ABSTRACT
Colon cancer is the third most common cancer worldwide. Many miRNAs have been reported to be involved in colon cancer progression. However, there are only a few studies on the role of miR-219a-1 in colon cancer, and the molecular mechanisms involved remain unclear. The aim of this study was to investigate the miR-219a-1 level in patients with colon cancer and to explore both the effects and regulatory mechanisms of miR-219a-1 in the malignancy of colon cancer cells. Real-time PCR and western blot analysis were used to analyze the expression levels of miR-219a-1 and mediator of ErbB2-driven cell motility 1. Cell Counting Kit-8, transwell and wound-healing assays were performed to investigate the malignant ability of colon cancer cells. A luciferase assay was performed to explore whether miR-219a-1 could directly bind to 3ʹ-UTR region of MEMO1. miR-219a-1 was found to be downregulated in colon cancer cell lines and in patients with colon cancer. Additionally, miR-219a-1 could inhibit colon cancer cell proliferation, invasion and migration. We identified MEMO1 as a novel potential target gene of miR-219a-1. Luciferase assays showed that miR-219a-1 could directly bind to 3′-UTR of MEMO1. Overexpression of miR-219a-1 in colon cancer cells could inhibit the expression of MEMO1. Furthermore, MEMO1 was upregulated in patients with colon cancer, which was inversely correlated with miR-219a-1 levels. In conclusion, our study revealed that miR-219a-1 exerts anti-tumor effects and regulates colon cancer cell proliferation, invasion and migration by targeting MEMO1, suggesting that miR-219a-1 could act as a therapeutic target in colon cancer.
Introduction
Colon cancer is the third most common cancer worldwide and is one of the leading causes of cancer-related death.Citation1 Studies have revealed that the pathogenesis of colon cancer is a multi-step process that includes changes of cancer-related genes or molecules .Citation2,Citation3 Although several genes, proteins, and molecules have been identified, research on new molecular biomarkers (including microRNAs) of colon cancer remains a major focus.
MicroRNAs (miRNAs) are a class of small (approximately 22 nucleotides), non-coding, single-strand RNA molecules that play an important regulatory role at the post-transcriptional level .Citation4,Citation5 Emerging evidence has shown that miRNAs have different expression patterns in malignant tumors compared with those in normal tissues or cells .Citation6,Citation7 The altered miRNA expression has been shown to be related to tumorigenesis or cancer progression .Citation8,Citation9 miR-219a-1, which is located on chromosome 6-NC_000006.12 ,Citation10 is recently found to be involved in the progression of various cancers, including malignant melanoma ,Citation11 osteosarcoma ,Citation12 hepatocellular carcinoma ,Citation13 papillary thyroid carcinoma ,Citation14 and breast cancer .Citation10 It has been reported that miR-219a-1 can inhibit the growth or migration of several cancer cells by targeting different genes in different cancer cells .Citation15–17
Mediator of ErbB2-driven cell motility 1 (MEMO1) is a 297 amino acid protein that plays a regulatory role in epithelial-mesenchymal transition in mammary epithelial cells .Citation18 MEMO1 interacts with ErbB2 and controls ErbB2-regulated microtubule dynamics .Citation19 However, whether MEMO1 can be regulated by miRNA in cancer cells especially in colon cancer, is unknown.
In China, colon cancer has caused an increasing number of deaths in recent years. Although Xiong et al. discovered miRNA-219-5p functions as a tumor suppressor in colorectal cancer ,Citation20 the functions of miR-219a-1-3p and the underlying molecular mechanisms in colon cancer are not fully understood. In the present study, we investigated the expression level of miR-219a-1 in patients with colon cancer and in healthy individuals, and found that miR-219a-1 was significantly downregulated in the peripheral blood of patients with colon cancer. We further explored the function and molecular regulatory mechanism of miR-219a-1 in colon cancer cells. A dual luciferase reporter assay showed that MEMO1 is a direct target of miR-219a-1. Moreover, miR-219a-1 overexpression significantly inhibit colon cancer cell proliferation, invasion and migration. In conclusion, this study provides new insights into the relationship between miR-219a-1 and MEMO1 in the development and progression of colon cancer.
