ABSTRACT
Small bowel cancer is a very rare disease. It can be treated by surgery at early stages; however, there is no standard therapeutic strategy established for the treatment of unresectable and/or metastatic diseases.
Here we present a case of metastatic small bowel adenocarcinoma, which progressed after sequential treatment with XELOX (capecitabine and oxaliplatin), FOLFIRI (fluorouracil, leucovorin, and irinotecan), cetuximab, HER-2 targeted therapy and apatinib and was then effectively controlled by fruquintinib. Genetic testing showed wild-type KRAS/NRAS/BRAF, HER-2 amplification, and microsatellite stable. Then the patient started to receive fruquintinib and has already achieved a 6-month progression-free survival. Till Jun 2019, the treatment with fruquintinib is still ongoing and no severe adverse effect has been seen so far.
Although fruquintinib is not, at present, a standard therapeutic strategy recommended by the treatment guideline for advanced small bowel adenocarcinoma, the significant curative effect has been seen in our clinical practice.
Introduction
Small bowel cancer is rare. Even among tumors of the digestive tract, only 3% occur in the small bowel despite the fact that the small intestine accounts for over 90% of the gastrointestinal tract.Citation1 Of the several histological types, adenocarcinomas and carcinoids (neuroendocrine tumors) are the most common.Citation2 The most common site of small bowel adenocarcinoma is duodenum (57%), followed by jejunum (29%) and ileum (10%).Citation3 Risk factors of small bowel adenocarcinoma include Crohn’s disease, celiac disease, small bowel adenoma, familial adenomatous polyposis (FAP), Peutz–Jeghers syndrome (PJS), smoking, and obesity.Citation4 Clinical manifestations of small bowel cancer are nonspecific abdominal discomforts, such as abdominal pain, nausea, vomiting, gastrointestinal bleeding, and intestinal obstruction, which result in a diagnosis delay of 6–10 months on average.Citation5 Due to its relatively low incidence, small bowel cancer lacks an effective screening method in comparison to colorectal cancer.
Here, we report a case of heavy-treated metastatic moderately differentiated adenocarcinoma located in the terminal ileum adenocarcinoma, which progressed after multi-line treatment and was effectively controlled by fruquintinib as a late-line therapy.
Case report
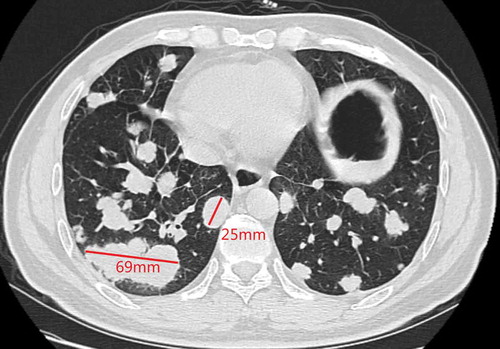
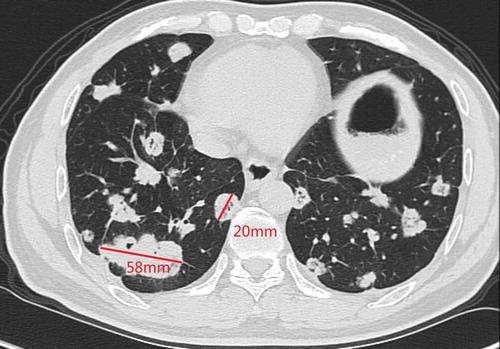
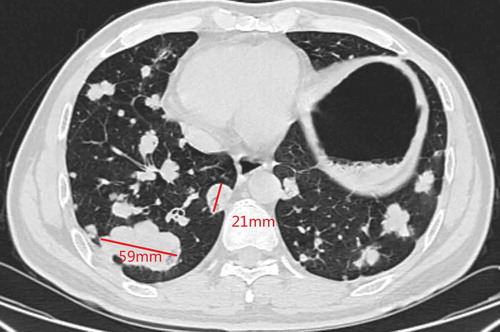
A 48-y-old man was admitted to our hospital due to the finding of elevated tumor markers carbohydrate antigen 19–9 (CA 19–9) and carbohydrate antigen 15–3 (CA15-3) in aroutine physical examination. Colonoscopy showed polypoid mucosal bulge at the terminal ileum and the pathological diagnosis of the lesion was adenocarcinoma. Distant metastasis was excluded through computed tomography (CT) scan of the chest and abdomen, and then laparoscopic radical surgery was performed in Jun2016. Posterior peritoneal metastases were found but unresectable during the surgery. Postoperative pathology showed that the lesion on the terminal ileum was moderately differentiated adenocarcinoma, ulcerative type, 4cm * 2.5cm, infiltrated into the sub-serosal layer and with positive nerve vessel invasion, the