ABSTRACT
Persistent activation of signal transducer and activator of transcription 3 (STAT3) is frequently reported in cancers and plays important roles in tumor progression. Therefore, directly targeting persistent STAT3 signaling is an attractive cancer therapeutic strategy. The aim of this study is to test the inhibitory efficacy of novel STAT3 small molecule inhibitors, LLY17 and LLL12B, in combination with irradiation in human medulloblastoma cells. Both LLY17 and LLL12B inhibit the IL-6-induced and persistent STAT3 phosphorylation in human medulloblastoma cells. Irradiation using 4 Gy alone exhibits some inhibitory effects on medulloblastoma cell viability, and these effects are further enhanced by combining with either STAT3 inhibitor. Irradiation alone also shows certain inhibitory effects on medulloblastoma cell migration and invasion and the combination of LLY17 or LLL12B with irradiation further demonstrates greater inhibitory effects than monotherapy. STAT3 inhibitor alone or irradiation alone exhibits some suppression of medulloblastoma tumorsphere growth, and the combination of LLY17 or LLL12B and irradiation exhibits greater suppression of tumorsphere growth than monotherapy. Combining either STAT3 inhibitor with irradiation reduces the expression of STAT3 downstream targets, Cyclin D1 and Survivin, and induces apoptosis in medulloblastoma cells. These results support that combination of a potent STAT3 inhibitor such as LLY17 or LLL12B with irradiation is an effective and novel therapeutic approach for medulloblastoma.
Introduction
Radiation therapy is commonly used in treating various tumors including medulloblastoma which is the most common embryonal brain tumor in children.Citation1–3 Medulloblastomas arise from distinct regions of the cerebellum and brainstem during development and have the potential for spread through cerebrospinal pathways.Citation4–6 The current treatment for medulloblastoma includes surgery, radiation therapy, and chemotherapy. With the modification of radiation therapy volumes and doses or followed by chemotherapy using cisplatin, lomustine (CCNU) and vincristine, the 5-year survival rate has improved.Citation7,Citation8 However, those who survive medulloblastoma often suffer severe long-term side effects due to the treatment regimen and they remain at risk for tumor recurrence.Citation7 Thus, there is an urgent need for investigating more effective and less toxic therapies to improve outcomes and reduce side effects for children with medulloblastoma.
In recent years, Signal Transducers and Activators of Transcription 3 (STAT3) has drawn attention as a potential target for tumor therapy. It is a latent cytoplasmic transcription factor that belongs to the STAT protein family and it participates in regulating target genes including Cyclin D1, c-Myc, Survivin, MMP2, VEGF, and NF-κB, which are related to cancer cell proliferation, survival, migration, invasion, angiogenesis, and immune surveillance.Citation9,Citation10 The activation of STAT3 is mediated through the binding of cytokines or growth factors to cell surface receptors, such as interleukin-6 (IL-6), interleukin-11 (IL-11), epidermal growth factor (EGF), and vascular endothelial growth factor (VEGF).Citation11,Citation12 Therefore, STAT3 as an aggregation point of various signaling pathways is an attractive target for cancer therapy.
STAT3 is persistently activated in various types of tumor cells including medulloblastoma.Citation13,Citation14 In medulloblastoma, STAT3 is activated in CD133+ medulloblastoma stem cells and promotes tumorigenesis of Group 3 medulloblastoma.Citation15 STAT3 expression contributes SHH medulloblastoma tumor onset in male mice and elevated STAT3 expression correlates with worse overall survival in male patients.Citation16 Targeting STAT3 or upstream kinases of STAT3 inhibits the growth of human medulloblastoma cells in vitro and in vivo.Citation17,Citation18 In addition, we have previously shown that the STAT3 inhibitors, LY5 and LLL12, inhibited STAT3 phosphorylation and exhibited antitumor activity in human medulloblastoma cells.Citation19,Citation20 The evidence suggests that STAT3 is rational strategy for medulloblastoma therapy.
To date, a number of STAT3 inhibitors have been developed and several inhibitors are undergoing clinical trials.Citation21 We have recently designed and synthesized several non-peptide compounds targeting STAT3 using Advanced Multiple Ligand Simultaneous Docking (AMLSD) methods to improve selectivity and druggability.Citation22 Currently, accumulating evidence shows that STAT3 is a molecular target capable of sensitizing tumors to radiation therapy.Citation23–29 However, the efficacy and mechanisms of STAT3 inhibitors with radiation treatment are not fully investigated in human medulloblastoma cells. In the present study, we investigated the combination effects of novel STAT3 small molecule inhibitors LLY17 or LLL12B with irradiation in human medulloblastoma cells. Our data show that STAT3 inhibitors inhibit IL-6 stimulated STAT3 phosphorylation. In addition, LLY17 or LLL12B combined with irradiation demonstrates enhanced suppression of cell viability, cell migration, invasion and tumorsphere growth. Furthermore, LLY17 or LLL12B with irradiation induces cell apoptosis and inhibits STAT3 downstream proteins. Therefore, our findings suggest that combination of STAT3 inhibitor LLY17 or LLL12B with irradiation therapy is a potential therapeutic strategy for medulloblastoma.
Materials and methods
Cell lines, reagents, STAT3 inhibitors, and radiation
Human medulloblastoma cell lines UW288, UW426, and D425 were kindly provided by Dr. Corey Raffel (University of California, San Francisco) and Dr. Hui-Wen Lo (Wake Forest University School of Medicine). Human medulloblastoma cell line D283 was purchased from American Type Culture Collection (Manassas, VA, USA). All cells were cultured in Dulbecco’s Modified Eagle Medium (Corning, NY, USA) with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA) and 1% Penicillin/Streptomycin (Sigma-Aldrich, St. Louis, MO, USA) and maintained in a humidified atmosphere with 5% CO2 at 37°C.
