ABSTRACT
Adenoviral vectors are superior to plasmid vectors in their gene transport efficiency. The A subunit of the diphtheria toxin (DTA) gene is a popular suicide gene in cancer gene therapy. However, DTA is seldom used in adenoviral therapy due to its great toxicity. The toxicity of DTA is so great that even a single molecule of DTA is enough to kill one cell. To avoid this highly toxic effect on normal cells, DTA should be controlled by tumor-specific promoters. The survivin promoter is a widely used tumor-specific promoter. But genes driven by the survivin promoter show a low level of basal gene expression in non-cancer cells. DTA driven by the survivin promoter in adenoviral vectors may be highly toxic not only to cancer cells but also to normal cells. Therefore, DTA should be attenuated when it is used in adenoviral vectors driven by the survivin promoter. In this study, we compared the three kinds of recombinant adenoviruses that carry DTA or its attenuated forms (DTA176 and DTA197) in the treatment of human lung cancer. The results showed that in comparison with both DTA and DTA176, DTA197 is more suitable for adenoviral cancer therapy controlled by the survivin promoter. In addition, Adsur-DTA197 (DTA197 delivered by an adenoviral vector with the survivin promoter) sensitized human lung cancer cells to cisplatin both in vitro and in vivo. These results indicated that Adsur-DTA197 may be a potential chemosensitizer in cancer therapy.
Introduction
The diphtheria toxin (DT) comes from a strain of Corynebacterium diphtheriae infected with a kind of phage.Citation1 DT consists of fragment A and fragment B. Fragment B recognizes the receptor on the cell surface and then transfers fragment A into the cell. Fragment A of the diphtheria toxin (DTA) is poisonous. DTA induces ADP-ribosylation of elongation factor 2 (EF-2), leading to the cell’s death. Mutation of G to A or G to R at codon 717 of the cell’s EF-2 gene leads to resistance to DT.Citation2,Citation3 DTA is a frequently used suicide gene in cancer gene therapy. Due to the lack of a B subunit, DTA cannot cross the cell membrane, and no bystander effect exists in gene therapy.Citation4 The absence of a bystander effect is a disadvantage in cancer gene therapy, but it also avoids the possibility of the surrounding normal cells being killed.
Adenoviral vectors are widely used in gene therapy because of their highly efficient gene expression and safety.Citation5-8 Many adenoviral vectors carrying a suicide gene have been applied in the therapy of various forms of cancer both in animal models and in clinical trials.Citation9,Citation10 Moreover, an adenovirus-mediated p53 has been used as a remedy in the treatment of patients with various cancers for several years.Citation11 Although DTA is a well-known suicide gene, it has seldom been used in adenoviral vectors for cancer gene therapy because of its great toxicity that affects both packaging cells and normal cells. Its toxicity is so great that even a single molecule of DTA is sufficient to kill one cell.Citation12 If it was packaged into recombinant viruses, DTA would kill such packaging cells as HEK293 and 293 T.
Target therapy aimed at cancer cells is an attractive goal for the researchers of cancer gene therapy. A major problem in cancer gene therapy is how to selectively kill the cancer cells without killing the normal cells. Utilizing a suicide gene under the control of the survivin promoter is such an approach of tumor-targeting therapy.Citation13,Citation14 For example, an interesting study reported that a mutant Bax gene (in which serine was deleted at position S184) driven by the survivin promoter in a plasmid vector showed an enhanced antitumor effect.Citation15 The problem with using the survivin promoter is that suicide genes controlled by it are expressed not only in the tumor cells, but also in the normal cells because of the low basal-level expression. Therefore, DTA must be attenuated when it is used with the survivin promoter in adenoviral vectors.
Cross-reacting material 176 (CRM176) is an attenuated mutant of DT, substituting aspartic acid for glycine at position 128. The toxicity of CRM176 is about one-tenth that of DT.Citation16 Cross-reacting material 197 (CRM197) is another attenuated mutant of DT. Its mutation is at position 52, replacing glutamic acid with glycine.Citation17 CRM197 was previously thought to be a nontoxic mutant of DT, but recent studies have demonstrated that CRM197 has a faint toxicity that can inhibit synthesis of proteins.Citation18 Its toxicity is about 106 times less than that of DT.Citation16
In the present study, either DTA or one of its attenuated forms (DTA176 or DTA197) were packaged into an adenoviral vector containing the survivin promoter. DT-resistant 293 cells were generated using a lentiviral vector encoding a mutant elongation factor 2 gene. The DT-resistant 293 cells were used to package adenovirus-mediated DTA with the survivin promoter (Adsur-DTA), as well as adenovirus-mediated DTA176 with the survivin promoter (Adsur-DTA176). In addition, common 293 cells were used to package adenovirus-mediated DTA197 with the survivin promoter (Adsur-DTA197), since DTA197 only has a weak cellular toxicity. We compared the three recombinant adenoviruses (Adsur-DTA, Adsur-DTA176 and Adsur-DTA197), and tried to find out which was most suitable for the therapeutic treatment of human lung cancer. We then combined a low dose of cisplatin with the recombinant adenovirus that was the most suitable for the treatment of lung cancer.
