ABSTRACT
Objective
To investigate the clinicopathologic features and immunophenotype of primary squamous cell carcinoma of the breast (PSCCB) with HER2 overexpression.
Methods Two cases of PSCCB with HER2 overexpression were retrospectively reviewed, and the pathological features, immunophenotype and prognosis were discussed.
Results The tumor showed malignant squamous cells arranged in sheets, groups and nests, forming keratin-pearl and intercellular bridges. Immunohistochemical (IHC) analysis showed that the tumor cells were positive for 34βE12, p63, CK5/6, E-cadherin and P120, while negative for ER and PR. Furthermore, HER2 overexpression showed strong continuous expression in cell membrane with a score of 3+ by IHC, or amplification by FISH.
Conclusions PSCCB is a rare tumor in breast cancer and HER2 overexpression is rather unusual in PSCCB. The diagnosis mainly depends on the clinicopathologic features together with the immunophenotype. HER2 positive indicates poor prognosis. However, targeted therapy for HER2 may be a new hope for patients.
Introduction
Primary squamous cell carcinoma of the breast (PSCCB) is a well-known malignant tumor composed of squamous cells. It is an extremely rare tumor, accounting for less than 0.1% of all breast carcinomas.Citation1 According to the WHO classification,Citation2 PSCCB is listed under metaplastic breast carcinoma. PSCCB accounts for 7.5% of the metaplastic breast carcinoma,Citation3 and most of them are triple-negative breast cancer,Citation4 HER2-positive PSCCB is even rarer, accounting for 10% of PSCCB.Citation5 In this article, we report two cases of PSCCB with HER2 overexpression and briefly review the related literature.
Clinical history
Case 1
A 54-year-old Chinese female presented with a lump in the left breast, which she had noticed with occasional pain for more than 10 days. The patient is a nonsmoker and claimed to have no history of radiation or any other family history.
Clinical examination revealed a firm lump of 2 × 2 cm in the upper inner and central quadrant of the left breast. There was no retraction or discharge from the nipple and the skin over the lump was normal. Right breast examination was normal and axillary lymph nodes were not enlarged.
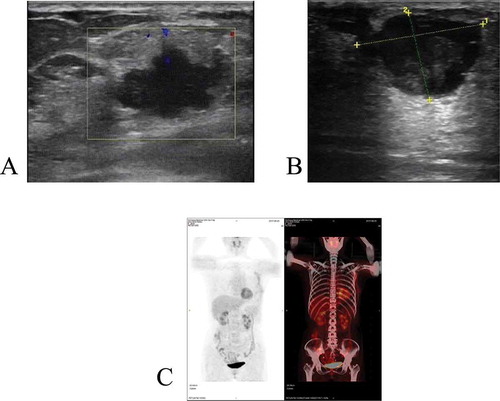
Ultrasonographic examination of the left breast showed an irregular-shaped hypoechoic lesion measuring 2.2 × 1.4 cm with an internal heterogeneous anechoic area, suggesting a malignant tumor (), which was classified as BI-RADS 4B.
Figure 1. (a) Ultrasonographic examination of the left breast showed an irregular-shaped hypoechoic lesion measuring 2.2 × 1.4 cm with an internal heterogeneous anechoic area(case 1); (b) Ultrasonographic examination of the right breast showed an irregular-shaped hypoechoic lesion measuring 2.5 × 2 cm with an internal mixed echo and punctiform strong echo(case 2); (c) The postoperative PET-CT show no mass lesion in other parts(case 1)
Ultrasound-guided core-needle biopsy from the lump in the left breast showed invasive ductal carcinoma. IHC for estrogen/progesterone receptor was negative and HER-2/Neu protein was continuously point positive in cell membrane with a score of 2 + . Thus, the lesion was considered to be primary invasive ductal carcinoma of the breast and the patient received a radical surgery of the left breast. The surgical specimen showed a 2-cm large, tan-white, solitary nodular mass located in the left breast. And the patient has remained disease free for 34 months post-operatively.