cutting edges were free of tumor cells, and 10/24 positive peri-intestinal lymph nodes were found. Combined with posterior peritoneal metastasis seen during operation, the disease was classified as stage IV (pT3N2M1). Genetic testing showed that KRAS, NRAS, and BRAF V600E were wild type. Systemic chemotherapy with XELOX regimen (oxaliplatin 130mg/m2 on d1, capecitabine 1000mg/m2 taken orally BID on d 1–14, 3 weeks/cycle) was performed for 4 cycles after the surgery from Jul2016 to Sep2016. Chest CT in Oct2016 showed newly appeared multiple lung nodules which are first considered to be lung metastases. Therapeutic effect evaluation: progressive disease (PD). Then the patient converted to thesecond-line treatment. The systemic chemotherapy with FOIFIRI regimen (irinotecan 180mg mg/m2 on d 1, l-leucovorin 400mg/m2 on d 1, 5-Fluorouracil 400mg/m2 on d 1, and bolus 2400mg/m2/46 h, 2 weeks/cycle) plus cetuximab (500mg/m2, 2 weeks/cycle) was performed for 4 cycles from Oct2016 to Dec2016. Chest CT in Dec2016 showed multiple lung nodules and the largest nodule slightly enlarged compared with that in Oct2016. Genetic testing was performed again, and the results suggested HER-2 amplification, microsatellite stable, and intermediate tumor mutation burden (12Muts/Mb). Then the patient was enrolled in several clinical studies of new drugs from Jan2017 to Dec2017 in amedical center in the UK (detailed information is not available). After the patient came back to China, positron emission computed tomography (PET-CT) scan was performed in Mar2018 and the result showed metastases in left supraclavicular, mediastinal, left hilar, retroperitoneal, and double lungs, indicating the progression of the disease. The patient then started to take orally apatinib 500mg from Apr2018 and obvious hand-foot syndrome (HFS) was seen. Chest CT in May2018 showed that the metastatic tumors of both lungs were increased in both number and sizes, and cavities were formed in some lesions. The patient then stopped apatinib by himself. Five months later, chest CT in Nov2018 showed that metastases in double lungs and lymph nodes were increased in both numbers and sizes (). After providing an off-label written informed consent, the patient started to receive fruquintinib (5mg, oral QD, 3 weeks on and 1 week off, 28 d/cycle) from Dec2018. Chest CT in Feb2019 showed that some lesions were smaller than previous, and cavities were formed (). Chest CT in Apr2019 showed that partial nodules were more solid than previous ().
According to the response evaluation criteria in solid tumors (RECIST) version 1.1, we selected two lesions with the longest diameters and observed their volume changes during treatment. The target lesion 1 was 69 mm and the target lesion 2 was 25 mm in Nov.2018 (). After receiving fruquintinib for 3 months, the target lesion 1 was 58 mm and the target lesion 2 was 20 mm in Feb 2019 (). There was a 17% decrease in the sum of diameters of target lesions. After receiving fruquintinib for 4 months, the target lesion 1 was 59 mm and the target lesion 2 was 21 mm (). There was a 2.5% increase in the sum of diameters of target lesions between Feb 2019 and Apr 2019. Therefore, the therapeutic effect evaluation was stable disease (SD) according to the RECIST version 1.1.
