ABSTRACT
Lung cancer remains the leading cause of cancer death in the United States. Since most lung cancers occur in aged individuals with chronic lung disorders characterized by inflammation and/or fibrosis, we hypothesized that aging and tissue inflammation/remodeling act in concert to promote lung cancer progression. To test this, we engaged in studies using young and aged C57BL/6 mice in conjunction with bleomycin treatment in a syngeneic model of lung cancer. Wildtype young (3 months) and aged (9 months) C57BL/6 mice were injected with Lewis Lung Carcinoma (LLC) cells at day 14 after injection with phosphate-buffered saline or bleomycin. Untreated aged mice were found to develop more lung metastases than young mice. Bleomycin induced weight loss and lung inflammation/remodeling in both young and aged mice, and it increased the number of lung metastases in aged lungs, but not in young lungs. Since aged lungs show alterations in the expression of fibronectin EDA, we repeated studies in aged WT and aged FN EDA KO mice. In the absence of tissue remodeling/inflammation, WT and FN EDA KO mice developed the same number of metastases when injected with LLC cells. However, the increase in lung metastasis due to bleomycin treatment was abolished in FN EDA KO mice, but only in aged and injured lungs. Together, these studies show increased lung cancer metastasis in aging animals and point to the influence of FN EDA and injury in this process.
Introduction
Cancer develops most commonly in the elderly with 78% of cancers occurring in patients over 55 years of age.Citation1 This is also the case for lung cancer, which remains the leading cause of cancer death in men and women in the United States and worldwide.Citation2 How exactly does aging promote cancer is unclear, but compared to young individuals, aging is characterized by oxidant stress, mitochondrial dysfunction, immunologic deficiencies, stem cell exhaustion, and accumulation of damage in cells, tissues, and organs, among other things.Citation3–6 Aging has also been characterized by a reduced capacity to respond to environmental injury.Citation7 Yet, how these processes contribute to carcinogenesis is unknown. Interestingly, most lung cancers occur in individuals with chronic lung disorders characterized by inflammation and tissue remodeling such as chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis.Citation8 Considering the emerging data linking inflammation and the tumor microenvironment in cancer development and progression,Citation9 it is not surprising that lung cancer develops most commonly in such settings.
Since the discovery of the first oncogene, Src, in 1970, and later of cellular proto-oncogenes, the prevailing paradigm of cancer development for decades was that cancer arises due to mutations in oncogenes and tumor suppressor genes of single cells. However, numerous studies performed in vitro and in vivo have now shown that, despite malignant cell transformation, tumor growth is dependent on processes such as inflammation and angiogenesis in the microenvironment. In fact, it has been shown that a malignant phenotype can be reversed by using integrin blocking antibodies.Citation10,Citation11 Thus, it can be said that genetic mutations are necessary, but not sufficient, to cause tumor growth.Citation12 It has been known for some time that chronic inflammatory diseases such as COPD, asbestosis and silicosis increase the risk of lung cancer.Citation13 For example, exposure to asbestos, and the resulting inflammatory response, have been linked to lung cancer and mesothelioma.Citation14 Indeed, a link between chronic inflammation and many cancers is now well establishedCitation15 and several studies have demonstrated a protective effect of non-steroidal anti-inflammatory drugs in cancer development.Citation16 Others have found that the ability of Rous sarcoma virus to form tumors in newly hatched chicks is dependent upon wounding and inflammation, and that the pro-fibrotic growth factor, transforming growth factor β (TGFβ), can replace wounding as a promoter of tumor development.Citation17
Despite the above, the contribution of chronic tissue inflammation and remodeling and their interaction in lung cancer have been difficult to study in humans considering the many confounding factors present, not the least of which is exposure to tobacco, perhaps the best known pro-oncogenic agent. It is for this reason that scientists have turned their attention to experimental models of lung cancer which, even though they do not exactly resemble the human condition, allow for the more careful exploration of the role of individual factors. We hypothesized that aging and tissue inflammation/remodeling act in concert to promote lung cancer progression, and we set out to test this in a syngeneic model of lung cancer. For this, we used aged mice, which have been shown to display alterations in inflammatory and tissue remodeling markers.Citation18 Aged lungs are characterized by, among other things, increased mRNA expression for TGFβ, matrix metalloproteinases, plasminogen activator inhibitor-1, and matrix molecules fibronectin and collagen I.Citation19,Citation20 These changes explain, at least in part, the alterations observed in the structure of the aging lung.