STAT3 small molecule inhibitors LLY17 and LLL12B were synthesized in Dr. Chenglong Li’s laboratory at the University of Florida College of Pharmacy, dissolved in sterile dimethyl sulfoxide (DMSO) to make a 20 mM stock solution and stored at −20°C. Human Interleukin-6 (IL-6) was purchased from Cell Signaling Technology (Danvers, MA, USA) and prepared according to the manufacturer’s instruction. For radiation treatment, cells were irradiated with X-Rad320 irradiator (Precision X-Ray, North Branford, CT, USA). Cells were seeded and grown overnight and then treated with STAT3 inhibitors LLY17 or LLL12B followed by irradiation at 4 Gray (Gy).
Western blot
Cells were harvested and extracted using cell lysis buffer (Cell Signaling Technology, Danvers, MA, USA). Whole cell lysates were separated by 10% SDS–PAGE gels and transferred onto PVDF membranes. Proteins were detected with primary antibodies (1:1000) at 4°C overnight after blocking in 5% nonfat milk, followed by horseradish peroxidase (HRP)-conjugated secondary antibodies. The primary antibodies for detecting P-STAT3 (Y705), STAT3, N-cadherin, SLUG, Cyclin D1, Survivin, cleaved Caspase-3, and GAPDH were obtained from Cell Signaling Technology (Danvers, MA, USA). SuperSignalTM West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, Waltham, MA, USA) were used and visualized signals using Amersham Imager 680 (GE Healthcare Life Sciences, Marlborough, MA, USA).
MTT cell viability assay
Cell viability was investigated using the MTT assay. Cells were plated at a density of 4000–8000 cells/well in a 96-well plate. On the next day, irradiated (4 Gy) and non-irradiated cells were treated with LLY17, LLL12B, or DMSO control (no STAT3 inhibitor, no irradiation). After 72 hours treatment, 20 μl of thiazolyl blue tetrazolium dye solution (Sigma-Aldrich, St. Louis, MO, USA) was added in each well and after 4 h of incubation at 37°C in the dark, 100 μl of N, N-dimethylformamide solution (Sigma-Aldrich, St. Louis, MO, USA) was added to dissolve the formazan at room temperature overnight in the dark. The absorbance was read at a 595 nm wavelength using EL808 Ultra Microplate Reader (BioTek, Winooski, VT, USA)
Wound healing assay
UW426 and UW288 cells were seeded in a 6-well plate and grown to full confluence. A straight scratch was made in individual wells using a pipette tip. After scratching, cells were washed with medium and non-irradiated or irradiated (4 Gy) cells were treated with either LLY17, LLL12B or DMSO until the scratch in non-irradiated DMSO treatment cells was filled with cells again. Images were taken using a Leica microscope and relative migration (%) was calculated by comparing with non-irradiated DMSO treated cells.
Cell invasion assay
Cell invasion assay was performed using Matrigel-coated chambers. The inserts (24-well insert, CELLTREAT Scientific Products, Pepperell, MA, USA) were coated with 1 mg/ml Matrigel (Corning, NY, USA) at 37°C overnight. 2 × 104 non-irradiated or irradiated (4 Gy) cells in 200 µl serum free medium were seeded in the inserts and treated with LLY17, LLL12B or DMSO. 500 µl culture medium with 10% FBS was placed in the lower chamber. Cells were cultured for 24 h and invasive cells on the lower surface of the inserts were stained with 0.1% crystal violet in 25% methanol at RT for 10 min. Invasive cells were counted and photographed using a light microscope under ×100 magnification objective.
Tumorsphere formation
D283 and D425 cells were seeded in PromoCell 3D tumorsphere medium XF supplemented with SupplementMix (PromoCell, Germany) at a density of 10,000 cells/ml in ultra-low attachment 6-well plate. Non-irradiated or irradiated (4 Gy) cells were treated with either LLY17, LLL12B or DMSO for 10 days. Half culture volume of fresh medium was added every 5 days. Non-irradiated cells treated with DMSO served as controls. Images were taken using a Leica microscope and tumorsphere numbers were counted.
Apoptosis assay
Annexin V apoptosis assay was performed using flow cytometry to investigate cell apoptosis. Non-irradiated or irradiated (4 Gy) cells were treated with either LLY17, LLL12B, or DMSO overnight. Cells were harvested by trypsinization and incubated with Annexin V-APC and PI using APC Annexin V Apoptosis Detection Kit (Biolegend, San Diego, CA, USA) according to the manufacturer’s instruction. Cells were observed using FACSCanto II cytometer (BD Biosciences, San Jose, CA, USA) and analyzed using FLOWJO software (BD Biosciences, San Jose, CA, USA).
Statistical analysis
Quantitative data were evaluated for statistical significance (P-value) between experimental and control groups using one-way analysis of variance (ANOVA). Each experiment was carried out in triplicate and all quantitative data are presented as mean ± SD. P < .05 was considered significant. Data were analyzed with GraphPad software (GraphPad Software Inc, San Diego, CA, USA).