Common adverse reactions such as hand-foot syndrome and high blood pressure have not appeared. Till Jun 2019, the treatment with fruquintinib is still ongoing and a 6-month progression-free survival (PFS) has been reached.
During the whole course of the disease, the patient did not have obvious drug-related adverse effects, such as cough, shortness of breath, hemoptysis, and so on. The performance status was kept on score 0–1. And also, during the treatment, the posterior peritoneal metastasis was kept stable.
From the last day of observation for this report, the patient continues receiving the same treatment and will be incessantly followed up.
Discussion
Tumor angiogenesis has been shown to provide nutrients and oxygen for tumor growth and promote uncontrolled cell proliferation and cancer metastasis.Citation6 Vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) are among the most important pro-angiogenic factors that mediate angiogenesis by stimulating vessel endothelial cell proliferation and facilitate cancer cell migration as well as enhance neovascularization in tumors.Citation7 Anti-angiogenic agents include VEGF inhibitors and VEGFR inhibitors, which inhibit angiogenesis, cause vascular degeneration, normalize and contract tumor blood vessels, and counteract the chemotherapy-induced VEGF.Citation8
Fruquintinib is an anti-VEGFR small molecule tyrosine kinase inhibitor (TKI) independently developed in China. It is highly potent and can selectively inhibit VEGFR 1, 2, and 3 and thus inhibits VEGF/VEGFR signaling and further attenuates cell proliferation of human umbilical vein endothelial cells (HUVECs) and human lymphatic endothelial cells (HLECs) as well as angiogenesis and lymphangiogenesis in tumors. Its anti-tumor efficacy has been confirmed in a variety of human tumor xenograft models.Citation9
Three clinical trials have demonstrated that fruquintinib has a significant ability to treat advanced colorectal cancer. The phase I and phase II clinical studies prior to the FRESCO study enrolled patients with metastatic colorectal cancer who had previously received at least two lines of treatment with standard chemotherapy regimens (including fluorouracil, oxaliplatin, or irinotecan). In the phase Ib study (NCT01975077), 42 patients received 5 mg fruquintinib once daily for 3 weeks on and 1 week off, every 28 days. The median PFS was 5.80 months and the median overall survival (OS) was 8.88 months. In the phase II study (NCT02196688), 71 patients were randomly assigned to receive fruquintinib or placebo (47 in the fruquintinib group, 24 in the placebo group). PFS was significantly improved by the treatment with fruquintinib plus best supportive care (BSC) (4.73 months; 95% CI 2.86–5.59) versus placebo plus BSC (0.99 months; 95% CI 0.95–1.58). The median OS was 7.72 versus 5.52 months (95% CI 0.38–1.34).Citation10 Based on phase I and phase II clinical trials, the FRESCO study enrolled 416 patients who had to have histologically and/or cytologically confirmed metastatic colorectal cancer that progressed after at least 2 standard chemotherapy regimens. Prior treatment with VEGF inhibitors or anti-epidermal growth factor receptor (EGFR) inhibitors was permitted but prior treatment with other VEGFR inhibitors was excluded. Compared with the placebo group, the fruquintinib group significantly prolonged the median OS (9.3 months [95% CI, 8.2–10.5] versus 6.6 months [95% CI, 5.9–8.1]). The median PFS of fruquintinib was also significantly increased (3.7 months [95% CI, 3.7–4.6] versus 1.8 months [95% CI, 1.8–1.8]), the objective response rate (ORR) was 4.7%, and the disease control rate (DCR) was 62.2%.Citation11
On September 4, 2018, fruquintinib was approved by the National Medical Products Administration (NMPA) of China, for the first time in the world, to treat the metastatic colorectal cancer (mCRC) progressed after at least 2 lines of prior systemic treatment with fluoropyrimidine, oxaliplatin, and irinotecan, with or without prior use of VEGF or EGFR therapies. Notably, this case is a moderately differentiated terminal ileum adenocarcinoma, which is not an indication of fruquintinib so far, but fruquintinib showed a curative effect with a PFS of 6 months. Therefore, the indications of fruquintinib may not be limited to colorectal cancer, and its great potential for the treatment of small bowel cancer was also seen.