Case 2
A 43-year-old Chinese female presented with a lump in the right breast, which she had noticed accidentally 7 days ago. The patient is a nonsmoker and claimed to have no history of radiation or any other family history.
Clinical examination revealed a firm lump of 3 × 2 cm in the lower inner areola of the right breast. There was no retraction or discharge from the nipple and the skin over the lump was normal. Left breast examination was normal and axillary lymph nodes were not enlarged.
Ultrasonographic examination of the right breast showed an irregular-shaped hypoechoic lesion measuring 2.5 × 2 cm with an internal mixed echo and punctiform strong echo, suggesting a malignant tumor (), which was classified as BI-RADS 4B. Thus, the lesion was considered to be malignant tumor of the breast and the patient received a radical surgery of the right breast. The surgical specimen showed a 2-cm large, tan-white, cystic solid mass located in the right breast. Lung metastasis was found 14 months after operation ().
Table 1. Clinicopathological features of two cases of primary SCC of the breast
Materials and methods
The specimen was fixed in 4% buffered formalin, routinely processed, with tissue sections embedded in paraffin. The sections were cut at 4 μm in thickness and were stained with hematoxylin and eosin (H&E). IHC was performed according to standard protocols. The following antibodies were used: estrogen receptors (ER) (Roche Swiss, SP1, prediluted), progesterone receptors (PR) (Roche Swiss, 1E2, prediluted), cytokeratin5/6 (CK5/6) (Roche Swiss, D5/16B4 prediluted), p63 (Roche Swiss, 4A4, prediluted), Ki67 (Roche Swiss, 30–9, prediluted), p53 (Roche Swiss, DO-7, prediluted), HER2 (Roche Swiss, 4B5, prediluted), E-cadherin
(Roche Swiss, 36, prediluted), P120 (Roche Swiss, 98, prediluted), and high molecular weight cytokeratin (34βE12) (Roche Swiss, 34βE12, prediluted).
Results
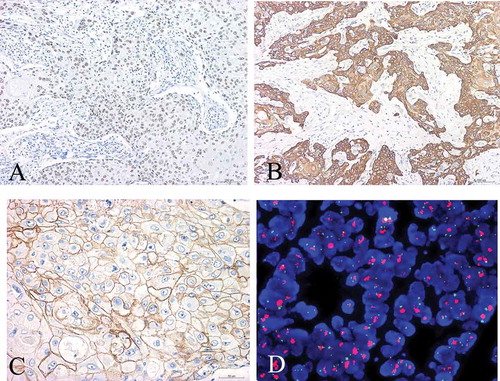
In case 1, the tumor showed malignant squamous cells arranged in sheets, groups and nests, with keratin-pearl formation and intercellular bridges (). All the masses were taken out and a small amount of intraductal carcinoma was found (). There was no component of obvious invasive ductal carcinoma or other features of metaplastic carcinoma such as spindle cells and osseous metaplasia. There was no involvement of the skin or the nipple. The tumor cells were positive for 34βE12, p63, CK5/6, E-cadherin and P120 ( They were negative for ER and PR (data not shown). The Ki67 labeling index was more than 60%. Furthermore, HER2 status was determined according to the IHC scoring criteriaCitation6 of the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP). HER2 was positive only for those tumors with more than 30% of cells (or>10% amplification by Fluorescence in situ hybridization FISH) with diffuse and intense circumferential staining. Thus, in case 1, the tumor cells were continuously point positive in cell membrane with a score of 2+ (). Subsequently, FISH detection of HER2 gene amplification revealed cluster amplification (). According to 2013 ASCO/CAP recommendations, HER2/CEP17 ratio >2.0 was considered HER2 gene amplification.