Results
Compared with DTA and DTA176, DTA197 is more suitable for adenoviral gene therapy under the control of the survivin promoter
In order to verify the expression of the various forms of DTA, Adsur-DTA, Adsur-DTA176-infected DT-resistant 293 cells and Adsur-DTA197-infected 293 cells were collected for western blot analysis. The cells infected with either Adsur-DTA, Adsur-DTA176 or Adsur-DTA197 expressed the various forms of DTA, but the cells infected with AdEmpty did not ()).
Figure 1. MTT assay of the cells infected with Adsur-DTA, Adsur-DTA176 or Adsur-DTA197. (a). A549, NCL-H292 or A427 cells were infected with Adsur-DTA, Adsur-DTA176, Adsur-DTA197, AdCMV-DTA197 or AdEmpty at 2, 10 or 50 MOI. (b). Western blot analysis of the DT-resistant 293 cells infected with Adsur-DTA or Adsur-DTA176, and of the 293 cells infected with Adsur-DTA197
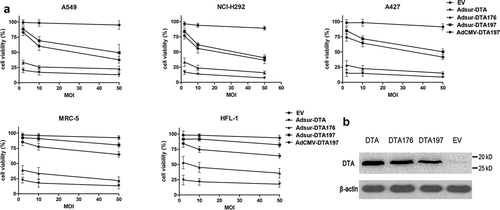
Adsur-DTA showed very potent toxicity at a very low titer (2 MOI) not only to cancer cells (A549, NCL-H292, A427) but also to normal cells (MRC-5 and HFL-1) ()). The toxicity of Adsur-DTA176 is lower in comparison with that of Adsur-DTA, but the toxicity of Adsur-DTA176 is still very strong at a very low titer (2 MOI), both to cancer cells (A549, NCL-H292, A427) and to normal cells (MRC-5 and HFL-1). These results showed that it is very difficult to control the high toxicity of Adsur-DTA or Adsur-DTA176 by the survivin promoter because of the basal gene expression driven by the survivin promoter.
Adsur-DTA197 showed moderate toxicity at a titer of 10 MOI in cancer cells (A549, NCL-H292, A427), and very low toxicity in normal cells (MRC-5 and HFL-1). These results indicate that in comparison with DTA and DTA176, DTA197 is more suitable for adenoviral cancer therapy with the survivin promoter due to its mild toxicity.
Although the antitumor activity of AdCMV-DTA197 was higher than that of Adsur-DTA197 (A549, NCl-H292 and A427), AdCMV-DTA197 was much more toxic to normal cells (MRC-5 and HFL-1) compared with Adsur-DTA197 (p < .01). In contrast to AdCMV-DTA197, Adsur-DTA197 showed a tumor-specific killing effect.
Adsur-DTA197 induces the apoptosis of lung cancer cells in vitro
Three days after being infected with Adsur-DTA197, the cells showed morphological changes associated with cell apoptosis. The Adsur-DTA197-infected cells were rounded or condensed, and some of the cells were floating ()). The cells stained with Hoechst 33258 showed the state of the nucleus. Cracked nuclei or condensed nuclei of the A549 cells appeared when they were infected with Adsur-DTA197 ()).
Figure 2. Induction of tumor cell apoptosis by Adsur-DTA197. (a). A549 cells under the microscope that were either untreated (Con), infected with AdEmpty (EV), or infected with Adsur-DTA197 (Adsur-DTA197). Scale bar, 50 µm. (b). A549 cells with Hoechst staining. Scale bar, 25 µm. The cracked nuclei or condensed nuclei of the A549 cells appeared when they were infected with Adsur-DTA197. (c). Expression of the caspases was analyzed by western blotting analysis. The activated caspase-9, activated caspase-8, activated caspase-3 and cleaved PARP appeared in the Adsur-DTA197-infected cells
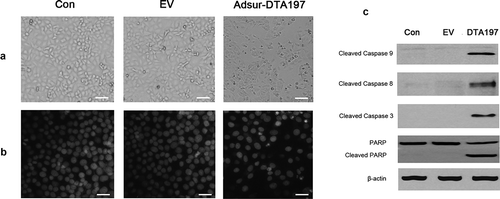
Expression of the caspases of the A549 cells was analyzed by western blotting ()). Activated caspase-9, caspase-8 and activated caspase-3 appeared in the Adsur-DTA197-infected cells. Cleaved PARP was also found in the Adsur-DTA197-infected cells.
