ABSTRACT
ONC201 demonstrated promising activity in patients with advanced endometrial cancer in a Phase I clinical trial. ONC201 activates the integrated stress response (ISR) and upregulates TRAIL and its receptor DR5. We hypothesized ONC201 upregulation of DR5 could sensitize tumors to TRAIL and combination of ONC201 and TRAIL would lead to enhanced cell death in endometrial cancer models. Five endometrial cancer cell lines AN3CA, HEC1A, Ishikawa, RL952, and KLE as well as a murine xenograft model were treated with ONC201 alone or in combination with TRAIL. ONC201 decreased the cell viability of all five endometrial cancer cell lines at clinically achievable low micro-molar concentrations (2–4 μM). ONC201 activated the ISR and induced protein expression of TRAIL and DR5 at the cell surface. Pretreatment with ONC201 sensitized endometrial cancer cell lines to TRAIL, leading to increased cell death induction compared to either agent alone. Tumor growth was reduced in vivo by the ONC201/TRAIL combination treatment in the xenograft model of endometrial cancer (p = .014). Mice treated with combination treatment survived significantly longer than mice from the three control groups (p = .018). ONC201 decreased cell viability in endometrial cancer cells lines primarily through growth arrest while the combination of ONC201 and TRAIL promoted cell death in vitro and in vivo. Our results suggest a novel cancer therapeutic strategy that can be further investigated in the clinic.
Introduction
Endometrial cancer is the most common gynecologic malignancy among women in the United States, with the incidence and mortality rate continuing to rise over recent years.Citation1 Curative treatment is feasible when the disease is caught early; however, 30% of cases will recur, leading to over 11,000 deaths from uterine cancer each year. For women with advanced stage or recurrent endometrial cancer, the prognosis is poorCitation2,Citation3 as current treatment at this stage has had limited efficacy.
The current treatment approach for women with recurrent, metastatic, or high-risk disease consists mainly of chemotherapy with or without radiation. There are only a handful of potentially effective therapies for these women and these are therapies with high toxicity profiles that provide a median progression free survival of around only 13 months. These results have led the National Comprehensive Cancer Network (NCCN) to strongly encourage participation in clinical trials for these patients.Citation4 Identification of a new effective therapy in endometrial cancer would therefore be of great impact clinically.
ONC201 is a well-tolerated, orally active small molecule in the imipridone class that has demonstrated anti-cancer activity in various advanced solid tumors in preclinical cancer models without significant toxicity.Citation5 Its promising activity in patients with advanced endometrial cancer in a Phase I clinical trial has led to it currently being tested as a single agent in multiple Phase II trials.Citation5 Clinical trials of ONC201 are also being conducted in patients with breast cancer. Our group has recently shown efficacy of ONC201 in breast cancer cell models (17) however not all cell lines were responsive to ONC201 as a single agent. Importantly, in a recent publication we were able to show that breast cell lines resistant to ONC201 alone were sensitive to ONC201 when used in combination with TRAIL (12). Gynecologic cancers often have limited response to single-agent therapies. We are therefore hopeful that this combination would be efficacious in endometrial cancer models as well.
ONC201 has not been well elucidated in endometrial cancer; however, its mechanism of action had been well studied in a variety of other model systems. ONC201 is able to induce transcription of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) through inhibition of the ERK and AKT pathway in a p53 independent manner.Citation6,Citation7 TRAIL ligand can then activate cell death by binding to death receptor 4 and 5 (DR4, DR5) which activates the extrinsic pathway leading to apoptosis.Citation8,Citation9 Importantly, TRAIL has been shown to selectively target transformed cellsCitation10 leading to the appeal of TRAIL activation as an anticancer therapeutic. In addition to increasing TRAIL expression, ONC 201 has the ability to upregulate the TRAIL receptor, DR5, through activation of the integrated stress response (ISR).Citation11 Both upregulation of TRAIL and its receptor DR5 may prime the cell for entry into the cell death pathway.
Our goal was to explore the efficacy of ONC201 in endometrial cancer in preclinical studies and to elucidate the mechanism of action in endometrial cancer in order to choose combination therapies that may improve clinical outcomes. We hypothesized that ONC201 upregulation of DR5 could sensitize tumor cells to exogenous TRAIL, as recently demonstrated in preclinical breast cancer models,Citation12 and that combination treatment of ONC201 with recombinant human TRAIL (rhTRAIL) would facilitate enhanced cell death in ONC201-treated endometrial cancer cells.
We demonstrate that ONC201 decreases cell viability in endometrial cancer cells lines primarily through growth arrest while the combination of ONC201 and rhTRAIL promotes cell death in endometrial cancer cells. Our results suggest a novel cancer therapeutic strategy that can be exploited in the clinic.
