ABSTRACT
Rituximab is popularly used in the treatment of B-cell lymphomas that bear CD20 antigen. Most of the adverse events (AEs) induced by rituximab are infusion-related symptoms. However, rituximab-induced acute thrombocytopenia (RIAT), which often develops within the 1–3 days after rituximab administration, is relatively unusual, severe, and usually self-recovering. Until now, most of the reports about RIAT were described as case reports and RIAT often occurred in patients with mantle cell lymphoma (MCL). Here, we report two patients who developed severe RIAT, one patient had a refractory and relapsed follicular lymphoma (FL), and the other patient was newly diagnosed with splenic marginal zone lymphoma (SMZL). RIAT is a rare, under-diagnosed but serious adverse event that should arouse attention to clinicians, and routine blood count monitoring should be considered after the administration of rituximab, especially for high-risk lymphoma patients or patient with splenomegaly.
Background
Rituximab is a well-tolerated and classic chimeric human/murine monoclonal antibody that contains murine light- and heavy-chain variable region sequences and human κ and IgG1 constant region sequences.Citation1 It is commonly used in the treatment of B-cell lymphoma that bears CD20 antigen such as diffuse large B cell lymphoma (DLBCL), follicular lymphoma (FL), Waldenstrom’s macroglobulinemia/lymphoplasmacytic lymphoma (WM/LPL), mantle cell lymphoma (MCL), and chronic lymphocytic leukemia (CLL), etc. Despite in lymphoma, rituximab plays an important role in the management of refractory and relapsed immune thrombocytopenia (ITP), autoimmune hemolytic anemia (AIHA), and other diseases triggered by an abnormal B lymphocyte population.Citation2,Citation3
The mechanisms of rituximab are as follows: complement-mediated cytotoxicity (CDC), induction of antibody-dependent cell cytotoxicity(ADCC) and cellular phagocytosis, direct antitumor effect via apoptosis or other cell death pathways.Citation4 Since rituximab is a chimeric murine/human monoclonal antibody that contains allogeneic protein, it may cause an allergic-like reaction. Most of the adverse events (AEs) induced by rituximab are infusion-related symptoms, such as fever, chills, asthenia, headaches, bone or muscle pain, chest pain, hypotonia, pruritus, nausea, dizziness, angioedema, and urticaria.Citation5,Citation6 However, severe thrombocytopenia after the administration of rituximab is unusual. Despite infusion-related reactions (IRRs), an increased risk of infection and pancytopenia have been reported in previous studies.Citation7,Citation8 Delayed-onset peripheral thrombocytopenia can develop several weeks after rituximab infusion. Chiara et al. reported the frequency, risk factor assessment, and possible pathogenesis of delayed-onset peripheral blood cytopenia after rituximab in 77 treatments.Citation9 Thrombocytopenia was observed in 8 out of 23 (10.4%) patients 21–180 days after the last dose of rituximab. Multivariate analysis showed that previous treatment with chemotherapy and more than four rituximab doses were significantly associated with a higher risk of post rituximab delayed-onset cytopenia. However, rituximab-induced acute thrombocytopenia (RIAT) is rarer than other side effects, and most of the reports of RIAT are described as case reports.Citation10–12 Among the current case reports, the largest population to develop RIAT was patients who suffered from MCL or hairy cell leukemia (HCL).Citation12,Citation13 By retrieving studies from the NCBI database, we found only two patients who were diagnosed with FL and developed RIAT.Citation11,Citation14 No former similar phenomenon in a patient with splenic marginal zone lymphoma (SMZL) has been reported. Here, we report two patients who developed severe RIAT; one patient had a refractory FL, and the other patient was newly diagnosed with SMZL. These patients share the same characteristic of notable splenomegaly. To our knowledge, this is the third reported case of FL and the first reported case of SMZL.