Adsur-DTA197 sensitized lung cancer cells to cisplatin in vitro
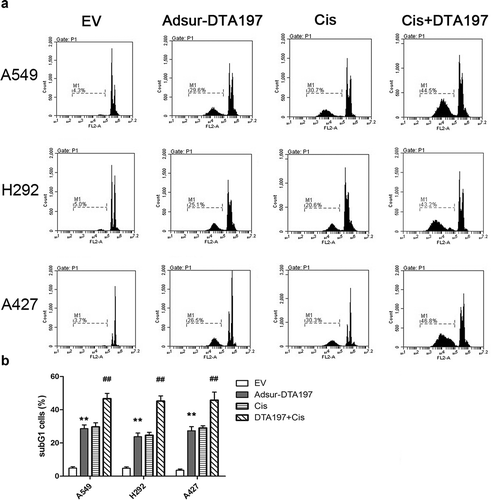
In order to evaluate the DNA damage effect, PI-stained cells were tested by flow cytometry ()). The sub-G1 proportion of the Adsur-DTA197-infected cells was much higher than it was in the AdEmpty-infected cells (p < .01). Cells that had the combined treatment showed more apoptotic cells than the cells treated with cisplatin alone (p < .01). These results suggested that Adsur-DTA197 sensitized lung cancer cells to cisplatin.
Figure 3. Flow cytometric analysis with PI staining. (a). Representative results of the flow cytometry with PI staining. (b). The sub-G1 proportion of the Adsur-DTA197-infected cells was much higher than that of the AdEmpty-infected cells (**p < .01). The cells treated with Adsur-DTA197 plus cisplatin showed more apoptotic cells than the cells treated with cisplatin alone (##p < .01)
Adsur-DTA197 showed how safe it is to normal cells in vivo
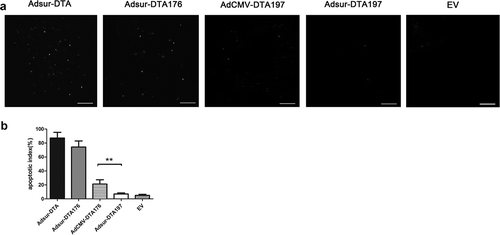
Adsur-DTA and Adsur-DTA176 were highly toxic to muscle tissue in a TUNEL assay. AdCMV-DTA197 had a small amount of toxicity to muscle tissue. In comparison with AdCMV-DTA197, Adsur-DTA 197 caused minimal toxicity in the muscle tissue of mice (p < .01) (). These data confirmed the safety of Adsur-DTA 197 for normal cells in vivo.
Figure 4. Adsur-DTA197 showed how safe it is for normal cells in vivo. (a). Representative fields from the immunohistochemical staining by means of TUNEL staining of the muscle tissue of the mice treated with adenoviruses. (b). Adsur-DTA and Adsur-DTA176 were highly toxic to the muscle tissue. In comparison with AdCMV-DTA197, Adsur-DTA197 caused minimal toxicity in the muscle tissue of the mice (**p < .01)
Adsur-DTA197 sensitized human lung cancer xenografts to cisplatin in vivo
Tumor volume and survival time were recorded. In comparison with the group treated with empty viruses, the tumor volume of the Adsur-DTA197-treated group was clearly inhibited (p < .01) (); and in both the A549 tumor-bearing mice and the NCL-H292 tumor-bearing mice, survival time was prolonged in the Adsur-DTA197-treated group in comparison with the AdEmpty-treated group (p < .05). In comparison with cisplatin treatment, cisplatin combined with Adsur-DTA197 treatment showed a significantly enhanced antitumor effect in terms of both tumor volume (p < .01) and survival time (p < .05). These results indicated that Adsur-DTA197 treatment inhibited tumor volume and prolonged the survival time of the tumor-bearing nude mice. A low dose of cisplatin combined with Adsur-DTA197 is very effective in the treatment of lung cancer.
Figure 5. Tumor volume (a) and survival time (b) of the mice treated with adenoviruses. The tumor volume of the Adsur-DTA197-treated group was clearly inhibited in comparison with that of the AdEmpty-treated group (*p < .01); and in both the A549 tumor-bearing mice and the NCL-H292 tumor-bearing mice, survival time was prolonged in the Adsur-DTA197-treated mice in comparison with the AdEmpty-treated mice (*p < .05). In comparison with the cisplatin treatment, the cisplatin combined with Adsur-DTA197 treatment showed a significantly enhanced antitumor effect both in tumor volume (##p < .01) and in survival time (#p < .05)
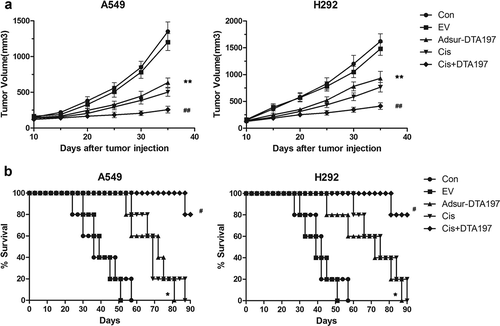
Apoptosis of the A549 or NCL-H292 tumor cells was tested by TUNEL assay (). More apoptotic cells could be detected in the Adsur-DTA197-treated mice than in the mice treated with empty viruses (p < .01). In comparison with cisplatin treatment, cisplatin combined with Adsur-DTA197 treatment resulted in more apoptotic cells (p < .01). The percentage of Ki67-positive cells decreased in the Adsur-DTA197 group in comparison with the two control groups (p < .01). The group treated with cisplatin plus Adsur-DTA197 showed more Ki67-positive cells than the cisplatin group (p < .01) ()).