Results
ONC201 decreases the viability of various endometrial cancer cell lines at μM concentrations
In order to determine the effectiveness of ONC201 against endometrial cancer we first conducted cell viability experiments in vitro. Five different endometrial cell lines with variation in disease pathology, AN3CA, HEC1A, KLE, Ishikawa and RL952, were subjected to vehicle or increasing doses of ONC201 at μM concentrations, and cell viability was determined by Cell Titer Glo assay. Dose response curves were then generated to determine the GI50, the concentration of drug that inhibits the growth of cancer cells by 50% (, ). GI50 for AN3CA, HEC1A, and KLE was approximately 2.5 μM and GI50 for Ishikawa and RL952 was approximately 4.0 μM. Endometrial cancer cell lines are therefore sensitive to ONC201 at micromolar doses previously shown in the first-in-human trial to be clinically achievable in advanced solid tumors.Citation5 Phase contrast imaging, shown in , demonstrates the effect of ONC201 at its GI50 concentration for each cell line. Next the impact of ONC201 at the GI50 on clonogenic survival was assessed. This assay is used to determine if a single cell can propagate and form a colony under the given condition and evaluates its long-term survival. Low-density single cells were plated for each cell line and treated with vehicle or with ONC201 at its GI50 for 72 hours followed by an additional 10 days in drug-free media (). In all five cell lines treated, long-term colony formation was completely abolished after treatment with ONC201 at low (i.e. <4 μM) concentrations.
Table 1. GI50 of the endometrial cancer cell lines
Figure 1. ONC201 decreases the cell viability of endometrial cancer cells at μM concentrations.
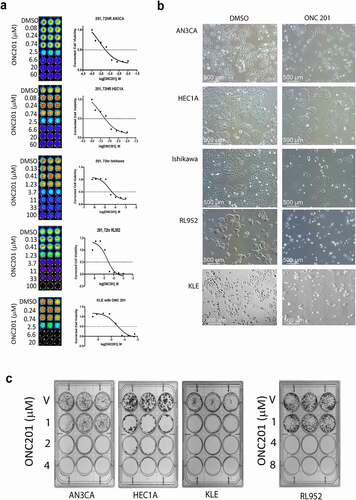
In endometrial cancer cell lines, the effect of ONC201 is predominantly antiproliferative, associated with cell cycle arrest and only slightly increased cell death
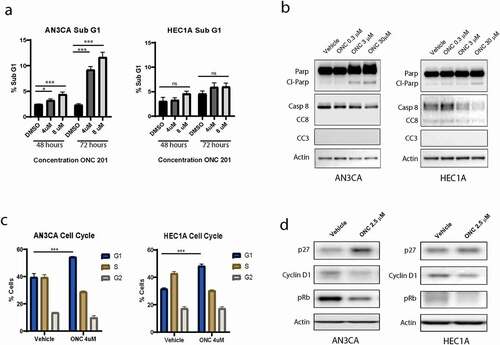
Decreased cell viability can result from either cell death or growth arrest. Therefore, we next performed cell death and cell cycle assessing assays on our treated cell lines to determine their response to ONC201. Cell death was only slightly increased following treatment with ONC201 for 72 hours, as demonstrated by the modest increase in Sub-G1 DNA content (increase by 9% with 8 μM ONC201 compared to control in AN3CA) and by the only slight increase in cleaved Parp by western blot (, a,b). Cleaved caspase 3 and 8 could not be detected following ONC201 treatment in the cell lines tested. Further assessment of the cell cycle following propidium iodide staining revealed that the cells arrested in the G0/G1 phase following ONC201 treatment, with the % of cells in G1 phase increasing from 40 to 55% in the AN3CA cell line and 32 to 48% in the HEC1A cell line when treated with 4 μM ONC201 (). This was associated with an increase in expression of cell cycle inhibitor p27 and a decreased expression of cyclin D1 and pRb, consistent with a G1 phase arrest (). Taken together these data suggest that ONC201 has a predominantly anti-proliferative effect on endometrial cancer cell lines when given as a single agent. This is in contrast to the strongly apoptotic effect ONC201 has demonstrated in a number of other cancer cell lines including colorectal and uterine serous cancer.Citation7,Citation11,Citation13
Figure 2. The effect of ONC 201 is predominantly antiproliferative, associated with cell cycle arrest and only slightly increased cell death
ONC201 activates the integrated stress response and induces protein expression of TRAIL and DR5 at the cell surface
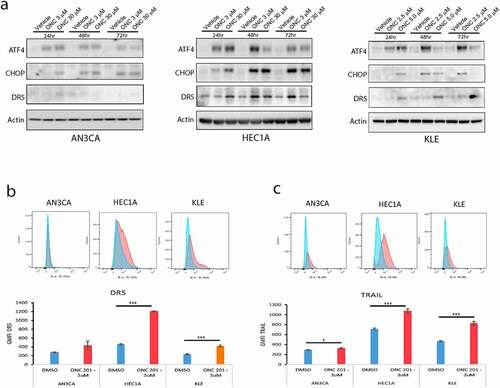
The apoptotic effect of ONC201 has been shown in prior studies to result from an increased expression of the endogenous tumor suppressor TRAIL as well as an increased expression of its receptor DR5.Citation7,Citation11 Increased cell surface expression of DR5 is mediated by ONC201 through activation of the integrated stress response involving activation of ATF4 and CHOP. Whereas increased expression of TRAIL is mediated by ONC201 through phosphorylation and subsequent cytoplasmic sequestration of Foxo3a.Citation7 We set out to determine if these pathways were activated by ONC201 in the endometrial cancer cell lines tested. We show by western blot in that ONC201 increases protein expression of ATF4, CHOP, and DR5 in endometrial cancer cell lines after 24, 48, or 72 hours of ONC201 exposure, signifying activation of the integrated stress response. We also observed an increase in both DR5 and TRAIL cell surface protein expression after ONC201 treatment compared with vehicle alone (, c). Of note, the increase in DR5 expression was less obvious in the AN3CA cell line as compared to in HEC1A or KLE. These results show that ONC201 activates both pathways in endometrial cancer cell lines and is consistent with ONC201 being able to prime the cell for TRAIL-mediated apoptosis.