Figure 2. Microscopic features of case 1. H&E × 100 staining showed malignant squamous cells arranged in sheets (a), keratin-pearl formation (b), and intercellular bridges (c) with H&E × 400 staining. Intraductal carcinoma (d) can be seen in the tumor with H&E × 200 staining
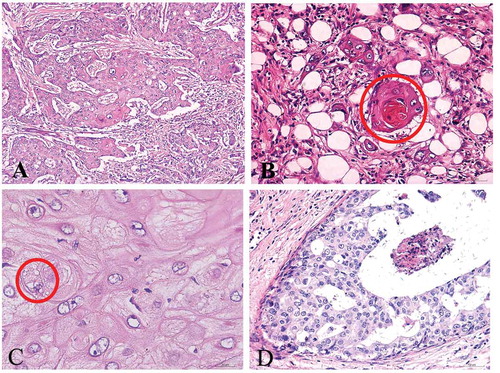
Figure 3. Immunohistochemistry and Fish detection of case 1. (a) Diffuse, positive expression of P63; (b) Diffuse, positive expression of CK5/6; (c) HER2 immunohistochemical staining showed that the tumor cells were continuously point positive in cell membrane with a score of 2+ (IHC with SP method×400) (d) FISH detection of HER2 gene amplification showed that red HER2 signals were linked into clusters
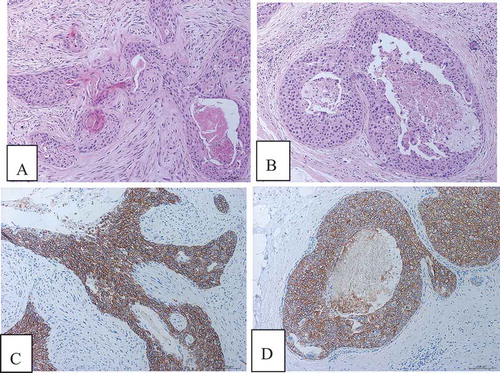
In case 2, the histological and immunohistochemical features of squamous cell carcinoma were similar to those of case 1. All the masses were taken out and a small amount of intraductal cancer was found. HER2 immunohistochemical staining showed strong continuous expression in the cell membrane with a score of 3+ ().
Figure 4. Microscopic features and immunohistochemistry of case 2. (a) Squamous cell carcinoma showed invasive growth. (b) A small amount of intraductal carcinoma. (c) HER2 immunohistochemical staining showed strong continuous expression in the cell membrane of invasive carcinoma with a score of 3+. (d) HER2 immunohistochemical staining showed strong continuous expression in the cell membrane of intraductal carcinoma with a score of 3+
According to the diagnosis criteria of breast squamous cell carcinoma:Citation7 1) more than 90% of the tumor cells were metaplastic squamous cells, 2) squamous cell carcinomas arising from the overlying skin or nipple of the breast were ruled out, and 3) metastasis of squamous cell carcinomas from other sites were ruled out. In both case1 and case 2, the combination of histological and immunohistochemical features led to the diagnosis of moderately differentiated squamous cell carcinoma. The postoperative PET-CT () did not show mass lesion or other evidence of metastatic disease, supporting the diagnosis of the patients’ lesion as PSCCB and one lymph node involvement was noted in two cases. The first patient presented no recurrence or metastasis at 34-month follow-up. Lung metastasis was found in the second patient 14 months after operation and the patient has undergone chemotherapy control for 32 months.
Discussion
Primary squamous cell carcinoma of the breast is uncommon, accounting for less than 0.1% of all breast carcinomas.Citation1 HER2-positive primary squamous cell carcinoma of breast is very rare. It has been listed under metaplastic breast carcinomas according to the World Health Organization Classification.Citation2 The origin of PSCCB may come from chronic breast lesion, such as breast epidermoid cyst, chronic abscess and complete metaplasia of breast tissue.Citation8,Citation9
Clinical features
PSCCB usually occurs in elderly women, who often accidentally notice the tumor on the breast. The ages of the two patients we reported are 54 and 43 years old, respectively. Gross examination: 50% of cases are cystic. In case 2, there was cystic in the lump. Many PSCCB arise from squamous metaplasia of adenocarcinoma, and squamous epithelial differentiation and keratinization occur in the center of the tumor, resulting in central necrosis and producing a cystic lesion.Citation4 Ultrasonographic examination showed that there was an irregular-shaped hypoechoic lesion in the breast. The cut surface of the tumor was a gray-white. Preoperative coarse needle biopsy is easily misdiagnosed as invasive ductal carcinoma or squamous metaplasia.Citation10 Therefore, routine tissue sections and IHC staining of PSCCB are particularly important for diagnosis.