Case report
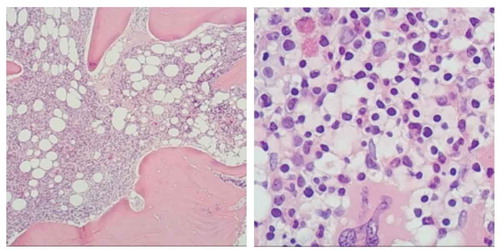
Patient 1: A 56-year-old woman who was diagnosed with stage IV FL was enrolled in our ward in 2011. She presented with symptoms of fatigue, anomaly, and bloating. Inguinal lymph node biopsy was performed, and the diagnosis was confirmed by immunohistochemistry (IHC), with results showing CD20(+), PAX-5(+), Bcl-2(+), CD10(+), Bcl-6(+), LCA(+), CD21(+), CD3(-), CD5(-), D45R0(-), Cyclin D(-), and Ki-67 30%. Bone marrow biopsy was also performed, and the results confirmed bone marrow infiltration. She was diagnosed with FL (grade 2, stage IV, B group), and the FLIPI score was 3 (high risk). Three cycles of the R-CHOP regimen (rituximab combined cyclophosphamide, adriamycin, vindesine, and prednisone) were carried out, and no obvious rituximab-associated serum sickness was observed. She only developed grade III neutropenia on day 9 in her first cycle of chemotherapy, and neutropenia was resolved promptly with granulocyte colony-stimulating factor (G-CSF). Anemia or thrombocytopenia had not been observed during chemotherapy. After three cycles of R-CHOP, the patient reached partial remission (PR), which was confirmed by enhanced contrast CT. She rejected receiving further therapy and was not reassessed until April 2018. She had experienced night sweats, fatigue, and floating during the preceding 7 months. Physical examination found multiple sites of shallow lymphadenopathy, especially in her right neck. Her spleen was 7.5 cm palpable below the left costal margin, and the ascites sign was positive (). Rebiopsy was performed in her right neck lymph node, and the IHC showed CD20(+), BCL-2(+), BCL-6(+), CD10(+), MUM-1 partially (+), CD21 FDC (+), CD3(-), CD5(-), and Ki-67 30%-40%. The level of LDH was 255 U/L (). The routine blood test showed a WBC of 2,710, neutrophil count of 1,100, a Hb of 90 g/L, and a platelet count of 84. The bone marrow biopsy showed lymphocytic cell infiltration (4%); the number of megakaryocytes was 121, and piles of platelets could be found. Flow cytometry analysis showed a group of unusual cells that expressed CD19, CD20, CD10, HLA-DR, cCD22, and cCD79a and partially expressed FMC7, CD23, and cLambda. The immunophenotype of malignant lymphocytes in the bone marrow was CD10(+), CD20(+), PAX-5(+), CD3(-), CD5(-), CD23(+), and Cyclin D1(-) (). Chromosomal analysis revealed 46, XX; thus, the diagnosis of relapsed FL (grade 3a, stage IV, B group, FLIPI 1 Score 4, high risk) was made.
Figure 2. (Right cervical lymph node) biopsy: Lymph node structure destruction, follicular hyperplasia, sleeve atrophy or disappearance, cell morphology in follicles tend to be consistent, mainly center cells, central mother cells >15/HPF. IHC: CD20(+), BCL-2(+), BCL-6(+), CD10(+), MUM-1 partially (+), CD21 FDC (+), CD3(-), CD5(-), Ki-67 30%-40%
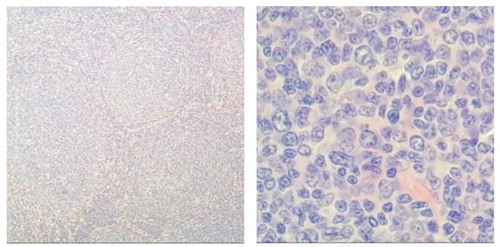
Figure 3. Bone marrow biopsy: HE and PAS staining showed more active bone marrow hyperplasia (70%), lymphocytosis (40–50%), focal and scattered distribution, infiltrating lesions were mostly located next to the trabecular, small cell bodies, irregular nuclei. The mature erythroid cells are scattered and the number of megakaryocytes is roughly normal. Reticulated fiber dyeing (MF-2 grade)
Considering that the patient had not received rituximab-containing chemotherapy within one year, we chose the R-CHOP regimen again, and rituximab was administered on day 0 with a dose of 600 mg. She developed chills and fever one hour after rituximab infusion, and these symptoms were solved with methylprednisolone. Blood culture showed no bacterial growth, and the inflammation index had no positive result. The next day, the repeat hemogram showed a WBC of 4,210, a Hb of 92 g/L, and a platelet count of 26,000. The coagulation profile was within normal limits. The patient did not show any signs of bleeding. She received thrombopoietin (TPO, 15,000 U) subcutaneous injection per day to prevent bleeding. The platelet count recovered to the baseline platelet level within one week. She then developed pancytopenia, which was considered secondary to cytotoxic chemotherapy, one week later. After the first cycle of R-CHOP, the symptoms of weakness and floating disappeared, and the splenomegaly improved.
She received a second cycle of R-CHOP on day 21. The follow-up blood cell counts were performed at regular intervals to monitor thrombocytopenia development. She had a similar episode during the second cycle of R-CHOP with a manifestation of chills and fever.