In contrast, apatinib, which the patient received previously, is also an anti-angiogenic small molecule TKI that selectively inhibits VEGFR-2, c-KIT, and c-SRC.Citation12 The NCT03190616 trial showed that apatinib can control refractory metastatic colorectal cancer and prolong PFS.Citation13 Also, a multicenter, single-arm, prospective study (Clinical trial information: ChiCTR1900020503) was present in the 55th American Society of Clinical Oncology (ASCO) held in this June. Forty-eight patients who had failed at least two lines of standard chemotherapies including fluorouracil, oxaliplatin, and irinotecan were recruited. Apatinib at a 500 mg dose was administered daily continuously. Each cycle was 4 weeks (28 d). The median PFS was 4.7 months [95% CI, 3.7–5.9]. The result showed that apatinib monotherapy shows promising efficacy in patients with chemotherapy-refractory metastatic colorectal cancer.Citation14 However, the patient could not tolerate the hand-foot syndrome induced by apatinib. In fact, VEGF and VEGF receptors are positively correlated with angiogenesis since the expression of VEGF and VEGF receptors are low in normal tissues and high in colorectal cancer.Citation15,Citation16 It is reported that at least 40% of gastrointestinal tumors have overexpression of VEGF and VEGF receptors.Citation17,Citation18 Apatinib only selectively inhibits VEGFR-2 in comparison to the potent and highly selective inhibition of VEGFR1, 2, 3 by fruquintinib. Apatinib has a limited inhibiting effect on VEGFR-3 and tumor lymphangiogenesis induced by VEGF-C, so its coverage of signaling pathways is not comprehensive. Resistance inevitably happens in subclonal populations that are suitable to hypoxic conditions and develops through the remaining signaling pathways.Citation19 Therefore, it is possible that single target inhibition of VEGFR is easy to be overcome, while multi-target inhibition of VEGFR may prolong the time to drug resistance.
In addition, the patient had a short PFS after treatment with cetuximab, which is considered to result from primary drug resistance. Using silver in situ hybridization (SISH) and immunohistochemistry, a clinical trial identified seven cases (4.9%) of HER-2 amplification among 142 patients with mCRC that received cetuximab after the prior treatment with oxaliplatin, irinotecan, and fluoropyrimidine failed. The median PFS was significantly different varied based on different HER-2 states. The result suggested that HER-2 amplified mCRC patients with wild-type RAS and BRAF genes might be resistant to cetuximab and have a shorter PFS.Citation20 Similar to colorectal cancer, HER-2 positive small bowel cancer patients might also have the primary resistance to cetuximab.
Conclusions
In summary, fruquintinib efficiently controlled a case of advanced small bowel cancer which progressed after several cytotoxicitic agents and apatinib treatment. Although small bowel cancer is not an indication of fruquintinib so far, the curative effect was shown. We still need further understanding of various types of TKIs. In addition, small bowel cancer, similar to colorectal cancer, may also show therapeutic resistance to cetuximab due to the existence of HER-2 amplification. All in all, further studies are needed in the future to clarify the related mechanisms.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. 2015. Cancer statistics, 2015. CA Cancer J Clin. 65:5–29. doi:10.3322/caac.21254.
- Pourmand K, Itzkowitz SH. 2016. Small bowel neoplasms and polyps. Curr Gastroenterol Rep. 18:23. doi:10.1007/s11894-016-0497-x.
- Halfdanarson TR, McWilliams RR, Donohue JH, Quevedo JF. 2010. A single-institution experience with 491 cases of small bowel adenocarcinoma. Am J Surg. 199:797–803. doi:10.1016/j.amjsurg.2009.05.037.
- Pan SY, Morrison H. 2011. Epidemiology of cancer of the small intestine. World J Gastrointest Oncol. 3:33–42. doi:10.4251/wjgo.v3.i3.33.
- Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. 2004. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. 101:518–526. doi:10.1002/cncr.20404.