One of the matrix molecules upregulated in the setting of aging is a fibronectin splicing variant, which is characterized by the inclusion of an extra exon termed Extra Type III Domain A (EDA). This is intriguing considering that data generated over the past three decades have linked fibronectin with cancers including breast, prostate, liver, and lung.Citation21–23 However, it has been difficult to study the role of fibronectin in vivo mainly because animals with knockout mutations in fibronectin are embryonic lethal.Citation24 More recently, animals with knockout mutations targeting fibronectin EDA were generated and their investigation suggests a role for this molecule in wound healing and aging.Citation25 Moreover, fibronectin EDA has been linked to lung inflammation and remodeling since its deletion is protective in the bleomycin model of lung injury and repair.Citation26 Considering these observations, we also tested animals lacking fibronectin EDA.
Our studies revealed that aged lungs were more susceptible to metastasis when compared to young lungs. Importantly, studies in young and aged animals exposed to bleomycin suggest that tissue remodeling/inflammation augment pulmonary metastasis, but only in the aging lung and not in the young lung. The effect of bleomycin was abolished in studies performed in FN EDA KO mice, despite the presence of fibrosis. Together, these observations suggest an interplay between lung aging and inflammation/remodeling in tumor progression, and that fibronectin EDA is an important molecule in this setting.
Results
Aging is associated with increased lung metastases
While studying a syngeneic model of lung cancer, we noticed a significant variability in the development of lung metastasis. A retrospective analysis of the data revealed that the age of the animals represented an important variable (not shown). To test the role of aging prospectively, wildtype C57BL/6 J mice at ages 3,5,7,9 and 12 months of age were injected subcutaneously with 1 × 106 LLC cells. Tumor growth was followed until tumors reached a size of ≥15 mm in any direction, after which time, all animals were sacrificed and lungs harvested and formalin fixed. We observed small differences in the size of the primary tumors, but these were not statistically significant (). As depicted in and 1C, mice failed to develop lung metastases until 7 months of age or older.
Figure 1. Aging is associated with increased lung metastases
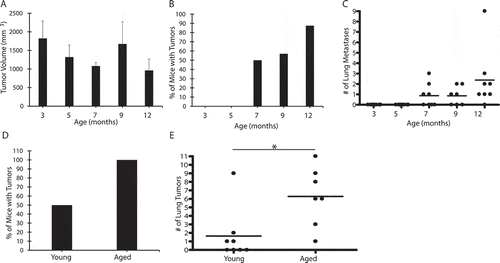
To circumvent the potential confounding effects of skin immunity, we repeated similar experiments in animals injected intravenously with the tumor cells. In these experiments, we observed that only 50% of young mice developed lung tumors compared to 100% of aged mice (). Aged mice also developed a greater number of lung tumors when compared to the number of tumors developed in the few affected young mice (). Thus, aged lungs are more susceptible to lung metastasis than young lungs.
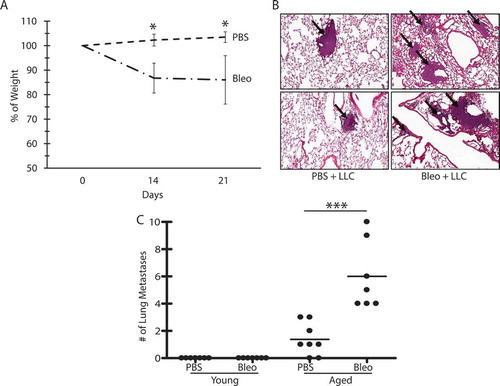
Bleomycin treatment is associated with increased metastases, but only in aged lungs
To examine the role of lung inflammation/remodeling in lung cancer progression, young and aged animals were administered bleomycin (1 mU/g) followed by injection of LLC cells 14 days after bleomycin treatment. Bleomycin does not recapitulate lung fibrosis as observed in humans and is not a good model to evaluate chronic lung disease. However, this model does allow for evaluation of the impact of lung injury and subsequent inflammation/remodeling in the processes being investigated. As expected, bleomycin-induced weight loss () and lung inflammation/remodeling () in both young and aged mice. In animals injected with LLC cells after bleomycin treatment, we again observed small differences in the size of subcutaneous tumors at the time of euthanasia, but these were not statistically significant (not shown). However, interestingly, bleomycin augmented the number of lung metastases in the aged lungs, but not in young lungs (), despite the presence of inflammation/remodeling in both groups.