Materials and methods
Human blood samples
The use of human peripheral blood samples from patients with colon cancer in this study was approved by the ethics committee of Shanghai Tenth People’s Hospital (2019-K-45). The peripheral blood samples of healthy people were collected and approved by the ethics committee of Nanjing Red Cross Blood Center (2011–01). The blood samples used for total RNA extraction and real-time PCR were samples remaining after clinical use. All volunteers (17 patients with colon cancer and 20 healthy individuals) signed an informed consent statement to approve the use of their sample.
Cell culture and transfection
The human colon cancer cell lines SW480 and SW620, and the human normal colon cell line FHC were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium (Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1% penicillin/streptomycin at 37 °C and 5% CO2.
miR-219a-1-3p mimics (miR10004567-1-5, target sequences: 5ʹ-GAGUUGAGUCUGGACGUCCCG-3ʹ) and negative control miRNA mimics (miR1N0000001-1-5), and miR-219a-5p inhibitors (miR20004567-1-5) and negative control miRNA inhibitor (miR2N0000001-1-5) were purchased from RiboBio (Guangzhou, China). miR-219a-1-3p mimics and inhibitors were transfected into cells using Lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer’s instructions.
Plasmids construction and cell transfection
The human MEMO1 3ʹuntranslated region (UTR) (containing the miR-219a-1 binding sequence 5ʹ-TCAACTCA-3ʹ) and a mutant 3ʹUTR (containing the mutated miR-219a-1 binding site 5ʹ-ACTCAACT-3ʹ) were synthesized and inserted into the pGL3-promoter luciferase reporter vector (Promega, Madison, WI, USA) by Generay Biotech (Shanghai, China) to produce pMEMO1-WT and pMEMO1-MUT constructs, respectively. The plasmids were confirmed by DNA sequencing.
The day before transfection, colon cancer cells (SW480 and SW620) were seeded in 24-well plates. Within 24 h, cells were transfected with 4 µg of each luciferase plasmid, 10 ng pRL-SV40 (internal control to normalize transfection efficiency) and 100 nM miR-219a-1 mimics or miR-NC (negative control) using Lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer’s instructions. The cell medium was replaced 6 h after transfection.
Luciferase reporter assay
Forty-eight hours after transfection, luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) with a Synergy™ 4 (BioTek, Winooski, VA, USA) and expressed as the ratio of firefly luciferase to Renilla luciferase activity. All experiments were performed in triplicate.
RNA extraction and real-time PCR
Total RNA from blood samples or cultured cells was extracted using TRIzol reagent (Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, USA) and TaKaRa MiniBEST Universal RNA Extraction kit (Takara Biotechnology (Beijing) Co. Ltd., Beijing, China), and small RNA was extracted using miRNeasy Mini kit (QIAGEN, Richmond, VA, USA). All RNA was reverse-transcribed into cDNA with the PrimScript RT reagent Kit (Takara Biotechnology (Beijing) Co. Ltd., Beijing, China) according to the manufacturer’s instructions. The reverse transcription primer of total RNA was oligo(dT). Real-time PCR was performed using Applied Biosystems StepOne™ System (Applied Biosystems, Foster City, USA). Amplification was performed according to the manufacturer’s instructions (Applied Biosystems, Foster City, USA). The gene expression values were normalized to glyceraldehyde-3-phosphate (GAPDH) or U6. miR-219a-1-3p and U6 primer sets (including RT primer, forward primer and reversed primer) were purchased from RiboBio (Guangzhou, China). MEMO1 and GAPDH primers sequences were as follows: MEMO1, forward: 5ʹ-GTCAAAGGTTCCGTTACAGTTAC-3ʹ; MEMO1, reversed: 5ʹ-CCATTCTTCTGGAGCTCTGTG-3ʹ; GAPDH, forward: 5ʹ-GGGAGCCAAAAGGGTCAT-3ʹ; GAPDH, reversed: 5ʹ-GAGTCCTTCCACGATACCAA-3ʹ.