Figure 3. ONC201 activates the integrated stress response and induces protein expression of TRAIL and DR5 at the cell surface
Pretreatment with ONC201 sensitizes endometrial cancer cell lines to TRAIL, leading to increased cell death induction as compared to either agent alone
Next, we asked whether treating the cells with exogenous TRAIL ligand, after they had been primed with ONC201 to upregulate the DR5 receptor, would push the balance from proliferation to apoptosis. To test this, endometrial cancer cell lines were treated with ONC201 for 72 hours followed by treatment with recombinant human TRAIL (rhTRAIL) for 4 hours. Cells were stained with propidium iodide and assessed for subG1 DNA content. shows that in AN3CA, RL952, Ishikawa and KLE cell lines there was a modest increase in cell death with ONC201 alone, but there was a robust increase in the proportion of cells undergoing cell death in the group that received the combination treatment (12-fold increase in AN3CA and a 16-fold increase in RL952 compared to control). TRAIL alone showed only little to modest effect on the cell lines tested. Both the intrinsic and extrinsic apoptotic cascades were activated following combination treatment with ONC201 and TRAIL as seen by increased cleavage of PARP, caspase-3, caspase-9, and caspase-8 (). Together this data show that pretreatment with ONC201 sensitizes endometrial cancer cell lines to TRAIL, leading to increased cell death induction compared to either agent alone.
Figure 4. Pre-treatment with ONC201 sensitizes endometrial cancer cell lines to TRAIL, leading to increased cell death induction compared to either agent alone
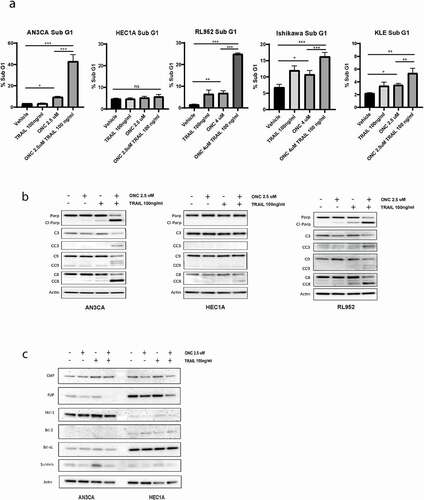
Of note, neither ONC201 alone or in combination with TRAIL resulted in activation of the apoptotic caspase cascade and showed only minimal increase in cell death by sub G1 in the HEC1A cell line (). Cancer cells have developed numerous mechanisms to overcome cell death. One mechanism includes dysregulating the equilibrium between anti- and proapoptotic family members. We therefore tested a panel of antiapoptotic proteins by Western blot analysis in our cell lines following dual treatment with ONC201 and TRAIL. As seen in expression of prosurvival proteins were similarly affected by ONC201 and TRAIL in both AN3CA and HEC1A cell lines. Decreased expression of FLIP was noted following dual treatment of ONC201/TRAIL compared to vehicle alone and there was no change in expression in Mcl-1, Bcl-2, Bcl-XL, CIAP, or survivin following treatment (). The differing response to ONC201/TRAIL treatment seen between HEC1A and AN3CA could not be explained by variances in Bcl-2 family modulators and warrants further investigation in future studies.