- Kerbel RS. 2008. Tumor angiogenesis. N Engl J Med. 358:2039–2049. doi:10.1056/NEJMra0706596.
- Dvorak HF. 2002. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 20:4368–4380. doi:10.1200/JCO.2002.10.088.
- Tampellini M, Sonetto C, Scagliotti GV. 2016. Novel anti-angiogenic therapeutic strategies in colorectal cancer. Expert Opin Investig Drugs. 25:507–520. doi:10.1517/13543784.2016.1161754.
- Sun Q, Zhou J, Zhang Z, Guo M, Liang J, Zhou F, Long J, Zhang W, Yin F, Cai H, et al. 2014. Discovery of fruquintinib, a potent and highly selective small molecule inhibitor of VEGFR 1, 2, 3 tyrosine kinases for cancer therapy. Cancer Biol Ther. 15:1635–1645. doi:10.4161/15384047.2014.964087.
- Xu RH, Li J, Bai Y, Xu J, Liu T, Shen L, Wang L, Pan H, Cao J, Zhang D, et al. 2017. Safety and efficacy of fruquintinib in patients with previously treated metastatic colorectal cancer: a phase Ib study and a randomized double-blind phase II study. J Hematol Oncol. 10:22. doi:10.1186/s13045-016-0384-9.
- Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, Yang L, Deng Y, Chen ZD, Zhong H, et al. 2018. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial. JAMA. 319:2486–2496. doi:10.1001/jama.2018.7855.
- Tian S, Quan H, Xie C, Guo H, Lu F, Xu Y, Li J, Lou L. 2011. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 102:1374–1380. doi:10.1111/j.1349-7006.2011.01939.x.
- Chen X, Qiu T, Zhu Y, Sun J, Li P, Wang B, Lin P, Cai X, Han X, Zhao F, et al. 2019. A single-arm, phase II study of apatinib in refractory metastatic colorectal cancer. Oncologist. doi: 10.1634/theoncologist.2019-0164.
- Wang FWS, Yuan X, Jia J, Bi X, Zhou Z, Zhou Q, Luo C, Deng M, Yi L, Li Y. 2019. Apatinib monotherapy for chemotherapy-refractory metastatic colorectal cancer: A multicenter, single-arm, prospective study. J Clin Oncol. 37: 3527-3527. doi:10.1200/JCO.2019.37.15_suppl.3527.
- Fan F, Wey JS, McCarty MF, Belcheva A, Liu W, Bauer TW, Somcio RJ, Wu Y, Hooper A, Hicklin DJ, et al. 2005. Expression and function of vascular endothelial growth factor receptor-1 on human colorectal cancer cells. Oncogene. 24:2647–2653. doi:10.1038/sj.onc.1208246.
- Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964–3968.
- Jayson GC, Kerbel R, Ellis LM, Harris AL. 2016. Antiangiogenic therapy in oncology: current status and future directions. Lancet. 388:518–529. doi:10.1016/S0140-6736(15)01088-0.
- Wadhwa R, Song S, Lee JS, Yao Y, Wei Q, Ajani JA. 2013. Gastric cancer-molecular and clinical dimensions. Nat Rev Clin Oncol. 10:643–655. doi:10.1038/nrclinonc.2013.170.
- Procaccio L, Damuzzo V, Di Sarra F, Russi A, Todino F, Dadduzio V, Bergamo F, Prete AA, Lonardi S, Prenen H, et al. 2019. Safety and tolerability of anti-angiogenic protein kinase inhibitors and vascular-disrupting agents in cancer: focus on gastrointestinal malignancies. Drug Saf. 42:159–179. doi:10.1007/s40264-018-0776-6.
- Jeong JH, Kim J, Hong YS, Kim D, Kim JE, Kim SY, Kim KP, Yoon YK, Kim D, Chun SM, et al. 2017. HER2 amplification and cetuximab efficacy in patients with metastatic colorectal cancer harboring wild-type RAS and BRAF. Clin Colorectal Cancer. 16:e147–e152. doi:10.1016/j.clcc.2017.01.005.