Cell proliferation assay
Colon cancer cell proliferation and proliferation were determined using the Cell Counting Kit-8 (CCK-8) (Beyotime, Shanghai, China). SW480 or SW620 cells transfected with miR-219a-1 mimics or inhibitors were seeded in 96-well plates at an initial density of 5 × 103 cells/well. After 1 h, 24 h, and 48 h, the cell cultured medium was replaced by serum-free DMEM, then 10 μl of CCK-8 solution was added into each well, and the plates were incubated for 3 h at 37 °C. Finally, the absorbance of each well was measured at 450 nm using a microplate reader and cell proliferation was measured. Experiments were performed in triplicate.
Transwell assay
The invasive ability of SW480 and SW620 cells transfected with miR-219a-1 mimics or inhibitors were evaluated using Matrigel-coated 8-μm pore polycarbonate membrane transwell chambers (Corning Co, Corning, NY, USA). SW480 and SW620 cells were transfected with miRNA mimics or inhibitors. About 6 h after transfection, 5 × 104 cells were resuspended in 100 μL serum-free DMEM and placed into cell culture insert, and 500 μL DMEM with 10% FBS was added to the bottom chamber. Cells were incubated for 18 h, after which noninvasive cells on the top of the membrane were scraped off. Invasive cells were fixed with 4% paraformaldehyde and stained with 1% crystal violet. Cells were counted under an optical microscope in eight randomly selected fields.
Wound-healing assay
SW480 or SW620 cells were seeded in 6-well plates overnight. The next day, cells were transfected with miR-219a-1 mimics or inhibitors. Eight hours after transfection, wounding was induced by scratching the cell monolayer with a 10 μl plastic pipette tip. Pictures were taken at 0 and 24 h using an optical microscope. The data was calculated by the distance. Experiments were performed in triplicate.
Western blot analysis
Colon cancer cells (SW480 and SW620) were harvested and the protein concentration was determined using a BCA protein assay kit (Beyotime, Shanghai, China). Equivalent quantities of protein were separated by SDS-PAGE and transferred to nitrocellulose membrane (Millipore, Billerica, MA, USA). After blocking in 5% nonfat milk, membranes were incubated with MEMO1 antibody (SAB2105059; Sigma-Aldrich, Germany) or GAPDH antibody (Beyotime, Shanghai, China). A peroxidase (HRP)-conjugated goat anti-mouse IgG or goat anti-rabbit IgG secondary antibodies (Zhongshan Biological, Beijing, China) was used, and the bands were detected using the ECL method (Millipore Corporation, Billerica, MA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation (SD). SPSS 17.0 software (SPSS, Chicago, IL, USA) was used to perform the statistical analysis. Statistically significant differences were performed using Student’s t-test or one-way ANOVA followed by SNK test. Pearson r was used to assess correlation analysis, and a P value < .05 was considered significant.
Results
Expression of miR-219a-1 in patients with colon cancer
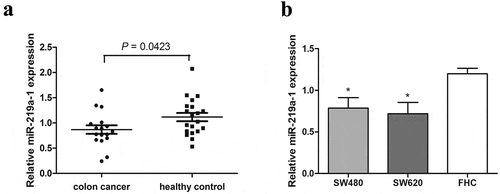
To explore the role of miR-219a-1 in colon cancer, the expression of miR-219a-1 was detected in blood samples of patients with colon cancer (n = 17) and healthy controls (n = 20) using real-time PCR. Results showed that miR-219a-1 was significantly downregulated in peripheral blood of patients with colon cancer (). The endogenous expression of miR-219a-1 was significantly decreased in colon cancer cells (SW480 and SW620) compared with that in FHC cells (). Therefore, we hypothesized that a decreased expression of miR-219a-1 is associated with the occurrence or development of colon cancer.