Results
LLY17 and LLL12B inhibit IL-6 stimulated STAT3 phosphorylation in human medulloblastoma cells
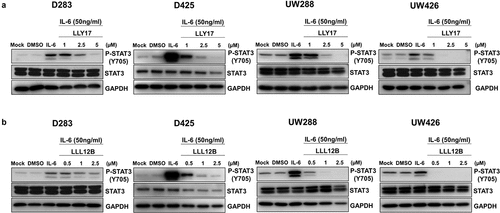
We examined the activity of LLY17 and LLL12B in inhibiting IL-6 stimulated STAT3 phosphorylation. D283, D425, UW288, and UW426 human medulloblastoma cells were treated with LLY17 (1, 2.5, and 5 μM), LLL12B (0.5, 1, and 2.5 μM), or DMSO in serum free medium for 4 hours. 50 ng/ml of IL-6 was added for an additional 30 minutes to induce STAT3 phosphorylation according to a previous study.Citation20 The results showed that IL-6 significantly induced STAT3 phosphorylation as expected and the induction of STAT3 phosphorylation was inhibited by LLY17 () and LLL12B () in D283, D425, UW288 and UW426 human medulloblastoma cells. These results indicate that STAT3 inhibitors LLY17 and LLL12B inhibit STAT3 phosphorylation induced by IL-6 in human medulloblastoma cells.
Figure 1. LLY17 and LLL12B inhibited IL-6 induced phosphorylation of STAT3 in human medulloblastoma cells. D283, D425, UW288, and UW426 human medulloblastoma cells were seeded and cultured overnight. Cells were pre-treated with LLY17 (1 µM, 2.5 µM and 5 µM), LLL12B (0.5 µM, 1 µM, and 2.5 µM) or DMSO for 4 hours in 0% FBS, then stimulated by 50 ng/ml IL-6 for additional 30 minutes and harvested for Western blot. Western blot analysis of P-STAT3 (Y705) and STAT3 in LLY17 (a) and LLL12B (b) treated cells. GAPDH was used as a protein loading control
LLY17 or LLL12B combined with irradiation reduces human medulloblastoma cell viability
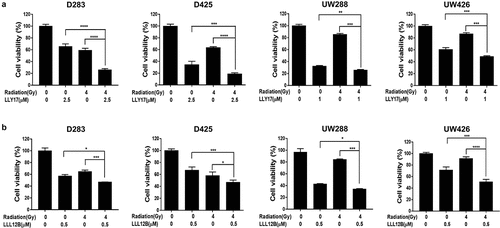
Various reports have shown the tumor growth inhibitory effect of STAT3 inhibitor and irradiation when either is used alone. Therefore, we used the MTT assay to investigate the combination effects of a STAT3 inhibitor and irradiation on cell viability in D283, D425, UW288, and UW426 medulloblastoma cells. After 72 hours treatment, 4 Gy dose of irradiation alone decreased cell viability compared to non-irradiation control level (). As expected, either LLY17 () or LLL12B () alone reduced cell viability in human medulloblastoma cells. Moreover, toxicity was significantly enhanced when STAT3 inhibitors and irradiation were used in combination compared to either irradiation, LLY17 () or LLL12B () alone. These results indicate that the STAT3 inhibitors, LLY17 and LLL12B sensitize human medulloblastoma cells to irradiation.
Figure 2. LLY17 or LLL12B and irradiation combination treatment significantly inhibited cell viability in human medulloblastoma cells. Cells were seeded and cultured overnight in 96-well plates. Non-irradiated cells or irradiated (4 Gy) cells were treated with LLY17, LLL12B, or DMSO for 72 hours. The combination effects of irradiation and LLY17 (a) or LLL12B (b) on cell viability were determined by MTT assay. Experiments were performed in triplicate and data are presented as means ± SD. *P < .05, **P < .01, ***P < .001, ****P < .0001
LLY17 or LLL12B combined with irradiation inhibits human medulloblastoma cell migration
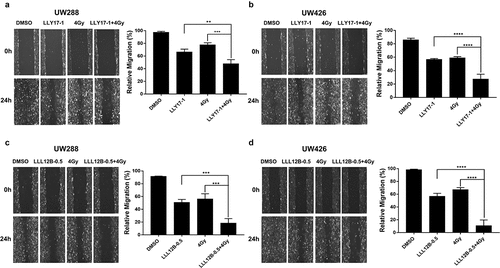
Next, we performed wound healing assay to investigate the effects of LLY17 or LLL12B followed by irradiation on cell migration in UW288 and UW426 medulloblastoma cells. In UW288 cells, 4 Gy dose of irradiation, LLY17 (1 μM), and LLL12B (0.5 μM) treatment alone inhibited cell migration compared to non-irradiated, DMSO treated cells ()). However, combining irradiation with LLY17 or LLL12B treatment resulted in greater inhibition of cell migration ()). Similarly, combining irradiation (4 Gy) with LLY17 (1 μM) or LLL12B (0.5 μM) resulted in greater inhibition of cell migration compared to single treatments in UW426 cells ()). These results confirm the inhibitory effects of LLY17 or LLL12B when combined with irradiation on human medulloblastoma cell migration.