Diagnosis
The tumor showed that malignant squamous cells were arranged in sheets, clusters and nests, forming keratin pearls and intercellular bridges. There was no obvious invasive ductal carcinoma component or other metaplastic carcinoma features and there was no skin or nipple involved. A small amount of intraductal cancer components can be seen in some areas. The tumor cells were positive for 34βE12, p63, CK5/6, E-cadherin and P120. They were negative for ER and PR. Furthermore, HER2 was positive by IHC or amplified by FISH detection. More than 90% of PSCCB are estrogen and progesterone receptors negative, HER2-positive PSCCB cases are rare,Citation5,Citation11 and ER-positive squamous cell carcinomas are also reported individually.Citation12 In both cases, the combination of histological, immunohistochemical features and PET-CT led to the diagnosis of squamous cell carcinoma of the breast and one lymph node involvement was noted in two cases.
Differential diagnosis
Invasive ductal carcinoma
Tumors have different shapes and lack regular structural features. Tumor cells are arranged in cords, clusters or trabeculae, and some tumors show syncytial infiltration with few stroma. Occasionally, some areas with monolayer linear infiltration or target annular structure can be seen.
Tumor cells have different shapes, but keratin pearls and intercellular bridges do not appear. However, the tumor cells of PSCCB have clear boundaries and intercellular bridges,keratin-pearl formation or a small amount of unicellular keratinization can be distinguished.Citation13 P63 is mainly expressed in myoepithelial cells in normal breast tissues and p63 is also expressed in some invasive ductal carcinomas. P63 maintains the characteristics of basal epithelium and is related to breast cancer progression, emphasizing its role in tumor cell invasion and stemness.Citation14 Positive expression of p63 also indicates higher tumor grade and poorer prognosis.
Squamous metaplasia
Most of the masses formed by extensive squamous metaplasia have a history of trauma. Under the microscope, squamous epithelial cells are mild and consistent, accompanied by infiltration of inflammatory cells, and their contours can still be seen in ducts and glands where squamous metaplasia occurs.
Treatment and prognosis
The treatment of PSCCB includes surgery and postoperative-related treatment. PSCCB has no established postoperative treatment plan, and most authors suggest that the adjuvant treatment of PSCCB should follow the guiding principles of invasive ductal carcinoma.Citation15 However, some authors considered that the biological behavior of PSCCB is more aggressive so that the conventional breast chemotherapy is not recommended.Citation16,Citation17
The prognosis of (triple-negative subtype) TN-PSCCB patients is worse than that of TNBC (triple-negative breast cancers) patients. Chemotherapy does not improve the survival rate of the TN-PSCCB. In contrast, non-TN PSCCB may benefit from chemotherapy and radiotherapy.Citation18 HER2 status, instead of hormone receptor status, is associated with improved survival. The survival rate of HER2-positive patients in PSCCB is similar to HER2-positive patients in invasive ductal carcinoma.Citation3 HER2-positive breast cancer is a subtype of human epidermal growth factor receptor 2 (HER2) protein that overexpresses and promotes the growth of cancer cells. Activation of HER2 mutation or HER2 overexpression is mainly due to gene amplification, which is associated with reproducible and strong response to HER2 targeted therapy in most malignant tumors (including breast cancer and gastric cancer). HER2 mutations and HER2 gene amplifications are rare in squamous cell carcinoma but have been reported in the head and neck squamous cell carcinomas.Citation19 Most of the PSCCB are usually hormone receptor negative and do not have HER2/Neu overexpression or amplification.Citation16 Pertuzumab is a new HER2/Neu receptor antagonist. Preclinical studies show that it acts as an anti-tumor agent by binding to HER2 receptor and blocking its pairing with other HER receptors, and it is an important targeted therapy for HER2-positive breast cancer patients.Citation20,Citation21
In our study, both cases had HER2 overexpression and were negative for ER and PR. In one case, HER2 expression pattern by IHC was continuously point positive in cell membrane with a score of 2+, which demonstrated HER2 gene amplified by FISH. In the other case, HER2 IHC staining showed strong continuous expression in cell membrane with a score of 3 + . This means hormone-based therapy may not be effective and HER2-based targeted therapy is likely to be effective. However, clinicians seldom encounter PSCCB, especially HER2-positive PSCCB, and there is no consistent treatment plan for such cases. Clinicians also recommend treatment plans based on the patient’s situation. Finally, the patient did not choose targeted treatment. In case 2, radical mastectomy with axillary clearance was performed with postoperative adjuvant therapy. Unfortunately, lung metastasis was found 14 months after operation.