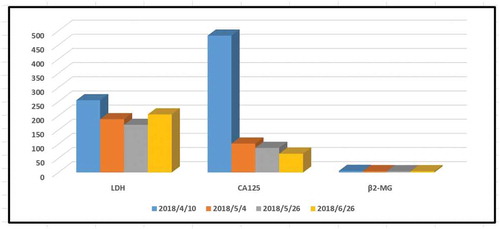
The changes in platelet count were shown in . Then, an assessment was performed, and PR had not been achieved. Therefore, we replaced R-CHOP with R-GDP (rituximab, cisplatin, gemcitabine, and dexamethasone) as salvage therapy. Thrombocytopenia developed again as we expected, and the infusion-related adverse effects occurred as before. Similar to the former treatment, acute thrombocytopenia did not result in any bleeding complications and did not meet the threshold criteria for platelet transfusion. Interestingly, the severity of thrombocytopenia was much weaker than the former cycles of treatment (). The trends of CA 125, LDH, and β2-microglobulin are shown in . No more intervention was given, and the platelet count recovered again within one week. After two cycles of R-GDP, no obvious response was achieved, and the patient died of the progression of the disease.
Figure 4. Platelet count during the rituximab contained chemotherapy period (Patient 1)
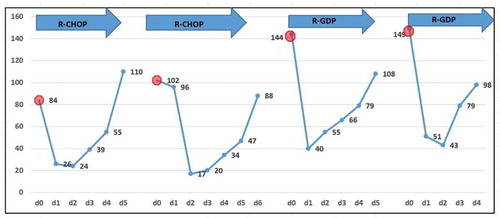
Figure 5. CA125, LDH, and β2-microglobulin during the chemotherapy period. After several cycles of chemotherapy including rituximab, the level of LDH and CA125 decreased to the normal level, indicating a rapid lysis of lymphoma cells occurred
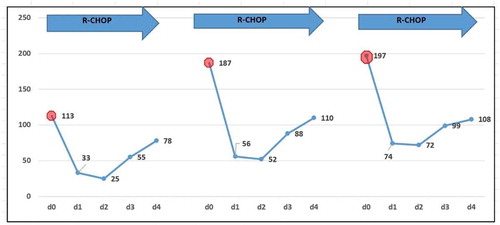
Patient 2: A 63-year-old female patient came to the clinic with the main complaints of bloating, underweight, and fatigue for approximately one year and double lower extremity edema for almost three months. The result of enhanced contrast CT of the abdomen showed hypersplenism (9.8 cm palpable below the left costal margin) with a small number of ascites. The blood test showed a WBC of 7,870, a Hb of 94 g/L, and a platelet count of 11,300. The lymphocytes in the peripheral blood were 38%, and the juvenile-like cells were approximately 5%. A bone marrow aspiration was conducted, and the results showed that abnormal lymphocytes accounted for 52% and were medium, round, or oval-shaped. The nuclear chromatin of these cells was more detailed; some cells had nucleoli, and the amount of cytoplasm was small. The number of megakaryocytes was 89, and platelets were easily found. Flow cytometry of the bone marrow showed a cluster of 46.11% abnormal monoclonal mature B cells with HLA-DR, CD19(+), CD5(+), CD20(+), FMC-7(+), CD22(+), IgM(+), cLambda(+), CD11c partially (+), and Ki-67 < 5% (). The pathologic result of the bone marrow aspiration confirmed a diagnosis of marginal zone lymphoma. The chromosome analysis showed 46, XX, del (7) (q22) [15]/46, XX [5]. The MYD88 L265P mutation was negative for this patient. An ultrasound indicated bilateral supraclavicular and bilateral inguinal lymphadenopathy. The CT scan in her chest showed multiple lymphadenopathies in her mediastinal. However, the PET/CT showed whole-body lymphadenopathy with no increase in FDG metabolism (). Obvious splenomegaly was confirmed again, and the SUV was approximately 2.3 with a Deauville Criteria 4 (the average SUV of the liver was 1.7). The diagnosis of SMZL (stage IV, B group) was confirmed, and R-CHOP was given to her. No rituximab infusion-related symptoms, such as fever and chills, were observed. However, on the next day after rituximab infusion, a routine blood test showed a decrease in the platelet count and the monitor of the blood test showed a transient recovery of the count of platelets with no neutropenia or anemia during thrombocytopenia (). She then developed pancytopenia, which was considered secondary to cytotoxic chemotherapy, one week later. The neutropenia resulted in a moderate fever, which was resolved by G-CSF and meropenem. The platelet count decreased to 21,000 at that time and recovered to normal levels on the fourth day of neutropenia. The patient received an additional two cycles of R-CHOP and developed RIAT each time. However, the extent of the RIAT was slight, and neutropenia did not occur with the use of long-lasting G-CSF prophylaxis (). After three cycles of R-CHOP, the splenomegaly improved significantly, and RIAT did not develop in the fourth cycle of R-CHOP. The patient is currently waiting for the fifth cycle of chemotherapy, and blood test monitoring is ongoing.