Figure 6. TUNEL staining of the xenografted tumor. (a). Representative fields of the TUNEL staining. (b). The Adsur-DTA197-treated mice showed more apoptotic cells in comparison with the mice treated with empty viruses (**p < .01). In comparison with the cisplatin treatment, the cisplatin combined with Adsur-DTA197 treatment caused more apoptotic cells (##p < .01). Scale bar, 50 µm
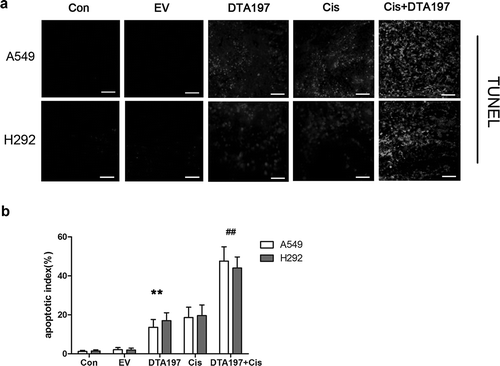
Figure 7. Histopathological analysis of the mice. (a). Representative fields of the immunohistochemical staining of the Ki67 from the A549- or NCL-H292-xenografted tumors. (b). Statistical data of the Ki67-positive cells. The Adsur-DTA197 treatment resulted in a decreased percentage of Ki67-positive cells in comparison with the two control groups (**p < .01). The cisplatin plus Adsur-DTA197 group showed fewer Ki67-positive cells in comparison with the cisplatin group (##p < .01). (c). Representative fields of the sections of the vital organs of the mice. No obvious changes in the sections were found under microscopic examination. Scale bar, 50 µm
Adsur-DTA was well tolerated in nude mice
The mice treated with Adsur-DTA197 acted as the normal mice. In comparison with the untreated mice, the mice treated with Adsur-DTA197 did not lose weight. Sections of the vital organs of the Adsur-DTA197-treated nude mice, such as the heart, the spleen, the kidney and the lungs, were examined under a microscope ()), and no obvious changes were found in these sections.
Discussion
Gene therapy has been developing very quickly in recent years, and it is a potential new option for the therapy of various diseases. Adenoviral vectors are the most frequently used vectors in gene therapy due to their high efficiency and low toxicity. Adenoviral vectors carrying suicide genes have been successfully applied in many preclinical trials, and some of them are now applied in clinical trials. For example, three types of adenovirus-mediated p53 have now been in clinical use or in clinical trials for several years. The first p53 adenovirus product in clinical use is Gendicine, which was approved for use in 2003, and another two Adp53 that are in clinical trials are Advexin and SCH-5850011. Because of the difficulty of packaging DTA into adenoviruses, only one report using DTA with an adenoviral vector was found. That study reported that DTA driven by a prostate-specific antigen (PSA) promoter in an adenoviral vector had a therapeutic effect on prostate cancer cells in the presence of an exogenous androgen.Citation19
A major problem in cancer gene therapy is how to selectively kill cancer cells without killing normal cells. According to the findings of this study, DTA and DTA176 are not suitable for use with the survivin promoter because they are highly toxic. Adsur-DTA and Adsur-DTA176 do not selectively kill cancer cells without also killing normal cells due to their high toxicity. Unlike DTA and DTA176, DTA197 possesses a mild cellular toxicity which is very suitable for use in adenoviral vectors containing the survivin promoter. Adenovirus-mediated DTA197 driven by the CMV promoter showed an antitumor effect in our previous studies.Citation20,Citation21 However, DTA197 under the control of the CMV promoter does not selectively kill tumor cells. Use of the survivin promoter in order to direct tumor-specific expression is well known, so the survivin promoter was used to selectively kill tumor cells in this study. In contrast to AdCMV-DTA197, Adsur-DTA197 showed its specificity and safety.
A recent report took the first step of using DTA197 in cancer gene therapy. It found that, under the control of the HSP promoter in the plasmid, DTA197 has lethal dose-related effects and is a promising suicide gene in cancer gene therapy.Citation22 In the present study, DTA197 was packaged into the adenoviruses containing the survivin promoter for the selective killing of the lung cancer cells. Adsur-DTA197 induced apoptosis of the A549, NCl-H292 and A427 lung cancer cells, and showed minimal toxicity in normal cells. We thought that the in vivo effect of Adsur-DTA197 could be evaluated by mice with two kinds of xenografted tumor cell lines, so A549 xenograft mice and NCl-H292 xenograft mice were treated with Adsur-DTA197. Adsur-DTA197 has shown its specificity and safety when used in experiments involving the treatment of mice.