Figure 2. Bleomycin treatment is associated with increased metastases, but only in aged lungs
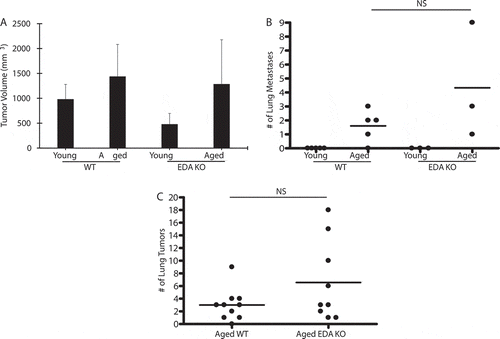
Fibronectin EDA is dispensable for aged-related lung cancer progression, but required for bleomycin-induced augmentation of metastasis
Since aged lungs show increased expression of fibronectin EDA (e.g., see ref. 20), and fibronectin is implicated in carcinogenesis, we hypothesized that this matrix molecule contributes to the aging effect. To test this, untreated aged wildtype and aged FN EDA KO mice were injected with LLC cells, both subcutaneously and intravenously. For subcutaneous tumors, growth was followed until tumors reached a size of ≥15 mm, after which time, all animals were sacrificed and lungs harvested and formalin fixed. In the subcutaneous model, no statistically significant differences were observed in the growth of the primary tumor () or in the number of lung metastases () when comparing aged wildtype and aged FN EDA KO animals. Likewise, when animals were injected intravenously, no differences were observed in the number of lung tumors when comparing aged wildtype and aged FN EDA KO animals (). Thus, despite being increased in aged lungs and being implicated in carcinogenesis and cancer progression, the absence of fibronectin EDA did not inhibit cancer progression in aged lungs.
Figure 3. Fibronectin EDA is dispensable for aged-related lung cancer progression
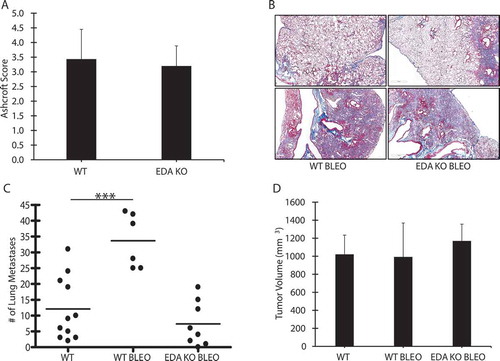
To test the role of fibronectin EDA in cancer progression in the setting of tissue remodeling/inflammation, we repeated experiments with bleomycin in aged FN EDA KO animals. Although fibronectin EDA has been reported to be essential for the development of lung fibrosis in the bleomycin model (e.g., see ref. 26), we observed no differences in the development of bleomycin-induced fibrosis in WT and FN EDA KO mice ( and 4B). This is consistent with our observation that the bronchoalveolar lavage fluid harvested from bleomycin-treated WT and FN EDA KO animals was similar in its ability to stimulate fibronectin expression in cultured fibroblasts (not shown). However, interestingly, when injected with LLC cells, the augmentation of pulmonary metastases in aged mice due to bleomycin was abolished in FN EDA KO mice (). There were no differences observed in the size of the primary tumor ().
Figure 4. Fibronectin EDA is required for bleomycin-induced augmentation of metastasis
Considering the above, we hypothesized that fibronectin EDA provides a scaffold for the proliferation, migration and organization of tumor cells within remodeled lung tissue. This is consistent with data showing that most tumor cells metastasizing to lung co-localized to areas of inflammation/fibrosis ( and 5B). Consistent with this, LLC migration was increased on plates coated with fibronectin EDA-containing cellular fibronectin compared to plastic (), and LLC proliferation was also increased when cells were cultured in the presence of cellular fibronectin ().