Figure 6. Bone marrow biopsy: HE and PAS staining showed that myeloid hyperplasia was normal (30–40%), small lymphocytes increased (40–50%), scattered and focal distribution, granulosa cells scattered in the mature stage, and the number of megakaryocytes was almost normal. Reticulated fiber staining (MF-2 grade, focal)
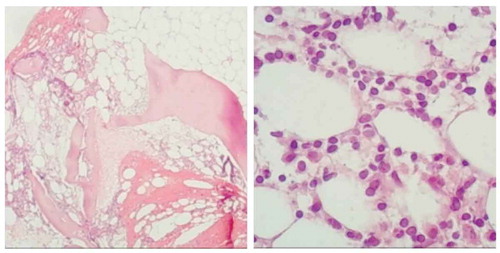
Figure 7. PET/CT showed an obvious splenomegaly was confirmed again and the SUV was about 2.3 with a Deauville Criteria 4 (the average SUV of the liver was 1.7)
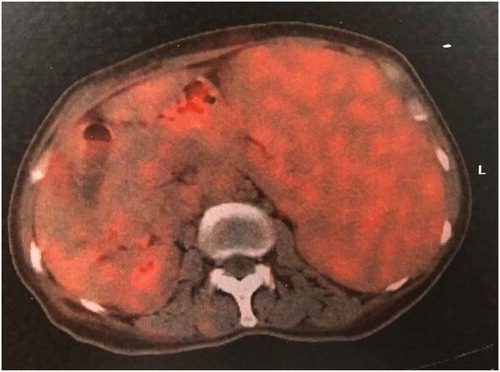
Figure 8. Platelet count during the rituximab contained chemotherapy period (Patient 2)
Discussion and conclusions
Rituximab is a well-tolerated and classic chimeric human/murine monoclonal antibody directed against CD20, a B cell-specific antigen, and rituximab has been proven to improve the prognosis of many types of invasive B cell lymphoma. Frequent clinical side effects of rituximab are infusion-associated serum sickness-like syndrome, which usually occurs simultaneously or several hours after rituximab administration. However, severe acute thrombocytopenia after the administration of rituximab is unusual. Here, we reported two female patients with massive tumor burden of FL or SMZL who both developed RIAT.
FL is a form of B cell lymphoma that accounts for approximately 8.1% to 23.5% of all subtypes of lymphoma in China.Citation15 The FLIPI scoring system is popularly used as a prognostic assessment tool. For patients in the medium- to high-risk FLIPI score groups, lymphadenopathy, splenomegaly, or involvement of extranodal is common.Citation16 For these patients, although R-CHOP is still the classic regimen as the first line of treatment, some patients will relapse, and when relapse occur7s, the disease is usually refractory.Citation17 SMZL is a low-grade B cell NHL characterized by massive splenomegaly, moderate lymphocytosis, rare involvement of peripheral lymph nodes, and indolent clinical course.Citation18 However, 30% of patients with SMZL develop aggressive disease with a median overall survival of only four years.Citation19 There is no standard strategy for the treatment of SMZL, and the classic R-CHOP regimen is used in patients with lymphadenopathy or B symptoms. Patients who met the following criteria were considered to be in the high tumor burden group: bulk (>7 cm) or 3 lymph nodes in distinct areas >3 cm; symptomatic splenic enlargement; organ compression by tumor, pleural or peritoneal effusion; elevated LDH or β2-microglobulin; and B symptoms.Citation17 High tumor burden patients usually respond poorly to the R-CHOP-like regimen even though these patients receive more intensive chemotherapy. Patient 1 in this report met all of the above items and exhibited no response to treatment containing rituximab.