Lung cancer is a very common tumor and has the highest mortality rate among human cancers.Citation23-28 It is classified into non-small-cell lung carcinoma and small-cell lung carcinoma according to its pathological characteristics.Citation29-31 Smoking and air pollution are the most common risk factors for lung cancer.Citation26,Citation28 The five-year survival rate for lung cancer is only about 17.4% in the USA. In most people with lung cancer, the tumor is first detected by CT imaging. Surgery, radiotherapy and chemotherapy are the traditional therapies for lung cancer. Chemotherapy is usually used before or after surgery. It is necessary to combine chemotherapy drugs with other treatments in order to enhance the antitumor effect and reduce the side effects. Cisplatin is one of the most commonly used chemotherapeutic drugs in the treatment of lung cancer.Citation32 In the present study, a low dose of cisplatin combined with Adsur-DTA197 achieved a very good therapeutic effect in the treatment of lung cancer. These results indicate that Adsur-DTA197 may be a potential chemosensitizer for cancer therapy.
Materials and methods
Cell culture
Human lung cancer A549, NCl-H292 and A427 cells and human normal cells MRC-5 and HFL-1 were cultured in DMEM containing 10% fetal bovine serum. These cells were placed in an incubator set at 5% CO2, and at 37°C.
Generation of the DT-resistant 293 cells
The mutant human EF-2 gene (G to R at codon 717) was chemically synthesized (by GenScript) and inserted into the lentiviral vector plasmid pCDH-CMV-MCS-EF1-copGFP-T2A-Puro (System Biosciences) at the EcoRI site and the BamHI site. This recombinant lentiviral vector plasmid which we named pCDH-mEF2 contained a CMV promoter driving the mutant EF-2 gene and contained an EF1 promoter driving a GFP gene as well as a puromycin resistant gene. Cotransfection of the pCDH- mEF2, the pCMVdeltaR8.91 and the pMD.G into the 293 T cells was performed to yield the lentivirus Lv-mEF2. Then the 293 cells were infected with Lv-mEF2, and the 293 cells stably expressing the mutant EF-2 gene were selected by puromycin and were confirmed by fluorescence microscopic imaging.
Virus packaging
The Admax system was used to generate the adenoviruses in this study. The nucleotide of the survivin promoter in the plasmid pSur-Bax was generously provided by Dr R Blumenthal. The survivin sequences was amplified by PCR. The primers were GAGTCTAGACTGGCCATAGAACCAGAGAAGTGA and CAAGAATTCCCACCTCTGCCAACGGGTCCCGCG. The CMV promoter of the pDC316 plasmid was excised by Xba I and EcoR I, and was changed by the survivin promoter to form pDCsur. A DTA, DTA176 or DTA197 gene was chemically synthesized and inserted into the pDCsur to generate pDCsur-DTA, pDCsur-DTA176 or pDCsur-DTA197.
The pDCsur-DTA197 and the pBHGloxdelE1,3cre were cotransfected into 293 cells to package the adenovirus Adsur-DTA197. Then the Adsur-DTA197 adenoviruses were amplified 3 times in 293 cells. The empty adenovirus for use on the control was packaged by the same method. Adsur-DTA and Adsur-DTA176 were packaged by a similar method, using the DT-resistant 293 cells for the packaging. The AdCMV-DTA197 adenoviruses were generated as previously described.Citation23,Citation24 All the adenoviruses were purified by CsCl gradient ultracentrifugation.
Preparation of polyclonal antibodies of DTA
The pCite-DTA197 plasmid (provided by Dr Manuel Caruso) was digested by the restriction endonuclease of NcoI and BamHI, and the DTA197 gene was inserted into pET32a+ (Novagen) to form the pET-DTA197 plasmid. The pET-DTA197 was transformed into E. coli BL21 and inducted with IPTG. The fusion proteins (His 6-DTA197) were confirmed by SDS-PAGE. After that, the fusion proteins (His 6-DTA197) were purified by a Ni-Sepharose column. In order to produce the polyclonal antibodies of DTA197, each BALB/c mouse was treated with a subcutaneous injection of 10 µg his 6-DTA197 protein mixed with Freund’s complete adjuvant. 14 days later, each mouse was treated with a subcutaneous injection of 5 µg his 6-DTA197 protein mixed with Freund’s incomplete adjuvant every two weeks. Two months after the first injection, the mice were sacrificed and their blood was collected. The titers of the anti-DTA197 antiserum were tested with ELISA.
Western blotting analysis
72 hours after infection with Adsur-DTA, Adsur-DTA176 or Adsur-DTA197, the expression of the DTA or the DTA176 in the DT-resistant 293 cells and of the DTA197 in the 293 common cells was tested using the anti-DTA described previously; and 72 hours after infection with Adsur-DTA197, the A549 cells were harvested for the following western blotting analysis. The protocol of western blotting analysis was described previously.Citation33 The antibodies of cleaved caspase-9, cleaved caspase-9, cleaved caspase-3 and PARP were purchased from Cell Signaling Technology.
Hoechst staining
72 hours after infection with the various adenoviruses, the A549 cells were stained with Hoechst 33258. The protocol of Hoechst staining was described previously.Citation34 The stained cells were examined under a fluorescence microscope.