Figure 3. The inhibitory effects of LLY17 or LLL12B combined with irradiation on cell migration in human medulloblastoma cells. Wound healing assay was performed to determine the migratory ability of irradiated and LLY17 or LLL12B treated UW288 and UW426 cells. Cells with combination treatment were compared to cells with single treatment or with DMSO control. Representative cell images showing the scratch at 0 h and 24 h after non-irradiation or irradiation and LLY17 (1 µM) or DMSO treatment in UW288 cells (a) and UW426 cells (b). Quantification of the migration area is shown in the right panel. Representative cell images showing the scratch at 0 h and 24 h after non-irradiation or irradiation and LLL12B (0.5 µM) or DMSO treatment in UW288 cells (c) and UW426 cells (d). Quantification of the migration area is shown in the right panel. Experiments were performed in triplicate and data are presented as means ± SD. **P < .01, ***P < .001, ****P < .0001
LLY17 or LLL12B combined with irradiation inhibits cell invasion and EMT in human medulloblastoma cells
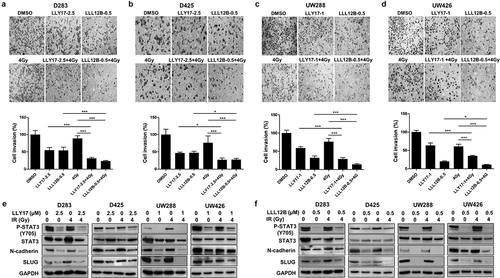
Tumor cell invasion is one of the hallmarks for metastasis. We performed cell invasion assay to study the combination effects of LLY17, LLL12B and irradiation on cell invasion in D283, D425, UW288, and UW426 medulloblastoma cells. As shown in ), LLY17 or LLL12B treatment enhanced efficacy of irradiation (4 Gy) on cell invasion. Due the fact that LLY17 or LLL12B combined with irradiation inhibits the migration and invasion of human medulloblastoma cells, we detected the expression of EMT markers to determine the effects of LLY17, LLL12B combined with irradiation on the EMT progression. As expected, Western blot results revealed that LLY17 () or LLL12B () treatment decreased STAT3 phosphorylation (Y705) in all cell lines. In addition, combined LLY17 or LLL12B and irradiation treatment down-regulated the expression of mesenchymal marker N-cadherin and transcription factor SLUG ()). These results indicate that LLY17 or LLL12B combined with irradiation inhibits cell invasion and EMT in human medulloblastoma cells.
Figure 4. The inhibitory effects of LLY17 or LLL12B combined with irradiation on cell invasion and EMT in human medulloblastoma cells. Invasion assay was performed to determine the invasion ability of irradiated and LLY17 or LLL12B treated human medulloblastoma cells. Cells were treated with DMSO, LLY17 or LLL12B alone, with or without irradiation (4 Gy) for 24 h. Representative images of D283 (a), D425 (b), UW288 (c), and UW426 (d) cells are shown. Quantification of cell invasion is shown in the bottom panel. Human medulloblastoma cells were treated with LLY17 (e) or LLL12B (f) alone, with or without irradiation (4 Gy) overnight and the expression levels of P-STAT3 (Y705), STAT3, N-cadherin, and SLUG were determined by Western Blot. GAPDH was used as a protein loading control. Experiments were performed in triplicate and data are presented as means ± SD. *P < .05, ***P < .001
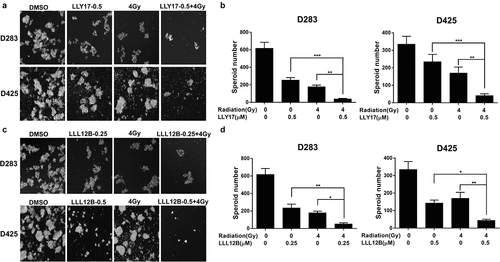
LLY17 or LLL12B combined with irradiation inhibits human medulloblastoma tumorsphere formation
Tumorsphere formation assay was performed to further evaluate the self-renewal capacity of D283 and D425 medulloblastoma cells. UW426 and UW288 cells were unable to form tumorspheres or neurospheres and therefore, were not tested in this assay. D283 and D425 cells were treated with LLY17 (0.5 μM) followed by irradiation (4 Gy) and compared to LLY17 alone, 4 Gy alone, and DMSO control. As shown, LLY17 and irradiation treatment alone suppressed tumorsphere formation in both cell lines and a greater reduction in tumorsphere formation was observed in the combination treatment groups ()). Similar results were noted in the cells treated with the combination of LLL12B (0.25 μM or 0.5 μM) and irradiation (4 Gy). The combination of LLL12B and irradiation exhibited a more pronounced suppression of tumorsphere growth than single treatment ()). Taken together, the results suggest that LLY17 or LLL12B combined with irradiation synergistically inhibits tumorsphere formation.
Figure 5. LLY17 or LLL12B combined with irradiation enhanced the inhibition of tumorsphere formation in human medulloblastoma cells. D283 and D425 cells were dissociated and plated in ultra-low attachment 6-wells at 10,000 cells/ml. Non-irradiated or irradiated (4 Gy) cells were treated with LLY17, LLL12B, or DMSO for 10 days. Cells with combination treatment were compared to cells with single treatment or with DMSO control. (a) Representative images of tumorspheres following LLY17 treatment without or with irradiation in D283 and D425 cells. (b) Quantification of tumorsphere numbers in D283 and D425 cells following LLY17 treatment without or with irradiation. (c) Representative images of tumorspheres following LLL12B treatment without or with irradiation in D283 and D425 cells. (d) Quantification of tumorsphere numbers in D283 and D425 cells following LLL12B treatment without or with irradiation. Experiments were performed in triplicate and data are presented as means ± SD. *P < .05, **P < .01, ***P < .001
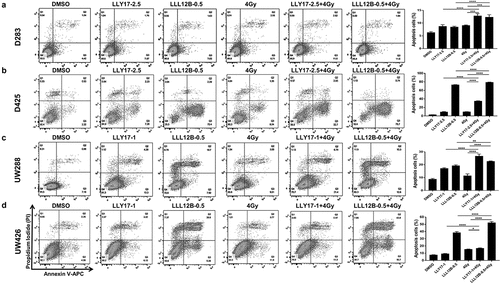
LLY17 or LLL12B combined with irradiation induces human medulloblastoma cell apoptosis
Next, we examined whether LLY17 and LLL12B sensitize human medulloblastoma cells to irradiation was associated with enhanced cell apoptosis. D283, D425, UW288, and UW426 medulloblastoma cells were treated with LLY17 or LLL12B followed by irradiation (4 Gy). After overnight treatment, cells were labeled with Annexin V-APC/PI and analyzed by flow cytometry. As shown in , the apoptotic effect of combined LLY17 or LLL12B and irradiation treatment was much greater than in the single treatment groups in D283, D425, UW288, and UW426 medulloblastoma cells. These results indicate that LLY17 or LLL12B in combination with irradiation induces human medulloblastoma cell apoptosis.