Figure 1. Expression of miR-219a-1 in patients with colon cancer and cell lines. The level of miR-219a-1 was detected by qRT-PCR in patients with colon cancer and healthy individuals (a), and different cell lines (b), including normal colon cell line FHC, colon cancer cell line SW480 and SW620. P = .0423 versus healthy control, t-test; * P < .05 versus FHC, one-way ANOVA followed by SNK test
miR-219a-1 inhibits SW480 and SW620 cell proliferation, invasion and migration
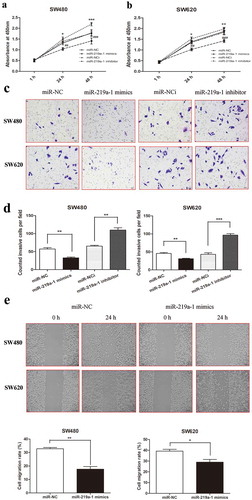
To explore the function of miR-219a-1 in colon cell proliferation, a CCK-8 assay was performed in SW480 and SW620 cells transfected with miR-219a-1 mimics or inhibitor. The results demonstrated that miR-219a-1 mimics inhibited SW480 and SW620 cell proliferation at 24 h and 48 h. In contrast, the miR-219a-1 inhibitor accelerated SW480 and SW620 cell proliferation at 24 h and 48 h( and b). Furthermore, we performed a transwell assay and wound-healing assay to investigate the role of miR-219a-1 in invasion and migration of colon cancer cells. Twenty-four hours after transfection with miR-219a-1 mimics, the numbers of SW480 and SW620 cells that invaded the lower side of the cell insert membrane were significantly decreased compared to those transfected with miR-NC cells (51.4 ± 8.2 vs. 33.5 ± 4.1 cells/field; 49.4 ± 7.7 vs. 35.6 ± 6.3 cells/field, respectively; and d). The wound-healing assay also revealed that cell mobility was significantly reduced in colon cancer cells transfected with miR-219a-1 mimics at 24 h (). When SW480 and SW620 transfected with miR-219a-1 inhibitor, an increased number of invasive cells was observed and the number of cells was counted under an optical microscope ( and d). These data revealed miR-219a-1 could inhibit colon cancer cell proliferation, invasion and migration.
Figure 2. miR-219a-1 inhibits SW480 and SW620 cell proliferation, invasion and migration. (a) miR-219a-1 mimics inhibited SW480 and SW620 cell proliferation. (b) miR-219a-1 inhibitor induced SW480 and SW620 cell proliferation. (c) miR-219a-1 mimics or inhibitor was transfected into SW480 or SW620 cells, and then transwell assay was performed. Representative images of SW480 and SW620 cell invasive ability after cells transfection. (d) Cell invasiveness was quantified by counting cells that passed through the Matrigel membrane, using a light microscope (×200). (e) Wound-healing assays were performed after SW480 and SW620 cells transfected with miR-219a-1 mimics or inhibitor. * or # P < .05, ** or ## P < .01, *** or ### P < .001
MEMO1 is the direct target of miR-219a-1 in colon cancer cells
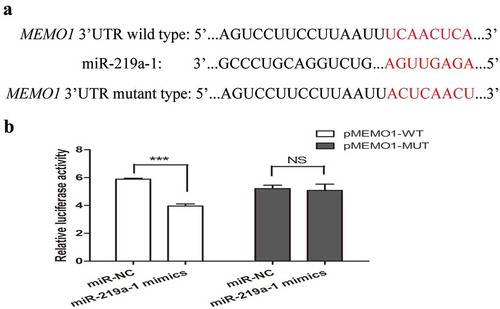
To further investigate the direct target gene of miR-219a-1, bioinformatics analysis using the TargetScan (http://www.targetscan.org/) database was used to predict the potential target gene of miR-219a-1. Based on this database, the MEMO1 3ʹUTR contains the miR-219a-1 binding site (). To validate whether miR-219a-1 could bind to the MEMO1 3ʹUTR, the DNA sequence of MEMO1 3ʹUTR, including the miR-219a-1 binding site, was cloned into a luciferase reporter plasmid (pGL3-promoter) named pMEMO1-WT. We also cloned the pMEMO1-MUT plasmid in which the miR-219a-1 binding site was mutant (). After luciferase plasmid (pMEMO1-WT or pMEMO1-MUT) and miR-219a-1 mimics were co-transfected into colon cancer cells, miR-219a-1 was shown to significantly decrease the luciferase activity of pMEMO1-WT in SW480 cells, while miR-219a-1 mimics had no effect on the luciferase activity of pMEMO1-MUT (). This result indicated that miR-219a-1 can directly bind to the 3ʹUTR of MEMO1 gene in colon cancer cells.