MTT assay
The cells were infected with Adsur-DTA, Adsur-DTA176 or Adsur-DTA197 at 2, 10 or 50 MOI, and were tested by MTT assay 72 h post infection. Bars in the figures represent the mean cell viability of five wells; the proliferation of the medium treated cells was defined as 100% cell viability.
Flow cytometric analysis
In order to estimate the apoptosis of the cells, flow cytometric analysis was performed as described previously.Citation34 Put briefly, the cells were infected with Adsur-DTA197 at 10 MOI. Cisplatin was treated at a concentration of 5 μg/mL.Citation33 We then tested the cells by cytometric analysis, staining them with PI 72 h post infection. We estimated the apoptosis rate of the cells by determining the proportion of sub-G1 cells.
Toxicity of Adsur-DTA197 to the muscle tissue of BALB/c mice
In order to test the toxicity of Adsur-DTA197 to normal cells in vivo, either Adsur-DTA, Adsur-DTA176, Adsur-DTA 197 or AdCMV-DTA197 was injected intramuscularly into the thigh of the left hind leg of BALB/c mice every three days. Three days after the third injection, the mice were sacrificed and the muscle tissue sections were detected by TUNEL assay. The protocol for the treatment of the mice was approved by the Animal Care and Use Committee of Chengdu Medical College and the Ethics Committee of Chengdu Medical College.
The experiments using the xenograft mice
Female BALB/c nude mice were provided by the Chinese Academy of Medical Science. Each mouse was 6 weeks old, and the weight of the mice ranged between 15 g and 20 g. Mice were randomly divided into 5 groups, with 5 mice in each group. The mice were raised in the SPF environment with adequate food and water.
A549 or NCl-H292 cells (5 × 106cells) were injected into each mouse subcutaneously in the right flank. On day 7, the mice were treated as follows: (1) Control group: intratumoral injection of 100 µL PBS twice a week; (2) Empty viruses group: intratumoral injection of 5 × 108 PFU empty viruses in 100 µL PBS twice a week; (3) Adsur-DTA197 group: intratumoral injection of 5 × 108 PFU Adsur-DTA197 in 100 µL PBS twice a week; (4) Cisplatin group: mice were intravenously injected with 5 mg/kg cisplatin; (5) Cisplatin plus AdDTA197 group: (3) and (4) combined. Tumor size = X× Y2 × 0.52; X represents the length, while Y represents the width. Finally, tumor tissues were collected from the mice for the histopathological experiments described in the following section.
Immunohistochemical analyses and TUNEL analyses
We cut the formaldehyde-fixed tissues into 4 µm paraffin sections. The sections were incubated with diluted anti-Ki67 (Abcam, Cambridge, MA, USA). After being incubated overnight, the sections were washed by PBS three times, and then incubated with diluted biotinylated rabbit anti-mouse antibody. Finally, we used streptavidin biotin reagents to stain the sections. We counted the number of active Ki67-positive cells from 20 randomly chosen fields, and recorded the percentage of active Ki67-positive cells from the specimens. We used the In SituCell Death Detection Kit (Roche) for TUNEL staining. The TUNEL-positive cells from 20 randomly chosen fields were counted in a blind manner. The apoptotic index represented the percentage of TUNEL-positive cells from the specimens.
Observation of side effects
We recorded weight change, appetite and behavior of the mice, as described by us previously, in order to observe the possible side effects on the mice treated with Adsur-DTA197. Samples taken from the vital organs – the heart, liver, lungs, spleen and kidney – were made into 4 μm paraffin sections and placed on slides. After H&E staining, the slides were observed at 400× magnification under a microscope.
Data analysis
We used SPSS 17.0 software to analyze the statistics. All the values shown in the figures represent means and standard deviations. Differences between groups were analyzed by one-way ANOVA, and then a Tukey–Kramer multiple comparison test was used to analyze the differences between the two groups.
Disclosure of potential conflicts of interest
The authors report no conflict of interest
Acknowledgments
The authors thank Dr. Hui Wang for his technical assistance in animal experiments and Dr. R Blumenthal for providing plasmids containing the survivin promoter.
Additional information
Funding
References
- Greenfield L, Bjorn MJ, Horn G, Fong D, Buck GA, Collier RJ, Kaplan DA. 1983. Nucleotide sequence of the structural gene for diphtheria toxin carried by corynebacteriophage beta. Proc Natl Acad Sci U S A. 80(22):6853–6857. doi:10.1073/pnas.80.22.6853.
- Kohno K, Uchida T. Highly frequent single amino acid substitution in mammalian elongation factor 2 (EF-2) results in expression of resistance to EF-2-ADP-ribosylating toxins. J Biol Chem. 1987;262(25):12298–12305.
- Gupta PK, Liu S, Leppla SH. 2010. Characterization of a Chinese hamster ovary cell mutant having a mutation in elongation factor-2. PLoS One. 5(2):e9078. doi:10.1371/journal.pone.0009078.