Figure 6. LLY17 or LLL12B combined with irradiation induced apoptosis in human medulloblastoma cells. Cells were treated with DMSO, LLY17, or LLL12B alone, with or without irradiation (4 Gy) overnight and subjected to the Annexin V apoptosis assay using flow cytometry. Representative results of the assay in D283 (a), D425 (b), UW288 (c) and UW426 (d) cells are shown. The percentage of apoptotic cells (early and late apoptosis) was quantified and shown in the right panel. Experiments were performed in triplicate and data are presented as means ± SD. *P < .05, **P < .01, ***P < .001, ****P < .0001
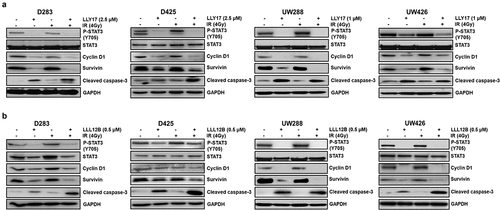
LLY17 or LLL12B combined with irradiation modulates the expression of STAT3 target and apoptosis proteins in human medulloblastoma cells
To further elucidate the combination effects of LLY17 or LLL12B and irradiation, we investigated the potential molecular mechanisms in D283, D425, UW288, and UW426 medulloblastoma cells treated with LLY17 or LLL12B and irradiation. Western blot results revealed expression levels of STAT3 downstream targets, Cyclin D1 and Survivin were reduced following LLY17 () or LLL12B () treatment with or without irradiation. Greater reduction of Survivin expression using combination treatment was observed in all four medulloblastoma cell lines (). Furthermore, cleaved Caspase-3 expression levels, a marker of apoptosis, were markedly increased with LLY17 or LLL12B treatment alone or when combined with irradiation.
Figure 7. LLY17 or LLL12B combined with irradiation inhibited STAT3 targets and induced cell apoptosis protein in human medulloblastoma cells. Non-irradiated or irradiated (4 Gy) human medulloblastoma D283, D425, UW288, and UW426 cells were treated with LLY17 (a), LLL12B (b) or DMSO overnight. Cells were harvested and analyzed by Western blot. The expression levels of P-STAT3 (Y705), STAT3, Cyclin D1, Survivin and cleaved Caspase-3 were determined. GAPDH was used as a protein loading control
Discussion
Radiation therapy has been used in cancer treatment for decades. In medulloblastoma, radiation therapy is typically performed after surgery to remove the remaining cancer cells and it is administered alone or combined with chemotherapy for older children.Citation7 The combination of different therapeutic strategies may enhance the efficacy and reduce the nonspecific toxicity due to what otherwise would be high dose monotherapy. Here, we tested the efficacy of novel STAT3 inhibitors, LLY17 and LLL12B in combination with irradiation in suppressing human medulloblastoma cells.
Medulloblastoma represents a heterogeneous disease with distinct histological and molecular characteristics.Citation30–32 Recently, the World Health Organization (WHO) grouped medulloblastoma to WNT-activated, SHH-activated TP53-mutant, SHH-activated TP53-wildtype, non-WNT/non-SHH group 3 and group 4 by histologic and molecular features.Citation33 This new classification system has benefited the development of targeted therapy for medulloblastoma, and now SHH-activated subtypes can be targeted using multiple Hedgehog pathway inhibitors.Citation34 However, targeted therapies are not well developed or even apparent in other medulloblastoma subtypes.
STAT3 is an attractive target for cancer therapy and persistently activated in different medulloblastoma subtypes. However, few selective STAT3 inhibitors have been approved for human treatment. To improve selectivity and the potential to be translated into clinical evaluations, we designed and synthesized novel small molecules targeting STAT3 named LLY17 and LLL12B using Advanced Multiple Ligand Simultaneous Docking (AMLSD) methods. In addition, radiation therapy is one of the standard cares of medulloblastoma. However, several published studies reported that persistent STAT3 signaling may confer radio-resistance in cancer cellsCitation27,Citation35–39 and thus compromise the efficacy of radiation therapy in cancer, and likely in medulloblastoma. The significance of our study to evaluate the combinatorial effects of LLY17 and LLL12B respectively with irradiation to provide experimental evidence to support the potential of our candidate STAT3 inhibitors with radiation as a more effective medulloblastoma therapy. We observed that STAT3 inhibitors LLY17 and LLL12B can enhance the inhibitory activity of irradiation in human medulloblastoma cells, which further support the translational potential of such combinational treatments.