Figure 3. MEMO1 is a potential target of miR-219a-1 (a) Human MEMO1 3ʹ-UTR fragment containing wide-type (WT) or mutated (MUT, indicated in red) miR-219a-1-binding sequence. (b) Luciferase reporter assays in SW480 cells, with cotransfection of pMEMO1-WT or pMEMO1-MUT and miR-219a-1 mimics or miR-NC as indicated. Luciferase activities were measured 48 h post-transfection. * P < .05, ** P < .01, *** P < .001
miR-219a-1 inhibits MEMO1 expression in SW480 and SW620 cells
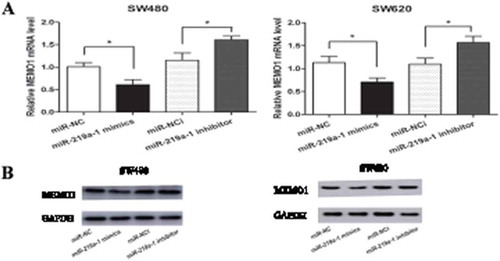
To confirm the regulatory role of miR-219a-1 in MEMO1 expression, we transfected miR-219a-1 mimics or inhibitor into SW480 and SW620 cells. Forty-eight hours after transfection, qRT-PCR and western blotting assay were performed to analyze the mRNA and protein expression level of MEMO1. Results showed that MEMO1 mRNA and protein levels were significantly decreased in colon cancer cells transfected with miR-219a-1 mimics ( and b), while the expression level of MEMO1 increased slightly in the cells transfected with miR-219a-1 inhibitor ( and b). These data revealed that in colon cancer cells, miR-219a-1 can inhibit MEMO1 expression.
Figure 4. miR-219a-1 inhibits MEMO1 expression in SW480 and SW620 cells. (a) MEMO1 mRNA level in SW480 and SW620 cells transfected with miR-219a-1 mimics or inhibitor was analyzed by qRT-PCR. (b) MEMO1 protein level in SW480 and SW620 cells transfected with miR-219a-1 mimics or inhibitor was analyzed by Western-blot. * P < .05, ** P < .01, *** P < .001
MEMO1 expression was inversely correlated with miR-219a-1 in patients with colon cancer
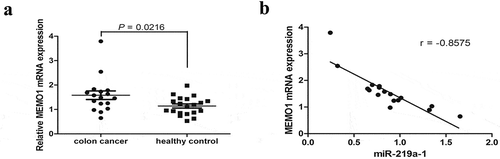
As MEMO1 was found to be regulated by miR-219a-1 in colon cancer cells in our study, we further evaluated the expression level of MEMO1 in patients with colon cancer. qRT-PCR results demonstrated that MEMO1 mRNA expression in 17 patients with colon cancer was significantly increased when compared with that in 20 healthy individuals (P < .05; ). Pearson’s correlation indicated an inverse correlation between miR-219a-1 and MEMO1 mRNA expression (n = 17, r = −0.8575, P < .0001, Pearson’s correlation; ) in patients with colon cancer.
Figure 5. MEMO1 expression was inversely correlated with miR-219a-1 in patients with colon cancer. (a) MEMO1 mRNA expression in patients with colon cancer (n = 17) and healthy individuals (n = 20) was detected by qRT-PCR. (b) A negative correlation was found between mRNA expression of MEMO1 and miR-219a-1 in patients with colon cancer (r = −0.8575; P < .0001, Pearson’s correlation)
Discussion
miR-219a-1 was recently reported to be involved in many solid tumors, such as papillary thyroid carcinoma, breast cancer, and osteosarcoma .Citation10,Citation12,Citation21 miR-219a-1 may play an anti-tumor role in tumorigenesis.Citation15,Citation17,Citation22 In this study, we examined the miR-219a-1 level in patients with colon cancer and investigated its role and molecular regulatory mechanism underlying tumorigenesis.