Figure 5. Lung metastases develop primarily in fibrotic areas
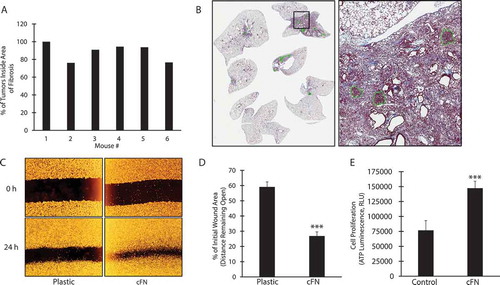
Role of Immunity
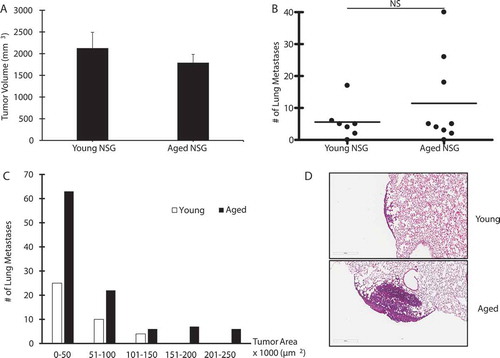
Finally, we wanted to test the role of immunity in our model to examine if it mediated the age-related effect on lung metastasis. For this, we injected LLC cells into young and aged NSG mice, which are extremely immunodeficient, lacking B and T cells, as well as functional NK cells. Dendritic cells and macrophages are present, but defective. We noted that both young and aged NSG mice developed tumors at the site of injection within 2 weeks, although no differences were observed between the groups when the animals were sacrificed at day 17 (). There were also no differences in metastases to the lung between young and aged mice (). Interestingly, however, the size of metastases to the lung was significantly larger in aged mice compared to young mice (). The location of the lung metastases in the NSG mice also differed from those observed in WT animals. While metastases in WT mice developed in areas adjacent to blood vessels, this was not the case in NSG mice, where the majority of metastases developed in the periphery of the lung (). These data suggest a role for innate immunity in tumor progression beyond the primary site in the syngeneic model.
Figure 6. Effect of Immunity on Lung Cancer Progression
Discussion
By the year 2020, it is estimated that 70% of all neoplasms will occur in individuals aged 65 years or older.Citation27 Among them, lung cancer remains the deadliest and occurs most commonly in individuals with chronic lung disorders characterized by inflammation and tissue remodeling such as chronic obstructive pulmonary disease and fibrosis.Citation8 The relative contribution of these processes to the pathogenesis of lung cancer, especially metastatic disease, has been difficult to study in part because of the concomitant exposure to tobacco, a well-known carcinogen.Citation28Citation29Citation30 Thus, we set out to study the role of aging and lung tissue inflammation/remodeling in an experimental syngeneic model of lung cancer. Several observations were made as summarized below.
First, we observed that aged lungs develop more metastatic lesions than young lungs, resembling the human condition. This was observed when LLC cells were injected subcutaneously or intravenously, which suggests that some inherent factor(s) in the aged lungs is responsible for this susceptibility, and not tumor implantation, growth, or metastasis from the primary tumor, nor does it relate to the ability of cancer cells to escape the immune system in the periphery. These data contribute to the notion that malignant cell transformation alone is not sufficient for tumor growth and progression. Young, healthy, immunocompetent animals are able to mount a successful defense in the lung to protect against lung lesions. However, aging appears to render the lung vulnerable to cancer progression. Note, however, that we did not examine for lung metastasis in other organs, but suspect that they too may experience increased metastasis based on the work by others showing that the intravascular injection of radiolabeled K1735M2 melanoma cells into senescence-accelerated mice prone 10 (SAMP10) results in increased metastatic colonies in aged animals, specifically in lung as well as in spleen and kidney. Using positron emission tomography, they found that the K1735M melanoma cells also tended to remain in the lungs of aged mice, whereas they rapidly disappeared in those of young mice, thereby supporting our findings, but also unveiling perhaps another mechanism for increased lung metastasis in the aging lung (increased retention in lung) (29). Thus, our data, especially data generated when tumor cells were injected into animals intravascularly, suggest that increased lung metastasis was likely related to aging promoting increased tumor cell entrance into tissues or increased retention, rather than enhanced tumor metastatic potential.