The medulloblastoma cell lines D283, D425, UW288, and UW426 belong to group 3/4, group 3, WNT-activated, and SHH-activated medulloblastoma subtype, respectively.Citation40 In our study, we showed that LLY17 and LLL12B suppressed cell viability and increased the sensitivity of different medulloblastoma subtypes to irradiation (). We also investigated STAT3 downstream targets involved in cell proliferation (Cyclin D1) and survival (Survivin) and found that both Cyclin D1 and Survivin expression levels were decreased after combination treatment with LLY17 or LLL12B and irradiation. Meanwhile, the induction of cell apoptosis () and cleaved Caspase-3 was increased (). These results could partially explain the significant decrease in cell viability with combination treatment and indicate that STAT3 is a viable therapeutic target for medulloblastoma therapy.
Medulloblastoma has a propensity to metastasize and active migration is a prerequisite for tumor cell invasion and metastasis.Citation41,Citation42 Therefore, we investigated the effects of combined LLY17 or LLL12B and irradiation on human medulloblastoma cell migration, invasion, and EMT. However, accumulative evidence showed that irradiation induced cell migration while others showed inhibited cell migration in medulloblastoma.Citation43–46 Our results showed a decrease in migration after irradiation, with combination treatment inducing a significant decrease in UW288 and UW426 cell migration (). Likewise, cell invasion and EMT related proteins N-cadherin and SLUG were inhibited with combination treatment in all human medulloblastoma cell lines (). This suggests that STAT3 inhibitors combined with irradiation could be beneficial in limiting medulloblatoma metastasis.
Cancer stem cells (CSCs) are a small subpopulation of cancer cells with self-renewing capacity when they are grown as tumorsphere or neurosphere and are responsible for cancer initiation, progression, resistance, and recurrence.Citation47,Citation48 Like many other solid tumors, medulloblastoma stem cells are capable of tumor initiation and display significant resistance to radiation therapy.Citation49,Citation50 Recent studies have shown that STAT3 is implicated in CSCs regulation and resistant to radiation.Citation10,Citation29 Chang et al.Citation51demonstrated that targeting STAT3 with cucurbitacin I significantly blocked STAT3 signaling, suppressed CSC-like properties, and improved chemoradiosensitivity in MB-CD133+ cells. Additionally, Yang et al.Citation52showed that STAT3-related genes were upregulated in medulloblastoma stem cells and celecoxib sensitized these cells to irradiation exposure by reducing STAT3 phosphorylation. To further explore LLY17 and LLL12B as possible treatments for medulloblastoma, we demonstrated that LLY17 and LLL12B can inhibit tumorsphere growth. Importantly, the combination of LLY17 or LLL12B with irradiation treatment exhibited greater suppression of tumorsphere growth than single treatment ().
In conclusion, STAT3 inhibitors, LLY17 and LLL12B combined with irradiation effectively inhibit cell viability, cell migration, invasion and tumorsphere growth and induce cell apoptosis of human medulloblastoma cells. These findings support that combination of a potent STAT3 inhibitor such as LLY17 or LLL12B with irradiation may be an effective therapeutic approach for medulloblastoma and is deserved for future development.
Authorship contribution statement
Perform experiments, L. P., R. Z., and L. M.; Funding acquisition, J. L.; Project administration, J. L.; Resources, C. L.; Supervision, C. L. and J. L.; Writing – original draft, L. P.; Writing – review & editing, J. L., C. P., and J. F. The manuscript was reviewed by all authors, and all authors have approved the final version of the article.
Supplemental Material
Download Zip (579.5 KB)Acknowledgments
We acknowledge Dr. Richard Eckert at University of Maryland School of Medicine for his assistance of using microscope for wound healing assay and cell invasion assay and Dr. A-Lien Lu-Chang at University of Maryland School of Medicine for her assistance of using Amersham Imager 680 for western blot.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Allen C, Her S, Jaffray DA. 2017. Radiotherapy for Cancer: present and Future. Adv Drug Deliv Rev. 109:1–2. 10.1016/j.addr.2017.01.004.
- Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019;21:v1–v100. 10.1093/neuonc/noz150.
- Millard NE, De Braganca KC. 2016. Medulloblastoma. J Child Neurol. 31:1341–1353. 10.1177/0883073815600866.
- Packer RJ, Cogen P, Vezina G, Rorke LB. 1999. Medulloblastoma: clinical and biologic aspects. Neuro Oncol. 1:232–250. 10.1093/neuonc/1.3.232.
- Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C, Kranenburg TA, Hogg T, Poppleton H, Martin J, et al. 2010. Subtypes of medulloblastoma have distinct developmental origins. Nature. 468(7327):1095–1099. DOI:10.1038/nature09587
- Gilbertson RJ, Ellison DW. 2008. The origins of medulloblastoma subtypes. Annu Rev Pathol. 3:341–365. 10.1146/annurev.pathmechdis.3.121806.151518.
- Padovani L, Horan G, Ajithkumar T. 2019. Radiotherapy Advances in Paediatric Medulloblastoma Treatment. Clin Oncol (R Coll Radiol). 31:171–181. 10.1016/j.clon.2019.01.001.
- Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. 2001. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children’s Cancer Group study. J Clin Oncol. 19:3470–3476. 10.1200/jco.2001.19.15.3470.
- Kamran MZ, Patil P, Gude RP. 2013. Role of STAT3 in cancer metastasis and translational advances. Biomed Res Int. 2013:421821. 10.1155/2013/421821.
- Lee H, Jeong AJ, Ye SK. 2019. Highlighted STAT3 as a potential drug target for cancer therapy. BMB Rep. 52:415–423. 10.5483/BMBRep.2019.52.7.152.