Here, we found that miR-219a-1 was downregulated in patients with colon cancer when compared with healthy individuals (). We hypothesized that miR-219a-1 inhibits colon cancer cell proliferation, invasion, and migration. We employed miRNA mimics or miRNA inhibitors to overexpress or downregulate miR-219a-1 levels in SW480 and SW620 cells to investigate the biological function of miR-219a-1. Our results confirmed that miR-219a-1 overexpression significantly inhibited the proliferation of colon cancer cells (SW480 and SW620), in addition to their ability to invade and migrate (). These malignant behaviors of colon cancer cells are closely associated with the progression of colon cancer. Although miR-219a-1 exhibits anti-tumor effects on colon cancer progression, its regulatory mechanism is still unclear.
Next, we explored the molecular mechanism of action of miR-219a-1 on the malignant behavior of colon cancer cells. By bioinformatics analysis, MEMO1 is predicted to be a potential direct target gene of miR-219a-1. It has been reported that MEMO1 is a novel HER2 effectorCitation23 and can regulate microtubule dynamics, actin network and adhesion site formation by localization of RhoA and mDia1 .Citation19,Citation24 To investigate the relationship between miR-219a-1 and MEMO1, we used a dual luciferase assay to confirm that miR-219a-1 can directly bind to 3ʹ-UTR region of MEMO1 (). After miR-219a-1 mimics or inhibitors transfection, both mRNA and protein expression levels of MEMO1 were downregulated or upregulated, which revealed that MEMO1 could be regulated by miR-219a-1 in colon cancer cells.
MEMO1 is related to epithelial-mesenchymal transition (EMT) in mammary epithelial cells and breast cancer .Citation18,Citation25 It can regulate breast cancer cell invasion and migration .Citation26 Here, we detected the expression level of MEMO1 in patients with colon cancer and found it to be significantly upregulated in patients with colon cancer compared to that in healthy people. Increased MEMO1 expression in colon cancer cells may regulate malignant behavior of cancer cells. Finally, we analyzed the correlation between miR-219a-1 and MEMO1 expression and found that they appeared to be inversely correlated with each other. These data indicated that miR-219a-1 could regulate MEMO1 expression by directly binding to its 3ʹUTR in colon cancer cells. In addition to our findings, MEMO1 was also found to be involved in the migration of colorectal cancer recently .Citation27 V Bogoevska illustrated that MEMO1 connected extracellular signals from membrane to the cytoskeletal actin network, and was an important downstream regulator of HER2-driven colorectal cancer cell migration and invasion. In our research, we discovered the potential connection between miR-219a-1 and MEMO1. Given that MEMO1 is involved in the EMT process of breast cancer, we hypothesized that miR-219a-1 modulated MEMO1 expression is also involved in the EMT process of colon cancer. However, this study needs to be validated by further research.
In this study, we found that the expression of miR-219a-1 in peripheral blood of patients with colon cancer was decreased and preliminarily confirmed that its low expression was associated with the proliferation and invasion of colon cancer cells. However, there are some limitations in our research. First, the sample size of our study is small. Based on our findings here, we will expand colon cancer blood samples to further explore the underlying molecular mechanisms. In addition, tumor tissue samples were not involved in this study. The expression of MEMO1 protein and miR-219a-1 in patients’ tumor tissues and controls require further investigation. Although this study did not investigate the correlation of miR-219a-1 and MEMO1 in tumor tissues, our findings in peripheral blood can be exploited for the development of novel diagnostic markers.
In this study, we used human blood samples from patients with colon cancer and healthy people to analyze the levels of miR-219a-1 and MEMO1 and revealed the anti-tumor activity of miR-219a-1 in colon cancer cells. We also identified a new target gene of miR-219a-1, MEMO1, and confirmed the role of miR-219a-1 in colon cancer cell proliferation, invasion and migration. Our results suggest that miR-219a-1 might be a therapeutic target in colon cancer.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Authors’ contributions
Y. L. designed research; K.–Q. X., J.S., D.-P. M., and Y.-H. Y. performed research; K.–Q. X. and Q. F. analyzed data; and Y. L. wrote the paper. All authors have read and approved the final manuscript.