Second, we showed that lung tissue inflammation/remodeling was associated with increased lung metastases, but only in aged animals suggesting an interplay between aging and lung injury. In our hands, bleomycin induces fibrosis in both young and aged mice, with aged mice showing greater injury.Citation20 Young mice, however, still failed to develop lung metastases, which suggests that inflammation and/or tissue remodeling is not sufficient for cancer progression in our model. We hypothesize that the aberrant extracellular matrix of an injured lung is partly responsible for the increased metastatic lesions observed in our model; this represents the main reason we injected the cells late in the model (14 days after bleomycin administration). Unfortunately, we have not performed experiments during early time points of bleomycin administration, but Orr and coworkers examined lung metastasis in rodents five days after bleomycin administration. They found increased lung metastasis in the bleomycin-treated animals even though these were young animals (30). However, two important differences in experimental design should be noted. First, they administered bleomycin intravascularly, which is more likely to promote vascular injury, while we injected bleomycin intratracheally. Second, they tested fibrosarcoma cells and not LLCs, as we did here. Independent of the above, the early inflammatory and late remodeling responses observed in the bleomycin model are not well circumscribed in time. This is the very reason we use the term ‘inflammatory/remodeling’ as we cannot formally assign effect to either one. Thus, in light of the fact that aged mice show greater injury in response to bleomycin (e.g., see ref. 20), it remains to be determined if increased inflammation or increased fibrosis contributes to the increased susceptibility to lung cancer. Other age-related factors capable of contributing to the effects observed are likely and are currently under investigation.
Third, we observed that, even though fibronectin EDA is increased in aged lungs and has been implicated in cancer, this matrix molecule was not required for cancer progression in normal lungs. Fibronectin EDA has been shown to be a vascular marker for solid tumors and metastases.Citation31 and immunization against fibronectin EDA in a therapeutic setting has also been recently shown to decrease tumor burden and lung metastases in the MMTV-PyMT transgenic model of metastatic mammary carcinoma.Citation32 Based on these observations, we hypothesized that the aging lung contains a pro-oncogenic microenvironment due to increased levels of fibronectin EDA that renders the host susceptible to lung cancer progression. However, this was not found to be the case in our model. We found tumors in the lung even in FN EDA KO mice suggesting that this matrix molecule is not required for lung metastasis. Of note, we did not evaluate lung metastasis in KO animals injected with tumor cells intravascularly, which prevents us from evaluating if the effects were related to alterations in tumor cell exit from the primary site or enhanced entry into the secondary site. In addition, other pro-inflammatory and pro-fibrotic factors differentially expressed in FN EDA KO mice could contribute to our observations and this is currently under investigation. For example, fibronectin EDA upregulates Arginase 1 through integrins in myeloid cells, resulting in increased cancer growth in another experimental model.Citation33
Fourth, although fibronectin EDA could not explain the increased susceptibility to lung metastasis in the aged lung, it was required for the augmentation of lung metastasis in response to tissue injury induced by bleomycin in aged animals. Of interest was the fact that, despite a report in the literature that fibronectin EDA is essential for bleomycin induced lung fibrosis (e.g., see ref. 26), in our hands, FN EDA KO mice developed the same degree of bleomycin-induced lung fibrosis as WT animals. In this regard, the bronchoalveolar lavage fluids harvested from bleomycin-treated WT and FN EDA KO animals were similar in their ability to stimulate matrix (e.g., fibronectin) expression in cultured fibroblasts. Thus, this might be related to differences in the age and sex of animals used, and batch of bleomycin administered in the different studies. Nevertheless, we did not observe an augmentation of lung metastasis in response to bleomycin in FN EDA KO animals, which suggests that this matrix molecule might play a role in lung cancer progression in the setting of aging coinciding with tissue remodeling/inflammation. Several mechanisms may explain the pro-oncogenic effects of fibronectin EDA as it may serve as a scaffold for the proliferation, organization, retention, and migration of tumor cells. Moreover, the presence of the EDA domain has been shown to enhance the binding of fibronectin to the α5β1 integrin, which we have previously shown to be required for LLC metastases to the lung.Citation34 Interestingly, however, fibronectin EDA is not necessary for the spontaneous development of tumors in the lung, as evidenced by the fact that FN EDA KO mice crossed with mice harboring mutations in Kras, still developed lung lesions (unpublished personal observations). In the end, our data point to a potential role for FN EDA in age-related lung metastasis in the setting of tissue inflammation/remodeling, but much work is required to define its exact role and the mechanisms involved.