RIAT is a rare adverse event compared to other common side effects, such as serum sickness-like syndromes, and RIAT usually occurs within 1 to 3 days after rituximab infusion, the time before chemotherapy-induced myelosuppression.Citation17 RIAT can bring a high bleeding risk because it is often underdiagnosed due to its low incidence. Although the mechanism of RIAT is still unclear, most of the patients who developed this rare comorbidity share the following common clinical characteristics: MCL as the predominant histological subtype; presenting infusion-associated symptoms, including fever, rigor, and chills, etc.; massive tumor burden, bone marrow infiltration, and splenomegaly; re-exposure to rituximab usually does not induce a repetitive RIAT but there are exceptions; RIAT is usually transient and self-limiting; and commonly, no platelet infusion is needed.Citation13,Citation14,Citation20 However, since the number of case reports of RIAT is small, a more accurate and convincing conclusion requires more clinical cases to confirm RIAT.
Theoretically, lymphomas that bear the CD20 antigen can develop RIAT as long as rituximab was used. MCL was the most frequent subtype of NHL associated with RIAT.Citation21 Currently, most of the articles about RIAT are case reports, except for one analysis performed by Medizinische and his partners.Citation10 The authors retrospectively analyzed the change in platelet counts following the administration of rituximab in 253 patients with non-Hodgkin’s lymphoma (NHL). The 30% decrease in the platelet count was chosen as a cutoff value. The authors found that for the entire patient group, a 30% decrease in the platelet count on at least one of the 3 days following rituximab infusion was 6.2% in 65 cycles. The relative decrease in platelet count was found more often in patients with MCL, Burkitt’s lymphoma (BL), and indolent lymphoma other than FL compared to DLBCL, FL, and other B cell lymphomas. Furthermore, the percentual decrease in the platelet count associated with rituximab was more pronounced when the initial platelet count was below 100*109/L. Patients with splenomegaly, bone marrow infiltration, and leukemic presentation of lymphoma also showed a notable decline in platelets. Interestingly, most of the patients who developed RIAT did not show a repetitive decrease in platelet count when exposed to rituximab in the following cycles.Citation10 Santhosh K. et al. reported five patients suffering from MCL who developed RIAT. Although most of the patients had no bleeding complications, four of the five patients received a platelet infusion. For all of the five patients, subsequent retreatment with rituximab did not result in RIAT again, except one patient who received only one cycle combined chemotherapy including rituximab.Citation21 Similarly, Hazem El-Osta et al. reported a patient who was diagnosed with MCL and developed acute thrombocytopenia after rituximab infusion. Her platelets dropped from 175 to 16*103/ul within hours of rituximab treatment and recovered to a normal level after 6 days without transfusion support. No RIAT occurred when the patient was given the subsequent 5 more cycles of chemotherapy containing rituximab; in addition, this patient received maintenance therapy with rituximab.Citation20 The explanation for the greater incidence rate of RIAT in MCL is unclear. Some explanations include the following: first, some researchers observed that the number of CD20 molecules per cell was higher in patients with MCL than in patients with other subtypes.Citation22,Citation23 Second, the level of CD20 expression was reported to be higher in peripheral lymphocytes than in bone marrow cells.Citation24 Thus, elevated expression of CD20 may contribute to an increase in circulating CD20, resulting in the relatively frequent occurrence of RIAT in MCL.
Compared to MCL, RIAT in FL is not a frequent adverse event. To the best of our knowledge, we found only two recent reports of RIAT in patients with FL. In 2016, Hiroshi Ureshino reported the first patient with FL who developed RIAT on the day after rituximab infusion. The authors speculated that the occurrence of infusion-related cytokine release syndrome in the rituximab-sensitive massive tumor burden FL contributed to the development of RIAT.Citation14 Another case report was performed by Yoshiyuki Omura and his colleagues.Citation11 The authors described a 74-year-old patient who was diagnosed with FL and developed RIAT. Their case was different from the previous cases in several ways. First, coagulation abnormalities that mimicked disseminated intravascular coagulation occurred during rituximab infusion with increased levels of D-dimer and PT-INR. Second, in the former studies, RIAT usually did not happen again when re-exposed to rituximab, and even if RIAT did occur, the extent of the thrombocytopenia was similar or less serious than the first occurrence. However, for this patient, the severity of RIAT in the 4th cycle of rituximab therapy was much greater than that observed RIAT during the 3rd cycle, even though the tumor burden decreased, indicating that the tumor burden might not independently explain the pathogenesis of RIAT. Third, the patient did not present an infusion reaction on the subsequent courses of rituximab therapy, except for the first cycle. Until now, the reason for these differences has been unknown. The authors correlated the coagulation to the fact the infused rituximab interacted with lymphoma cells, which might activate immune components and cause consumption coagulopathy, thus expending platelets. For our patient, RIAT was transient, self-limiting, and repeatable; however, the extent of thrombocytopenia was less serious in later cycles compared to the first cycle. Although the bone marrow aspiration was almost normal, the splenomegaly and massive tumor burden did not improve, indicating that RIAT might be associated with disease progression.