Competing interests
The authors declare no conflict of interest.
Consent for publication
The authors provided consent for publication
Ethical approval and consent to participate
The use of human peripheral blood samples of colon cancer patients in this study was approved by the ethics committee of Shanghai Tenth People’s Hospital (2019-K-45). And the peripheral blood samples of healthy people were collected and approved by the ethics committee of Nanjing Red Cross Blood Center (2011-01). All the participants are consent to participate.
Acknowledgments
We wish to thank the help given by Ms. Shuling Fan from Shanghai tenth People’s Hospital in sample collection.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. 2018. Cancer statistics, 2018. CA Cancer J Clin. 68(1):7–30. doi:10.3322/caac.21442.
- Fearon ER. 2011. Molecular genetics of colorectal cancer. Annu Rev Pathol. 6:479–507. doi:10.1146/annurev-pathol-011110-130235.
- Chen W, Swanson BJ, Frankel WL. 2017. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol. 12(1):24. doi:10.1186/s13000-017-0613-8.
- Guo H, Ingolia NT, Weissman JS, Bartel DP. 2010. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 466(7308):835–840. doi:10.1038/nature09267.
- Iorio MV, Croce CM. 2017. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med. 9(6):852. doi:10.15252/emmm.201707779.
- Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, Diehn M, Liu H, Panula SP, Chiao E, et al. 2009. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 138(3):592–603. doi:10.1016/j.cell.2009.07.011.
- Rokavec M, Horst D, Hermeking H. 2017. Cellular model of colon cancer progression reveals signatures of mRNAs, miRNA, lncRNAs, and epigenetic modifications associated with metastasis. Cancer Res. 77(8):1854–1867. doi:10.1158/0008-5472.CAN-16-3236.
- Braicu C, Zimta AA, Harangus A, Iurca I, Irimie A, Coza O, Berindan-Neagoe I. The function of non-coding RNAs in lung cancer tumorigenesis. Cancers (Basel). 2019;11(5). doi:10.3390/cancers11050605.
- Boufraqech M, Nilubol N, Zhang L, Gara SK, Sadowski SM, Mehta A, He M, Davis S, Dreiling J, Copland JA, et al. 2015. miR30a inhibits LOX expression and anaplastic thyroid cancer progression. Cancer Res. 75(2):367–377. doi:10.1158/0008-5472.CAN-14-2304.
- Zhuang C, Yuan Y, Song T, Wang H, Huang L, Luo X, He H, Huo L, Zhou H, Wang N, et al. 2017. miR-219a-5p inhibits breast cancer cell migration and epithelial-mesenchymal transition by targeting myocardin-related transcription factor A. Acta Biochim Biophys Sin (Shanghai). 49(12):1112–1121. doi:10.1093/abbs/gmx114.
- Long J, Menggen Q, Wuren Q, Shi Q, Pi X. 2017. MiR-219-5p inhibits the growth and metastasis of malignant melanoma by targeting BCL-2. Biomed Res Int. 2017:9032502. doi:10.1155/2017/9032502.
- Zhu X, Chen L, Lin J. 2018. miR-219a-5p represses migration and invasion of osteosarcoma cells via targeting EYA2. Artif Cells Nanomed Biotechnol. 46(sup3):S1004- S1010. doi:10.1080/21691401.2018.1525391.
- Yang J, Sheng YY, Wei JW, Gao XM, Zhu Y, Jia HL, Dong QZ, Qin LX. 2018. MicroRNA-219-5p promotes tumor growth and metastasis of hepatocellular carcinoma by regulating cadherin 1. Biomed Res Int. 2018:4793971. doi:10.1155/2018/4793971.
- Rao SA, Arimappamagan A, Pandey P, Santosh V, Hegde AS, Chandramouli BA, Somasundaram K. 2013. miR-219-5p inhibits receptor tyrosine kinase pathway by targeting EGFR in glioblastoma. PLoS One. 8(5):e63164. doi:10.1371/journal.pone.0063164.