Finally, we observed that the increased susceptibility of aged mice to lung metastasis in the LLC model was not seen in NSG mice. This suggests that the increased number of metastases observed in aged WT mice is somehow dependent on alterations in immune responses involving B, T and/or NK cell–related mechanisms. Of interest, however, was the observation that aged NSG mice developed metastases to the lung that were larger in size compared to metastases in young NSG mice, again, indicating an effect of aging. This requires further investigation, but the model was tested because of concerns that immunity could affect tumor cell exit from the primary site, clearance of tumor cells before they establish colonies in the lung, or entry into the lung. We recognize that the approach is somewhat limited, however, the data further emphasize that host factors are important in lung metastasis in our model.
Overall, we report that aging acts in concert with lung injury to further increase the risk of lung cancer progression. This work adds to a growing body of literature implicating both aging and lung injury as risk factors for lung cancer development and progression.Citation35 While aging is thought to promote lung cancer progression through immune-mediated factors, the amplifying effects of lung injury in aging appear dependent on fibronectin EDA. These data, along with those of Sueblinvong, et al. (e.g., see ref. 20), suggest that young mice are able to successfully repair bleomycin-induced lung injury, while aged mice are predisposed for disrepair, and thus more susceptible to lung cancer progression. Our data suggest that this aberrant repair in the aged lung is facilitated by fibronectin EDA, which is not only increased in the aged lung, but also increased in the aged lung in response to bleomycin, when compared to young lungs. It is important to note that these mechanisms may vary in different cancers as aging does not appear to affect tumor progression similarly when different tumors are tested.Citation36 Taken together, our data suggest that age-dependent host immune factors influence lung cancer progression. Importantly, lung inflammation and tissue remodeling augment pulmonary metastasis in the aging lung, but not in young lungs, through mechanisms involving fibronectin EDA, which perhaps provides a scaffold for tumor cell migration/organization and proliferation. This points to an interplay between lung aging and inflammation/remodeling in lung tumor progression. Admittedly, strategies for targeting fibronectin in the clinic would be limited due to the high concentration of fibronectin in the plasma and other organs, but much work is being done in this area.Citation34 Perhaps a better approach would be to target fibronectin recognition by blocking its association with its various integrins.Citation34 This report underscores the need for further studies in this area to unveil new anti-lung cancer therapies.
Materials and methods
Reagents and cell culture
Lewis Lung Carcinoma (LLC) cells were purchased from ATCC (CRL-1642) and grown in Dulbecco’s modified Eagle’s medium (Corning DMEM, 10013 CM) with 10% fetal bovine serum (Atlanta Biologicals, S11150) at 37°C in a humidified 5% CO2 incubator. Cells were used within 3 months of resuscitation, and the presence of mycoplasma in cell cultures was monitored by use of the Lonza Mycoplasma Test KIT (Fisher Scientific, NC9725794). Bleomycin (B8416) and cellular fibronectin (cFN; F2518) were purchased from Sigma Aldrich. Sutures for surgery were purchased from Ethicon (K833).
Young and aged animals
WT C57BL/6 J and NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NOD scid gamma, NSG) mice (all female) were obtained from Jackson Laboratories and housed in pathogen-free facility at the University of Louisville, with access to food and water ad libitum. FN EDA KO mice (female) were obtained from Dr. Eric White (University of Michigan) and verified by PCR analysis and sequencing. They were developed in 2003 by Muro, et al., and are in a C57BL/6 background.Citation25 All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Louisville.
LLC model of lung cancer
LLC cells were injected (1 x 106/100 μL sterile PBS) subcutaneously into the hind flank of mice. In parallel experiments, animals were injected with LLC cells intravenously (1 x 105/100 µL sterile PBS). Afterward, tumors were monitored and the length, width, and height, measured weekly, using digital calipers. A tumor size >15 mm in any direction was considered the endpoint, per IACUC regulations. All animals were then sacrificed, and tissues were harvested for analysis. The same procedure and number of cells was performed for injections of LLC cells into NSG mice.