Previous studies reported that RIAT was not always associated with infusion reactions, suggesting that the pathogenesis of these two adverse events might be different.Citation13 For our two patients in this report, one patient had a high tumor burden of FL and exhibited infusion reactions, while the other patient had SMZL and did not show signs of infusion-related symptoms. The accurate mechanism of RIAT is unknown, and the possible causes might be as follows: (1) The patient has soluble CD20 antigen in the circulation, binding them to platelets that causes immune-mediated cell lysis, especially in patients with advanced-stage tumors and bone marrow involvement.Citation25 (2) Platelets have an Fc receptor and CD20 antigen, and rituximab antibodies that bind to lymphoma cells also bind to platelets through their Fc receptors, leading to platelet degradation.Citation13 (3) With rapid lysis of lymphoma cells and changes in the levels of cytokines and complements, thrombocytopenia can be initiated by the induction of disseminated intravascular coagulation.Citation26 (4) Splenomegaly also contributes to the delay and destruction of platelets. (5) P38 mitogen-activated protein kinase and Akt antiapoptotic survival pathways might play important roles in causing cell deathCitation21. In vitro studies have demonstrated that rituximab-induced signaling results in the inhibition of P38 mitogen-activated protein kinase, extracellular signal-regulated kinase 1/2, and Akt antiapoptotic survival pathways leading to cell death. This signaling pathway is believed to be synergistic with cytotoxic chemotherapyCitation4. (6) Endothelial barrier disruption plays an important role in RIAT, especially in patients with massive tumor burden. Lymphoma cells massively infiltrate into the spleen (and other organs) and induce structural alterations in the endothelium and subendothelial space. Then, rituximab rapidly eradicates lymphoma cells, and the subendothelium is exposed to the flowing bloodCitation14. Cytokines released from normal and neoplastic lymphocytes and endothelial cells affect the integrity of the endothelial barrier, inducing platelet activation and aggregation, leading to transient acute thrombocytopenia, which is recovered with endothelial repairCitation14. RIAT is often transient and self-limiting, and corticosteroids used in the following days might help with recovery from thrombocytopenia. For our patient, a high level of LDH in the serum was observed, which indicated a high tumor burden to some extent. After several cycles of chemotherapy, which included rituximab, the level of LDH decreased to the normal level, indicating rapid lysis of lymphoma cells. Tumor lysis can initiate thrombocytopenia by the induction of disseminated intravascular coagulation, which might partially be interpreted as the mechanism of platelet elimination.
RIAT occurs in MCL, hairy cell leukemia, and FL, and theoretically, RIAT can be developed in all kinds of B cell lymphomas. M Bobot reported that a patient with chronic lymphocytic leukemia (CLL) developed RIATCitation27. Pamuk GE reported that a 75-year-old patient diagnosed with prolymphocytic leukemia developed RIAT four hours after the completion of rituximab infusionCitation28. Similarly, platelets in this patient recovered three days after rituximab infusion without any intervention. RIAT not only is observed in hematopoietic diseases but also occurs in other types of diseases, such as autoimmune diseases. Yushiro Endo reported a RIAT case in a patient with granulomatosis with polyangiitisCitation11. The patient developed RIAT from the first cycle of rituximab administration. In the two cycles of therapy, this patient showed no signs of hemorrhage. However, three days after the third cycle of rituximab infusion, her platelet count dropped again, and she developed bloody stools. Platelet infusion was given to the patient, and the bleeding stopped promptly. They correlated the RIAT to cytokine release syndrome (CRS).
Here, we reported that two patients developed repetitive and self-recovering RIAT. One patient was diagnosed with R/R FL with a massive tumor burden, and the other patient was newly diagnosed with SMZL. These two patients share the same characteristic of hypersplenism, even though the outcomes are distinctly different. The reason for this short-term phenomenon cannot be explained by the concomitant administration of chemotherapy because RIAT occurred on the next day after rituximab infusion and before chemotherapy administration. Consistent with the former studies, massive tumor burden, bone marrow infiltration, and splenomegaly might be risk factors for developing RIAT in these patients. Usually, rare patients with RIAT exhibit major bleeding episodes and tolerate subsequent rituximab and chemotherapy cycles well, suggesting that a change of regimen might be unnecessary. Furthermore, whether this rare and unique side effect of rituximab affects the overall response is not known. The acute mechanisms of RIAT need to be investigated in larger clinical samples in the future. Whether RIAT indicates the prognosis of the associated lymphoma should also be studied further. Overall, this rare, under-diagnosed but serious adverse event should arouse attention to clinicians, and routine blood count monitoring should be considered after the administration of rituximab, especially for high-risk lymphoma patients or patients with splenomegaly.