- Shibata MA, Miwa Y, Miyashita M, Morimoto J, Abe H, Otsuki Y. 2005. Electrogene transfer of an Epstein-Barr virus-based plasmid replicon vector containing the diphtheria toxin A gene suppresses mammary carcinoma growth in SCID mice. Cancer Sci. 96(7):434–440. doi:10.1111/j.1349-7006.2005.00070.x.
- Delhove J, Osenk I, Prichard I, Donnelley M. 2020. Public acceptability of gene therapy and gene editing for human use: a systematic review. Hum Gene Ther. 31(1–2):20–46. doi:10.1089/hum.2019.197.
- Flotte TR, Gao G. 2020. 2020: gene therapy enters its fourth decade. Hum Gene Ther. 31(1–2):2–3. doi:10.1089/hum.2019.29101.trf.
- Xia Y, Wang L, Ma X, Li X. 2020. Investigation on the genomic characterization of uterine sarcoma for rAd-p53 combined with chemotherapy treatment. Hum Gene Ther. 31(15–16):881–890. doi:10.1089/hum.2019.305.
- Zou H, Tuhin IJ, Monty MA, Luo S, Shao J, Yan Z, Yu L. 2020. Gene therapy for hepatocellular carcinoma using adenoviral vectors delivering a gene encoding IL-17A-neutralizing antibody fragments. Hum Gene Ther. 31(19–20):1074–1085. doi:10.1089/hum.2019.169.
- Gahéry-Ségard H, Molinier-Frenkel V, Le Boulaire C, Saulnier P, Opolon P, Lengagne R, Gautier E, Le Cesne A, Zitvogel L, Venet A, et al. 1997. Phase I trial of recombinant adenovirus gene transfer in lung cancer. Longitudinal study of the immune responses to transgene and viral products. J Clin Invest. 100(9):2218–2226. doi:10.1172/jci119759.
- Dinney CP, Fisher MB, Navai N, O’Donnell MA, Cutler D, Abraham A, Young S, Hutchins B, Caceres M, Kishnani N, et al. 2013. Phase I trial of intravesical recombinant adenovirus mediated interferon-α2b formulated in Syn3 for Bacillus Calmette-Guérin failures in nonmuscle invasive bladder cancer. J Urol. 190(3):850–856. doi:10.1016/j.juro.2013.03.030.
- Pearson S, Jia H, Kandachi K. 2004. China approves first gene therapy. Nat Biotechnol. 22(1):3–4. doi:10.1038/nbt0104-3.
- Yamaizumi M, Mekada E, Uchida T, Okada Y. 1978. One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell. 15(1):245–250. doi:10.1016/0092-8674(78)90099-5.
- Chen JS, Liu JC, Shen L, Rau KM, Kuo HP, Li YM, Shi D, Lee YC, Chang KJ, Hung MC. 2004. Cancer-specific activation of the survivin promoter and its potential use in gene therapy. Cancer Gene Ther. 11(11):740–747. doi:10.1038/sj.cgt.7700752.
- Huang R, Zhao Z, Ma X, Li S, Gong R, Kuang A. 2011. Targeting of tumor radioiodine therapy by expression of the sodium iodide symporter under control of the survivin promoter. Cancer Gene Ther. 18(2):144–152. doi:10.1038/cgt.2010.66.
- Garg H, Salcedo R, Trinchieri G, Blumenthal R. 2010. Improved nonviral cancer suicide gene therapy using survivin promoter-driven mutant Bax. Cancer Gene Ther. 17(3):155–163. doi:10.1038/cgt.2009.63.
- Kageyama T, Ohishi M, Miyamoto S, Mizushima H, Iwamoto R, Mekada E. 2007. Diphtheria toxin mutant CRM197 possesses weak EF2-ADP-ribosyl activity that potentiates its anti-tumorigenic activity. J Biochem. 142(1):95–104. doi:10.1093/jb/mvm116.
- Uchida T, Pappenheimer AM Jr., Greany R. Diphtheria toxin and related proteins. I. Isolation and properties of mutant proteins serologically related to diphtheria toxin. J Biol Chem. 1973;248(11):3838–3844.
- Qiao J, Ghani K, Caruso M. 2008. Diphtheria toxin mutant CRM197 is an inhibitor of protein synthesis that induces cellular toxicity. Toxicon. 51(3):473–477. doi:10.1016/j.toxicon.2007.09.010.
- Li Y, McCadden J, Ferrer F, Kruszewski M, Carducci M, Simons J, Rodriguez R. Prostate-specific expression of the diphtheria toxin A chain (DT-A): studies of inducibility and specificity of expression of prostate-specific antigen promoter-driven DT-A adenoviral-mediated gene transfer. Cancer Res. 2002;62(9):2576–2582.
- Dai LX, Yang J, Liu JM, Huang S, Wang BN, Li H, Zhao ZY, Cao K, Li MY, Li M-Y. 2019. Adenovirus-mediated CRM197 sensitizes human glioma cells to gemcitabine by the mitochondrial pathway. Cancer Biother Radiopharm. 34(3):171–180. doi:10.1089/cbr.2017.2363.