The prognosis of PSCCB is still regarded controversial. Since most squamous cell carcinomas are triple-negative breast cancer, the prognosis is worse than invasive ductal carcinoma.Citation22,Citation23 HER2 overexpression is rare in PSCCB and HER2 positive indicates poor prognosis. However, targeted therapy for HER2 may be a new hope for HER2-positive patients. More clinicopathological data and molecular studies are needed in order to decide the best treatment options and postoperative adjuvant options, as well as prediction of recurrence risk.
Abbreviations
PSCCB Primary squamous cell carcinoma of the breast
IHC Immunohistochemical
FISH Fluorescent in situ hybridization
WHO World Health Organization
ER Estrogen receptors
PR Progesterone receptors
CK5/6 Cytokeratin5/6
34βE12 High molecular weight cytokeratin
TNBC Triple-negative breast cancers
Disclosure of potential conflicts of interest
The authors declare that they have no competing interests.
References
- Gupta C, Malani AK, Weigand RT, Rangineni G. Pure primary squamous cell carcinoma of the breast: a rare presentation and clinicopathologic comparison with usual ductal carcinoma of the breast. Pathol Res Pract. 2006;202:465–469. doi:10.1016/j.prp.2006.01.006.
- Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO classification of tumours of the breast. 4th ed. Lyon: IARC Press. 2012. ISBN 978-92-832-2433-4.
- Schroeder MC, Rastogi P, Geyer CE Jr, Miller LD, Thomas A. Early and locally advanced metaplastic breast cancer: presentation and survival by receptor status in surveillance, epidemiol ogy, and end results (SEER) 2010–2014. Oncologist. 2018;23:481–488. doi:10.1634/theoncologist.2017-0398.
- Honda M, Saji S, Horiguchi SI, Suzuki E, Aruga T, Horiguchi K, Kitagawa D, Sekine S, Funata N, Toi M, et al. Clinicopathological analysis of ten patients with metaplastic squamous cell carcinoma of the breast. Surg Today. 2011;41:328–332. doi:10.1007/s00595-009-4276-2.
- Shui RH, Li AQ, Yang F, Zhou XY, Yu BH, Xu XL, Yang WT. Primary squamous cell carcinoma of the breast with unusual basal-HER2 phenotype. Int J Clin Exp Pathol. 2014;7:5203–5209.
- Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/Col- lege of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi:10.1200/JCO.2013.50.9984.
- Behranwala KA, Nasiri N, Abdullah N, Trott PA, Gui GP. Squamous cell carcinoma of the breast: clinico-pathologic implications and outcome. Eur J Surg Oncol. 2003;29:386–389. doi:10.1053/ejso.2002.1422.
- Motoyama T, Watanabe H. Extremely well differentiated squamous cell carcinoma of the breast. Report of a case with a comparative study of an epidermal cyst. Acta Cytol. 1996;40:729–733. doi:10.1159/000333947.