Abbreviations
| RIAT | = | Rituximab-induced acute thrombocytopenia |
| MCL | = | Mantle cell lymphoma |
| FL | = | Follicular lymphoma |
| SMZL | = | Splenic marginal zone lymphoma (SMZL) |
| ITP | = | Immune thrombocytopenia |
| CDC | = | Complement-mediated cytotoxicity |
| ADCC | = | Antibody-dependent cell cytotoxicity |
| ATs | = | Adverse events |
| IRRs | = | Infusion-related reactions |
| CRS | = | Cytokine release syndrome |
Authors’ contributions
Y.J.J. contributed to the concept development and drafted the manuscript. J.Q.S. provided the image data. N.W., D.Y., and L.L.F. collected the clinical data. H.T.Q. and J.F. contributed to figure preparation. All authors read and approved the final manuscript.
Consent for publication
Consent to publish this report was obtained from the patients.
Ethics approval and consent to participate
This study was approved by the ethics committee of Shandong Provincial Hospital Affiliated to Shandong University, and written informed consent was obtained from either the patients.
Acknowledgments
We express our sincere gratitude to the patients and their families for willingness to participate in this study.
Data availability statement
The datasets used for analysis during the current study are available from the corresponding author on reasonable request.
Disclosure statement
The authors declare no competing financial interests for this study.
Additional information
Funding
References
- Salles G, Barrett M, Foa R, Maurer J, O’Brien S, Valente N, Wenger M, Maloney DG. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther. 2017;34:2232–2273. doi:10.1007/s12325-017-0612-x.
- Ran NA, Payne AS. Rituximab therapy in pemphigus and other autoantibody-mediated diseases. F1000Research. 2017;6:83. doi:10.12688/f1000research.9476.1.
- Rodrigo C, Rajapakse S, Gooneratne L. Rituximab in the treatment of autoimmune haemolytic anaemia. Br J Clin Pharmacol. 2015;79:709–719. doi:10.1111/bcp.12498.
- Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47:115–123. doi:10.1053/j.seminhematol.2010.01.011.
- Kasi PM, Tawbi HA, Oddis CV, Kulkarni HS. Clinical review: serious adverse events associated with the use of rituximab - a critical care perspective. Crit Care. 2012;16:231. doi:10.1186/cc11304.
- Hainsworth JD. Safety of rituximab in the treatment of B cell malignancies: implications for rheumatoid arthritis. Arthritis Res Ther. 2003;5(Suppl 4):S12–6. doi:10.1186/ar1008.
- Nixon A, Ogden L, Woywodt A, Dhaygude A. Infectious complications of rituximab therapy in renal disease. Clin Kidney J. 2017;10:455–460. doi:10.1093/ckj/sfx038.
- Gobert D, Bussel JB, Cunningham-Rundles C, Galicier L, Dechartres A, Berezne A, Bonnotte B, DeRevel T, Auzary C, Jaussaud R, et al. Efficacy and safety of rituximab in common variable immunodeficiency-associated immune cytopenias: a retrospective multicentre study on 33 patients. Br J Haematol. 2011;155:498–508. doi:10.1111/j.1365-2141.2011.08880.x.
- Cattaneo C, Spedini P, Casari S, Re A, Tucci A, Borlenghi E, Ungari M, Ruggeri G, Rossi G. Delayed-onset peripheral blood cytopenia after rituximab: frequency and risk factor assessment in a consecutive series of 77 treatments. Leuk Lymphoma. 2006;47:1013–1017. doi:10.1080/10428190500473113.
- Wilop S, Galm O, Dada R, Osieka R, Jost E. Rituximab-associated changes in platelet count in patients with non-Hodgkin lymphoma. Leuk Lymphoma. 2008;49:2116–2124. doi:10.1080/10428190802503377.
- Omura Y, Shimazu H, Takahashi T. Rituximab-induced acute thrombocytopenia in a patient with follicular lymphoma: a case report and review of the literature. Internal Med. 2018;57:1151–1154. doi:10.2169/internalmedicine.9628-17.
- Dhand S, Bahrain H. Rituximab-induced severe acute thrombocytopenia: a case report and review of literature. Cancer Invest. 2008;26:913–915. doi:10.1080/07357900802010509.
