthi V, Zhang RR, McKee PA. Suppression of tumor growth in mice by rationally designed pseudopeptide inhibitors of fibroblast activation protein and prolyl oligopeptidase. Neoplasia. 2015;17:43–54.
- Duan L, Ying G, Danzer B, Perez RE, Shariat-Madar Z, Levenson VV, Maki CG. The prolyl peptidases PRCP/PREP regulate IRS-1 stability critical for rapamycin-induced feedback activation of PI3K and AKT. J Biol Chem. 2014;289:21694–21705.
- Nakajima T, Ono Y, Kato A, Maeda J, Ohe T. Y-29794–a non-peptide prolyl endopeptidase inhibitor that can penetrate into the brain. Neurosci Lett. 1992;141:156–160.
- Reuveni H, Flashner-Abramson E, Steiner L, Makedonski K, Song R, Shir A, Herlyn M, Bar-Eli M, Levitzki A. Therapeutic destruction of insulin receptor substrates for cancer treatment. Cancer Res. 2013;73:4383–4394.
- Metz HE, Houghton AM. Insulin receptor substrate regulation of phosphoinositide 3-kinase. Clin Cancer Res. 2011;17:206–211.
- Manning BD, Toker A. AKT/PKB Signaling: navigating the Network. Cell. 2017;169:381–405.
- Yoneyama Y, Inamitsu T, Chida K, Iemura SI, Natsume T, Maeda T, Hakuno F, Takahashi SI. Serine Phosphorylation by mTORC1 Promotes IRS-1 Degradation through SCFbeta-TRCP E3 Ubiquitin Ligase. iScience. 2018;5:1–18.
- Haruta T, Uno T, Kawahara J, Takano A, Egawa K, Sharma PM, Olefsky JM, Kobayashi M. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Molec Endocrinol. 2000;14:783–794.
- Waumans Y, Baerts L, Kehoe K, Lambeir AM, De Meester I. The dipeptidyl peptidase family, prolyl oligopeptidase, and prolyl carboxypeptidase in the immune system and inflammatory disease, including atherosclerosis. Frontiers Immunol. 2015;6:387.