- Hasleton PS, Misch KA, Vasudev KS, George D. Squamous carcinoma of the breast. J Clin Pathol. 1978;31:116–124. doi:10.1136/jcp.31.2.116.
- Gupta RK, Dowle CS. Cytodiagnosis of pure primary squamous-cell carcinoma of the breast by fine-needle aspiration cytology. Diagn Cytopathol. 1997;3:197–199. doi:10.1002/(SICI)1097-0339(199709)17:3<197::AID-DC5>3.0.CO;2-C.
- Karamouzis MV, Fida A, Apostolikas N, Rigatos G. A case of Her-2 positive squamous cell breast carcinoma: an unusual presentation of an unusual clinical entity. EJSO. 2006;32:1250–1251. doi:10.1016/j.ejso.2006.05.008.
- Pribish AM, Saglam O, Weinfurtner RJ. Estrogen receptor-positive primary squamous cell carcinoma of the breast. Radiol Case Rep. 2017;12:211–214. doi:10.1016/j.radcr.2017.01.008.
- Cardoso F, Leal C, Meira A, Azevedo R, Mauricio MJ, Leal Da Silva JM, Lopes C, Pinto FE. Squamous cell carcinoma of the breast. Breast. 2000;9:315–319. doi:10.1054/brst.1999.0145.
- Gatti V, Bongiorno-Borbone L, Fierro C, Annicchiarico-Petruzzelli M, Melino G, Peschiaroli A. p63 at the crossroads between stemness and metastasis in breast cancer. Int J Mol Sci. 2019;20(11):2683. doi:10.3390/ijms20112683.
- Bhatt L, Fernando I. Primary squamous cell carcinoma of the breast: achieving long-term control with cisplatin-based chemotherapy. Clin Breast Cancer. 2009;9:187–188. doi:10.3816/CBC.2009.n.031.
- Hennessy BT, Krishnamurthy S, Giordano S, Buchholz TA, Kau SW, Duan Z, Valero V, Hortobagyi GN. Squamous cell carcinoma of the breast. J Clin Oncol. 2005;23:7827–7835. doi:10.1200/JCO.2004.00.9589.
- Menes T, Schachter J, Morgenstern S, Fenig E, Lurie H, Gutman H. Primary squamous cell carcinoma (SqCC) of the breast. Am J Clin Oncol. 2003;26:571–573. doi:10.1097/01.coc.0000045809.85995.3B.
- He XX, Ji JL, Dong RR, Liu H, Dai XL, Wang CJ, Esteva FJ, Jim YSC. Prognosis in different subtypes of metaplastic breast cancer: a population-based analysis. Breast Cancer Res Treat. 2019;173:329–341. doi:10.1007/s10549-018-5005-6.
- Del Campo JM, Hitt R, Sebastian P, Carracedo C, Lokanatha D, Bourhis J, Temam S, Cupissol D, De Raucourt D, Maroudias N, et al. Effects of lapatinib monotherapy: results of a randomised phase II study in therapy-naive patients with locally advanced squamous cell carcinoma of the head and neck. Br J Cancer. 2011;105:618–627. doi:10.1038/bjc.2011.237.
- Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi:10.1016/S1535-6108(02)00097-1.
- Capelan M, Pugliano L, De Azambuja E, Bozovic I, Saini KS, Sotiriou C, Loi S, Piccart-Gebhart MJ. Pertuzumab: new hope for patients with HER2-positive breast cancer. Ann Oncol. 2013;24:273–282. doi:10.1093/annonc/mds328.
- Grabowski J, Saltzstein SL, Sadler G, Blair S. Squamous cell carcinoma of the breast: a review of 177 cases. Am Surg. 2009;75:914–917. doi:10.1177/000313480907501010.
- Nayak A, Wu Y, Gilcrease MZ. Primary squamous cell carcinoma of the breast: predictors of locoregional recurrence and overall survival. Am J Surg Pathol. 2013;37:867–873. doi:10.1097/PAS.0b013e3182877569.