- Ram R, Bonstein L, Gafter-Gvili A, Ben-Bassat I, Shpilberg O, Raanani P. Rituximab-associated acute thrombocytopenia: an under-diagnosed phenomenon. Am J Hematol. 2009;84:247–250. doi:10.1002/ajh.21372.
- Ureshino H, Nishioka A, Kojima K, Suzuki M, Kizuka H, Sano H, Shindo T, Kubota Y, Ando T, Kimura S, et al. Rituximab-induced acute thrombocytopenia in high tumor burden follicular lymphoma. Internal Med. 2016;55:2061–2064. doi:10.2169/internalmedicine.55.6140.
- Huang HQ, Guan ZA, Shen T, Shen ZX, Jiang WQ. Guidelines for the diagnosis and treatment of follicular lymphoma in China. Cancer Biol Med. 2013;10:36–42
- Czuczman MS, Leonard JP, Jung S, Johnson JL, Hsi ED, Byrd JC, Cheson BD. Phase II trial of galiximab (anti-CD80 monoclonal antibody) plus rituximab (CALGB 50402): follicular Lymphoma International Prognostic Index (FLIPI) score is predictive of upfront immunotherapy responsiveness. Ann Oncol. 2012;23:2356–2362. doi:10.1093/annonc/mdr620.
- MacDonald D, Prica A, Assouline S, Christofides A, Lawrence T, Sehn LH. Emerging therapies for the treatment of relapsed or refractory follicular lymphoma. Curr Oncol. 2016;23:407–417. doi:10.3747/co.23.3405.
- Huh J. Epidemiologic overview of malignant lymphoma. Korean J Hematol. 2012;47:92–104. doi:10.5045/kjh.2012.47.2.92.
- Gao X, Li J, Lin J, Liu D, Yu L, Wang Q. High-grade transformation in a splenic marginal zone lymphoma with a cerebral manifestation. Am J Case Rep. 2017;18:611–616. doi:10.12659/AJCR.903679.
- El-Osta H, Nair B. Rituximab-induced acute thrombocytopenia: an underappreciated entity. Leuk Lymphoma. 2013;54:2736–2737. doi:10.3109/10428194.2013.784972.
- Sadashiv SK, Rao R, Fazal S, Lister J. Rituximab-induced acute severe thrombocytopenia: a case series in patients with mantle cell lymphoma. Clin Lymphoma Myeloma Leuk. 2013;13:602–605. doi:10.1016/j.clml.2013.04.013.
- Hilal T, Wang Z, Almader-Douglas D, Rosenthal A, Reeder CB, Jain T. Rituximab maintenance therapy for mantle cell lymphoma: a systematic review and meta-analysis. Am J Hematol. 2018;93:1220–1226. doi:10.1002/ajh.25226.
- Huh YO, Keating MJ, Saffer HL, Jilani I, Lerner S, Albitar M. Higher levels of surface CD20 expression on circulating lymphocytes compared with bone marrow and lymph nodes in B-cell chronic lymphocytic leukemia. Am J Clin Pathol. 2001;116:437–443. doi:10.1309/438N-E0FH-A5PR-XCAC.
- D’Arena G, Musto P, Cascavilla N, Dell’Olio M, Di Renzo N, Carotenuto M. Quantitative flow cytometry for the differential diagnosis of leukemic B-cell chronic lymphoproliferative disorders. Am J Hematol. 2000;64:275–281. doi:10.1002/1096-8652(200008)64:4<275::AID-AJH7>3.0.CO;2-Y.
- Otrock ZK, Mahfouz RA, Oghlakian GO, Salem ZM, Bazarbachi A. Rituximab-induced acute thrombocytopenia: a report of two cases. Haematologica. 2005;90(Suppl):ECR23.
- Rafei H, Nassereddine S, Garcia IF. Disseminated intravascular coagulation-like reaction following rituximab infusion. BMJ Case Rep. 2017;01. doi:10.1136/bcr-2016-218443
- Bobot M, Benzaquen M, Rouby F, Lebowitz D, Serratrice J, Durand JM. [Rituximab-induced acute thrombocytopenia in a patient with chronic lymphocytic leukemia]. La Revue De Medecine Interne. 2017;38:344–346. doi:10.1016/j.revmed.2016.08.008.
- Pamuk GE, Donmez S, Turgut B, Demir M, Vural O. Rituximab-induced acute thrombocytopenia in a patient with prolymphocytic leukemia. Am J Hematol. 2005;78:81. doi:10.1002/ajh.20218.