- Dai L, Pan Q, Peng Y, Huang S, Liu J, Chen T, Wang X, Chen D, Wang J, Zhu Y, et al. 2018. p53 plays a key role in the apoptosis of human ovarian cancer cells induced by adenovirus-mediated CRM197. Hum Gene Ther. 29(8):916–926. doi:10.1089/hum.2017.186.
- Fogar P, Navaglia F, Basso D, Zambon CF, Moserle L, Indraccolo S, Stranges A, Greco E, Fadi E, Padoan A, et al. 2010. Heat-induced transcription of diphtheria toxin A or its variants, CRM176 and CRM197: implications for pancreatic cancer gene therapy. Cancer Gene Ther. 17(1):58–68. doi:10.1038/cgt.2009.48.
- Hou H, Zhang C, Qi X, Zhou L, Liu D, Lv H, Li T, Sun D, Zhang X. 2020. Distinctive targetable genotypes of younger patients with lung adenocarcinoma: a cBioPortal for cancer genomics data base analysis. Cancer Biol Ther. 21(1):26–33. doi:10.1080/15384047.2019.1665392.
- Wang X, Guo S, Zhao R, Liu Y, Yang G. 2019. STAT3-activated long non-coding RNA lung cancer associated transcript 1 drives cell proliferation, migration, and invasion in hepatoblastoma through regulation of the miR-301b/STAT3 Axis. Hum Gene Ther. 30(6):702–713. doi:10.1089/hum.2018.146.
- Xie Z, Gu Y, Lin X, Ouyang M, Qin Y, Zhang J, Liu J, Mai S, Zhou C. 2019. Unexpected favorable outcome to etoposide and cisplatin in a small cell lung cancer transformed patient: a case report. Cancer Biol Ther. 20(9):1172–1175. doi:10.1080/15384047.2019.1617561.
- Hu X, Gu Y, Zhao S, Hua S, Jiang Y. 2019. Elevated circulating CD4(+)CD25(-)Foxp3(+) regulatory T cells in patients with nonsmall cell lung cancer. Cancer Biother Radiopharm. 34(5):325–333. doi:10.1089/cbr.2018.2672.
- Mittal S, Rajala MS. 2020. Heat shock proteins as biomarkers of lung cancer. Cancer Biol Ther. 21(6):477–485. doi:10.1080/15384047.2020.1736482.
- Huang Y, Zhuang Q, Zhuang W. 2020. Mortal obligate RNA transcript inhibits cancer cell invasion and migration in lung adenocarcinoma by downregulating miRNA-223. Cancer Biother Radiopharm 35(5):345–350. doi:10.1089/cbr.2019.3244.
- Li M, Zhou CZ, Yang JJ, Lu S, Zheng D, Hu J, Zeng H, Lu Y, Lu KH, Li SA, et al. 2019. The in cis compound EGFR mutations in Chinese advanced non-small cell lung cancer patients. Cancer Biol Ther. 20(8):1097–1104. doi:10.1080/15384047.2019.1595280.
- Lu H, Han X, Ren J, Ren K, Li Z, Sun Z. 2020. Circular RNA HIPK3 induces cell proliferation and inhibits apoptosis in non-small cell lung cancer through sponging miR-149. Cancer Biol Ther. 21(2):113–121. doi:10.1080/15384047.2019.1669995.
- Dong Z, Liu H, Zhao G. 2020. Long noncoding RNA SNHG6 promotes proliferation and inhibits apoptosis in non-small cell lung cancer cells by regulating miR-490-3p/RSF1 Axis. Cancer Biother Radiopharm. 35(5):351–361. doi:10.1089/cbr.2019.3120.
- Gardner EE, Lok BH, Schneeberger VE, Desmeules P, Miles LA, Arnold PK, Ni A, Khodos I, de Stanchina E, Nguyen T, et al. 2017. Chemosensitive relapse in small cell lung cancer proceeds through an EZH2-SLFN11 Axis. Cancer Cell. 31(2):286–299. doi:10.1016/j.ccell.2017.01.006.
- Yuan Z, Cao K, Lin C, Li L, Liu HY, Zhao XY, Liu L, Deng HX, Li J, Nie CL, et al. 2011. The p53 upregulated modulator of apoptosis (PUMA) chemosensitizes intrinsically resistant ovarian cancer cells to cisplatin by lowering the threshold set by Bcl-x(L) and Mcl-1. Mol Med. 17(11–12):1262–1274. doi:10.2119/molmed.2011.00176.
- Cao K, Yang J, Lin C, Wang BN, Yang Y, Zhang J, Dai J, Li L, Nie CL, Yuan Z, et al. 2012. Noxa enhances the cytotoxic effect of gemcitabine in human ovarian cancer cells. Cancer Biother Radiopharm. 27(4):259–266. doi:10.1089/cbr.2011.1126.