- Li C, Dong J, Han Z, Zhang K. 2017. MicroRNA-219-5p represses the proliferation, migration, and invasion of gastric cancer cells by targeting the LRH-1/Wnt/beta-catenin signaling pathway. Oncol Res. 25(4):617–627. doi:10.3727/096504016X14768374457986.
- Shi JA, Lu DL, Huang X, Tan W. 2014. miR-219 inhibits the proliferation, migration and invasion of medulloblastoma cells by targeting CD164. Int J Mol Med. 34(1):237–243. doi:10.3892/ijmm.2014.1749.
- Ma Q. 2019. MiR-219-5p suppresses cell proliferation and cell cycle progression in esophageal squamous cell carcinoma by targeting CCNA2. Cell Mol Biol Lett. 24:4. doi:10.1186/s11658-018-0129-6.
- Sorokin AV, Chen J. 2013. MEMO1, a new IRS1-interacting protein, induces epithelial-mesenchymal transition in mammary epithelial cells. Oncogene. 32(26):3130–3138. doi:10.1038/onc.2012.327.
- Zaoui K, Honore S, Isnardon D, Braguer D, Badache A. 2008. Memo-RhoA-mDia1 signaling controls microtubules, the actin network, and adhesion site formation in migrating cells. J Cell Biol. 183(3):401–408. doi:10.1083/jcb.200805107.
- Xiong GB, Zhang GN, Xiao Y, Niu BZ, Qiu HZ, Wu B, Lin GL, You L, Shu H. 2015. MicroRNA-219-5p functions as a tumor suppressor partially by targeting platelet-derived growth factor receptor alpha in colorectal cancer. Neoplasma. 62(6):855–863. doi:10.4149/neo_2015_104.
- Huang C, Cai Z, Huang M, Mao C, Zhang Q, Lin Y, Zhang X, Tang B, Chen Y, Wang X, et al. 2015. miR-219-5p modulates cell growth of papillary thyroid carcinoma by targeting estrogen receptor alpha. J Clin Endocrinol Metab. 100(2):E204–13. doi:10.1210/jc.2014-2883.
- Huang LX, Hu CY, Jing L, Wang MC, Xu M, Wang J, Wang Y, Nan KJ, Wang SH. 2017. microRNA-219-5p inhibits epithelial-mesenchymal transition and metastasis of colorectal cancer by targeting lymphoid enhancer-binding factor 1. Cancer Sci. 108(10):1985–1995. doi:10.1111/cas.13338.
- Qiu C, Lienhard S, Hynes NE, Badache A, Leahy DJ. 2008. Memo is homologous to nonheme iron dioxygenases and binds an ErbB2-derived phosphopeptide in its vestigial active site. J Biol Chem. 283(5):2734–2740. doi:10.1074/jbc.M703523200.
- Marone R, Hess D, Dankort D, Muller WJ, Hynes NE, Badache A. 2004. Memo mediates ErbB2-driven cell motility. Nat Cell Biol. 6(6):515–522. doi:10.1038/ncb1134.
- Jiang K, Yang Z, Cheng L, Wang S, Ning K, Zhou L, Lin J, Zhong H, Wang L, Li Y, et al. 2013. Mediator of ERBB2-driven cell motility (MEMO) promotes extranuclear estrogen receptor signaling involving the growth factor receptors IGF1R and ERBB2. J Biol Chem. 288(34):24590–24599. doi:10.1074/jbc.M113.467837.
- MacDonald G, Nalvarte I, Smirnova T, Vecchi M, Aceto N, Dolemeyer A, Frei A, Lienhard S, Wyckoff J, Hess D, et al. 2014. Memo is a copper-dependent redox protein with an essential role in migration and metastasis. Sci Signal. 7(329):ra56. doi:10.1126/scisignal.2004870.
- Bogoevska V, Wolters-Eisfeld G, Hofmann BT, El Gammal AT, Mercanoglu B, Gebauer F, Vashist YK, Bogoevski D, Perez D, Gagliani N, et al. 2017. HRG/HER2/HER3 signaling promotes AhR-mediated Memo-1 expression and migration in colorectal cancer. Oncogene. 36(17):2394–2404. doi:10.1038/onc.2016.390.