- Furtek SL, Backos DS, Matheson CJ, Strategies RP. 2016. Approaches of Targeting STAT3 for cancer treatment. ACS Chem Biol. 11:308–318. 10.1021/acschembio.5b00945.
- Debnath B, Xu S, Neamati N. 2012. Small molecule inhibitors of signal transducer and activator of transcription 3 (Stat3) protein. J Med Chem. 55:6645–6668. 10.1021/jm300207s.
- Schaefer LK, Ren Z, Fuller GN, Schaefer TS. 2002. Constitutive activation of Stat3alpha in brain tumors: localization to tumor endothelial cells and activation by the endothelial tyrosine kinase receptor (VEGFR-2). Oncogene. 21:2058–2065. 10.1038/sj.onc.1205263.
- Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945–954.
- Garg N, Bakhshinyan D, Venugopal C, Mahendram S, Rosa DA, Vijayakumar T, Manoranjan B, Hallett R, McFarlane N, Delaney KH, et al. 2017. CD133(+) brain tumor-initiating cells are dependent on STAT3 signaling to drive medulloblastoma recurrence. Oncogene. 36:606–617. 10.1038/onc.2016.235.
- White CL, Jayasekara WSN, Picard D, Chen J, Watkins DN, Cain JE, Remke M. Gough DJ. A sexually dimorphic role for STAT3 in Sonic Hedgehog Medulloblastoma. Cancers (Basel). 2019;11. doi:10.3390/cancers11111702.
- Ajeawung NF, Wang HY, Gould P, Kamnasaran D. 2012. Advances in molecular targets for the treatment of medulloblastomas. Clin Invest Med. 35:E246. 10.25011/cim.v35i5.18697.
- Wei J, Ma L, Li C, Pierson CR, Finlay JL, Lin J. 2019. Targeting upstream kinases of STAT3 in human medulloblastoma cells. Curr Cancer Drug Targets. 19:571–582. 10.2174/1568009618666181016165604.
- Ball S, Li C, Li PK, Lin J. 2011 The small molecule, LLL12, inhibits STAT3 phosphorylation and induces apoptosis in medulloblastoma and glioblastoma cells. PLoS One. 6:e18820. 10.1371/journal.pone.0018820
- Xiao H, Bid HK, Jou D, Wu X, Yu W, Li C, Houghton PJ, Lin J. 2015. A novel small molecular STAT3 inhibitor, LY5, inhibits cell viability, cell migration, and angiogenesis in medulloblastoma cells. J Biol Chem. 290:3418–3429. 10.1074/jbc.M114.616748.
- Qin JJ, Yan L, Zhang J, Zhang WD. 2019. STAT3 as a potential therapeutic target in triple negative breast cancer: a systematic review. J Exp Clin Cancer Res. 38:195. 10.1186/s13046-019-1206-z.
- Yu W, Li C, Zhang W, Xia Y, Li S, Lin JY, Yu K, Liu M, Yang L, Luo J, et al. 2017. Discovery of an orally selective inhibitor of signal transducer and activator of transcription 3 using advanced multiple ligand simultaneous docking. J Med Chem. 60:2718–2731. 10.1021/acs.jmedchem.6b01489.
- Xie B, Zhang L, Hu W, Fan M, Jiang N, Duan Y, Jing D, Xiao W, Fragoso RC, Lam KS, et al. Dual blockage of STAT3 and ERK1/2 eliminates radioresistant GBM cells. Redox Biol. 2019;24:101189. 10.1016/j.redox.2019.101189.
- Oweida AJ, Darragh L, Phan A, Binder D, Bhatia S, Mueller A, Court BV, Milner D, Raben D, Woessner R, et al. STAT3 modulation of regulatory T cells in response to radiation therapy in head and neck cancer. J Natl Cancer Inst. 2019;111:1339–1349. 10.1093/jnci/djz036.
- Kim KW, Mutter RW, Cao C, Albert JM, Shinohara ET, Sekhar KR, Lu B. Inhibition of signal transducer and activator of transcription 3 activity results in down-regulation of survivin following irradiation. Mol Cancer Ther. 2006;5:2659–2665. 10.1158/1535-7163.mct-06-0261.
- Spitzner M, Roesler B, Bielfeld C, Emons G, Gaedcke J, Wolff HA, Rave-Fränk M, Kramer F, Beissbarth T, Kitz J, et al. 2014. Overexpression of STAT3 potentiates growth, survival, and radioresistance of non-small-cell lung cancer (NSCLC) cells. Int J Cancer. 134:997–1007. 10.1002/ijc.28429.
- Yin ZJ, Jin FG, Liu TG, Fu EQ, Xie YH, Sun RL. 2011. Overexpression of STAT3 potentiates growth, survival, and radioresistance of non-small-cell lung cancer (NSCLC) cells. J Surg Res. 171:675–683. 10.1016/j.jss.2010.03.053.
- Bonner JA, Trummell HQ, Willey CD, Plants BA, Raisch KP. 2009. Inhibition of STAT-3 results in radiosensitization of human squamous cell carcinoma. Radiother Oncol. 92:339–344. 10.1016/j.radonc.2009.06.022.
- Spitzner M, Ebner R, Wolff HA, Ghadimi BM, Wienands J, Grade M. 2014. STAT3: a novel molecular mediator of resistance to chemoradiotherapy. Cancers (Basel). 6:1986–2011. 10.3390/cancers6041986.
- Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins CE, French P, et al. 2011. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 29:1408–1414. 10.1200/jco.2009.27.4324.