Bleomycin model of lung injury and repair
Bleomycin sulfate was reconstituted in sterile PBS at 1 mU/g of body weight. Animals were anesthetized using a mixture of ketamine and xylazine. Under aseptic technique, the neck of the mouse was dissected, and the trachea was visualized. Bleomycin or PBS was instilled in the trachea, using a 27 G needle, in a total volume of 40 uL. The wound was sutured and animals were monitored and allowed to recover on a heating pad.
Tissue processing and histological analysis
Lungs were flushed with PBS, inflated at standard pressure with formalin, isolated, formalin-fixed, processed, paraffin-embedded, and sectioned (6 um) using a JUNG RM2055 microtome (Leica). They were then transferred onto charged, glass microslides (Fisher Scientific, 6776215) for histological analysis. Sections were deparaffinized, rehydrated, and stained with H&E (Fisher Scientific, 22050201 and 22050197) to evaluate lung tumors, or Mason’s trichrome (Fisher Scientific, 22110648) to evaluate collagen content for lung fibrosis determination. Five blinded reviewers used the semi quantitative Ashcroft scoring scale to score pulmonary fibrosis.Citation37
Wound healing (Scratch Assay)
LLC cells (2.5 x 105) were plated in 24-well plates coated with cellular fibronectin (10 ug/mL) or plastic alone for 24 hours. A scratch was then created using a 1 mL pipette tip. Media (2 mL) was then replaced and photographs taken at 0 and 24 hours. Quantification was performed using ImageJ software.
Analysis of data
Means plus standard deviations of the mean were calculated for all experimental values, unless otherwise noted. Significance was assessed by using the Student’s t test.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Disclosure of interest
The authors report no conflict of interest.
Acknowledgments
The authors would like to thank Aneesha Carter for her participation in this project and Dr. Eric White at the University of Michigan for kindly providing FN EDA KO mice for use in this research.
Additional information
Funding
References
- American Cancer Society. Cancer facts & figures 2017. Vol. 2017. Atlanta, GA USA: 2017 American Cancer Society.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29.
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300.
- Krishnan KJ, Greaves LC, Reeve AK, Turnbull D. The ageing mitochondrial genome. Nucleic Acids Res. 2007;35:7399–7405.
- Makinodan T, Marguerite K. Age influence on the immune system. Adv Immunol. 1980;29:287–330.
- Campisi J. Cancer and ageing: rival demons? Nat Rev Cancer. 2003;3:339–349.
- Kirkwood TBL. Understanding the odd science of aging. Cell. 2005;120:437–447.
- Yao H, Rahman I. Current concepts on the role of inflammation in COPD and lung cancer. Curr Opin Pharmacol. 2009;9:375–383.
- Park CC, Bissell MJ, Barcellos-Hoff MH. The influence of the microenvironment on the malignant phenotype. Mol Med Today. 2000;6:324–329.
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in Vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245.
- Wang F, Hansen RK, Radisky D, Yoneda T, Barcellos-Hoff MH, Petersen OW, Turley EA, Bissell MJ. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J Natl Cancer. 2002;94:1494–1503.
- Sato M, Vaughan MB, Girard L, Peyton M, Lee W, Shames DS, Ramirez RD, Sunaga N, Gazdar AF, Shay JW, et al. Multiple oncogenic changes (K-RAS(V12), p53 knockdown, mutant EGFRs, p16 bypass, telomerase) are not sufficient to confer a full malignant phenotype on human bronchial epithelial cells. Cancer Res. 2006;66:2116–2128.
- Tockman MS, Anthonisen NR, Wright EC, Donithan MG. Airways obstruction and the risk for lung cancer. Ann Intern Med. 1987;106:512–518.
- Yang H, Bocchetta M, Kroczynska B, Elmishad AG, Chen Y, Liu Z, Bubici C, Mossman BT, Pass HI, Testa JR, et al. TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc Natl Acad Sci U S A. 2006;103:10397–10402.