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. 10.1007/s00401-007-0243-4.
- Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, et al. 2012. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 123:465–472. 10.1007/s00401-011-0922-z.
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. 10.1007/s00401-016-1545-1.
- Liu X, Ding C, Tan W, Zhang A. Medulloblastoma: Molecular understanding, treatment evolution, and new developments. Pharmacol Ther. 2020;210:107516. 10.1016/j.pharmthera.2020.107516
- You S, Li R, Park D, Xie M, Sica GL, Cao Y, Xiao Z-Q, Deng X. 2014. Disruption of STAT3 by niclosamide reverses radioresistance of human lung cancer. Mol Cancer Ther. 13:606–616. 10.1158/1535-7163.mct-13-0608.
- Kim JS, Kim HA, Seong MK, Seol H, Oh JS, Kim EK, Chang JW, Hwang S-G, Noh WC. 2016. STAT3-survivin signaling mediates a poor response to radiotherapy in HER2-positive breast cancers. Oncotarget. 7:7055–7065. 10.18632/oncotarget.6855.
- Park SY, Lee CJ, Choi JH, Kim JH, Kim JW, Kim JY, Nam JS. The JAK2/STAT3/CCND2 Axis promotes colorectal Cancer stem cell persistence and radioresistance. J Exp Clin Cancer Res. 2019;38:399. 10.1186/s13046-019-1405-7.
- Wang X, Zhang X, Qiu C, Yang N. 2020. STAT3 contributes to radioresistance in cancer. Front Oncol. 10:1120. 10.3389/fonc.2020.01120.
- Otero DC, Poli V, David M, Rickert RC. 2006. Cutting edge: inherent and acquired resistance to radiation-induced apoptosis in B cells: a pivotal role for STAT3. J Immunol. 177:6593–6597. 10.4049/jimmunol.177.10.6593.
- Ivanov DP, Coyle B, Walker DA, Grabowska AM. 2016. In vitro models of medulloblastoma: choosing the right tool for the job. J Biotechnol. 236:10–25. 10.1016/j.jbiotec.2016.07.028.
- Martirosian V, Chen TC, Lin M, Neman J. 2016. Medulloblastoma initiation and spread: where neurodevelopment, microenvironment and cancer cross pathways. J Neurosci Res. 94:1511–1519. 10.1002/jnr.23917.
- Entschladen F, TLt D, Lang K, Joseph J, Zaenker KS. 2004. Tumour-cell migration, invasion, and metastasis: navigation by neurotransmitters. Lancet Oncol. 5:254–258. 10.1016/s1470-2045(04)01431-7.
- Nalla AK, Asuthkar S, Bhoopathi P, Gujrati M, Dinh DH, Rao JS. Suppression of uPAR retards radiation-induced invasion and migration mediated by integrin β1/FAK signaling in medulloblastoma. PLoS One. 2010;5:e13006. 10.1371/journal.pone.0013006
- Rieken S, Rieber J, Brons S, Habermehl D, Rief H, Orschiedt L, Lindel K, Weber KJ, Debus J, Combs SE. Radiation-induced motility alterations in medulloblastoma cells. J Radiat Res. 2015;56:430–436. 10.1093/jrr/rru120.
- Konings K, Vandevoorde C, Belmans N, Vermeesen R, Baselet B, Walleghem MV, Janssen A, Isebaert S, Baatout S, Haustermans K. The combination of particle irradiation with the Hedgehog inhibitor GANT61 differently modulates the radiosensitivity and migration of cancer cells compared to X-ray irradiation. Front Oncol. 2019;9:391. 10.3389/fonc.2019.00391.
- Asuthkar S, Nalla AK, Gondi CS, Dinh DH, Gujrati M, Mohanam S, Rao JS. 2011. Gadd45a sensitizes medulloblastoma cells to irradiation and suppresses MMP-9-mediated EMT. Neuro Oncol. 13:1059–1073. 10.1093/neuonc/nor109.
- Visvader JE, Lindeman GJ. 2008. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 8:755–768. 10.1038/nrc2499.
- Najafi M, Mortezaee K, Majidpoor J. 2019. Cancer stem cell (CSC) resistance drivers. Life Sci. 234:116781. 10.1016/j.lfs.2019.116781.
- Lu KH, Chen YW, Tsai PH, Tsai ML, Lee YY, Chiang CY, Kao C-L, Chiou S-H, Ku -H-H, Lin C-H, et al. 2009. Evaluation of radiotherapy effect in resveratrol-treated medulloblastoma cancer stem-like cells. Childs Nerv Syst. 25:543–550. 10.1007/s00381-009-0826-6.
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. 2004. Identification of human brain tumour initiating cells. Nature. 432(7015):396–401. 10.1038/nature03128.
- Chang CJ, Chiang CH, Song WS, Tsai SK, Woung LC, Chang CH, Jeng S-Y, Tsai C-Y, Hsu -C-C, Lee H-F, et al. 2012. Inhibition of phosphorylated STAT3 by cucurbitacin I enhances chemoradiosensitivity in medulloblastoma-derived cancer stem cells. Childs Nerv Syst. 28:363–373. 10.1007/s00381-011-1672-x.
- Yang MY, Lee HT, Chen CM, Shen CC, Ma HI. 2014. Celecoxib suppresses the phosphorylation of STAT3 protein and can enhance the radiosensitivity of medulloblastoma-derived cancer stem-like cells. Int J Mol Sci. 15:11013–11029. 10.3390/ijms150611013.