- Philip M, Rowley DA, Schreiber H. Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol. 2004;14:433–439.
- Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316.
- Martins-Green M, Boudreau N, Bissell MJ. Inflammation is responsible for the development of wound-induced tumors in chickens infected with Rous sarcoma virus. Cancer Res. 1994;54:4334–4341.
- Aoshiba K, Nagai A. Chronic lung inflammation in aging mice. FEBS Lett. 2007;581:3512–3516.
- Calabresi C, Arosio B, Galimberti L, Scanziani E, Bergottini R, Annoni G, Vergani C. Natural aging, expression of fibrosis-related genes and collagen deposition in rat lung. Exp Gerontol. 2007;42:1003–1011.
- Sueblinvong V, Neujahr DC, Mills ST, Roser-page S, Ritzenthaler JD, Guidot D, Rojas M, Roman J. Predisposition for disrepair in the aged lung. Am J Med Sci. 2012;344:41–51.
- Zheng Y, Ritzenthaler JD, Roman J, Han S. Nicotine stimulates human lung cancer cell growth by inducing fibronectin expression. Am J Respir Cell Mol Biol. 2007;37:681–690.
- Malik G, Knowles LM, Dhir R, Xu S, Yang S, Ruoslahti E, Pilch J. Plasma fibronectin promotes lung metastasis by contributions to fibrin clots and tumor cell invasion. Cancer Res. 2010;70:4327–4334.
- Pontiggia O, Sampayo R, Raffo D, Motter A, Xu R, Bissell MJ, De Kier Joffé EB, Simian M. The tumor microenvironment modulates tamoxifen resistance in breast cancer: A role for soluble stromal factors and fibronectin through β1 integrin. Breast Cancer Res Treat. 2012;133:459–471.
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091.
- Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, Stanta G, Baralle FE. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J Cell Biol. 2003;162:149–160.
- Muro AF, Moretti FA, Moore BB, Yan M, Atrasz RG, Wilke CA, Flaherty KR, Martinez FJ, Tsui JL, Sheppard D, et al. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:638–645.
- Balducci L, Extermann M. Cancer and aging. An Evolving Panorama. Hematol Oncol Clin North Am. 2000;14:1–16.
- Hecht SS. Tobacco smoke carcinogen and lung cancer. J Natl Cancer Inst. 1999;91:1194–1210.
- Shimizu K, Shimizu NK, Asai T, Tsukada H, Oku N. Enhanced experimental tumor metastasis with age in senescence-accelerated mouse. Biol Pharm Bull. 2008;31:847–851.
- Orr FW, Adamson IYR, Young L. Promotion of pulmonary metastasis in mice by bleomycin-induced endothelial injury. Cancer Res. 1986(46):891–897.
- Rybak JN, Roesli C, Kaspar M, Villa A, Neri D. The extra-domain A of fibronectin is a vascular marker of solid tumors and metastases. Cancer Res. 2007;67:10948–10957.
- Femel J, Huijbers EJM, Saupe F, Cedervall J, Zhang L, Roswall P, Larsson E, Olofsson H, Pietras K, Hellman L, et al. Therapeutic vaccination against fibronectin ED-A attenuates progression of metastatic breast cancer. Oncotarget. 2014;5:12418–12427.
- Rossnagl S, Altrock E, Sens C, Kraft S, Rau K, Milsom MD, Giese T, Samstag Y, Nakchbandi IA. EDA-Fibronectin Originating from Osteoblasts Inhibits the Immune Response against Cancer. PLoS Biol. 2016;14:1–32.
- Roman J, Ritzenthaler JD, Roser-Page S, Sun X, Han S. Alpha5Beta1-Integrin Expression Is Essential for Tumor Progression in Experimental Lung Cancer. Am J Respir Cell Mol Biol. 2010;43:684–691.
- Arenberg D, Luckhardt TR, Carskadon S, Zhao L, Amin MA, Koch AE. Macrophage migration inhibitory factor promotes tumor growth in the context of lung injury and repair. Am J Respir Crit Care Med. 2010;182:1030–1037.
- Wang JP, Hielscher A. Fibronectin: how Its Aberrant Expression in Tumors May Improve Therapeutic Targeting. J Cancer. 2017;8:674–682.
- Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41:467–470.