Tumor growth is reduced in vivo by combination treatment with ONC201 and recombinant TRAIL in a xenograft model of endometrial cancer
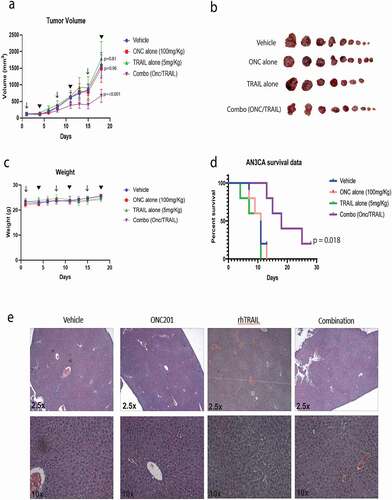
Next, the antitumorigenic effects of ONC201 with TRAIL were tested in vivo using a xenograft mouse model. Female mice yielding xenograft endometrial tumors were treated with either a vehicle control or 100 mg/kg ONC201 via oral gavage followed by either a vehicle control or recombinant TRAIL at 5 mg/kg IV 72 hours later. This dosing scheme was repeated weekly. The mice that received the combination therapy grew significantly smaller tumors () compared to those who received vehicle (p < .001) or either agent alone (p = .002 and p < .001) on day 18. Additionally, Kaplan-Meier survival analysis showed a significant survival benefit with the combination treatment (p = .018). There was no significant difference found between Vehicle, ONC201 alone or TRAIL alone control groups (). Mouse weights were measured over time as a surrogate measure for drug toxicity. No difference in mouse body weight was seen between treatment groups over time (). In addition, no evidence of liver toxicity was seen by H&E staining following treatment (). This is consistent with a nontoxic profile of either drug alone or combination therapy. Together this data demonstrated that combination treatment with ONC201 with recombinant TRAIL is effective at inhibiting tumor growth and is not toxic in vivo.
Figure 5. Tumor growth is reduced in vivo by combination treatment with ONC201 and recombinant TRAIL in a xenograft model of endometrial cancer
Discussion
Uterine cancer is the most common gynecologic malignancy among US women and the incidence is on the rise with over 63,000 new cases identified each year. Although prognosis for early stage endometrial cancer is favorable, the prognosis for those with metastatic or recurrent disease remains poor leading to over 11,000 deaths annually. For these patients the optimal adjuvant therapy has yet to be established, and chemotherapy in this group has had only modest response rates. In recent years, there has been a push to identify novel therapeutic approaches for these patients.
One novel drug therapy, ONC201, showed promising activity in patients with advanced endometrial cancer when tested in a variety of solid tumors during a phase I trial.Citation5 This has steered interest in ONC201 for its use as a therapeutic agent in endometrial cancer.
Data presented herein show that ONC201, a well-tolerated, orally active small molecule, has antitumorigenic properties in endometrial cancer models. In particular, we show that (1) ONC201 decreases cell viability in endometrial cancer cells lines at clinically achievable low micromolar concentrations, primarily through growth arrest when used as a single agent and that (2) combining ONC201 with TRAIL has the effect of pushing cells toward cell death. Moreover, (3) the effect of combining ONC201 with TRAIL is retained in vivo without demonstrated toxicity.
We established that cell proliferation was arrested in all five endometrial cell lines after treatment with ONC201, suggesting that ONC201 is effective universally in endometrial cancer. Moreover, the effective doses of ONC201 were in the micromolar range which have previously been shown to be clinically achievable.Citation5 This is consistent with the findings in type II serous endometrial cancer cells lines.Citation13 The effect on proliferation in our cell lines was associated with an arrest in the G1 phase of the cell cycle with an increase in G1 phase inhibitor p27 and decreased cyclin D1 and pRb.
Cyclin D1 plays an important role in activating cyclin-dependent kinases 4/6 to initiate the G1-> S phase transition required for cell proliferation. Aberration in CDK4 expression is seen in 35%–77% of endometrioid endometrial cancersCitation14 and cyclin D1 over expression has been shown to be associated with poorer prognosis in endometrial cancer.Citation15 This has led to the development of CDK inhibitors as targeted therapy in endometrial cancer, with clinical trials underway. We demonstrated that ONC201 decreased the expression of cyclin D1 and pRb, similar to the mechanism shown with ONC201 in colorectal cancer (Kline et al., 2016) and in endometrial cancer by CDK4 inhibitors,Citation16 further supporting a role for ONC201 in endometrial cancer.
Although the cell cycle was arrested after ONC201 treatment, this failed to translate into a significant increase in cell death, an outcome which was similarly found in a subset of breast cancer cell lines.Citation17 However, Ralff et al. demonstrated that in nontriple negative breast cancer cells, the antiproliferative phenotype could be pushed toward apoptosis with the combination treatment of ONC201 and TRAIL.Citation12
ONC201’s antitumor effect has been shown in nonendometrial cell lines to be p53-independent and mediated through inhibition of ERK and AKT leading to upregulation of TRAILCitation7 as well as by activation of the integrated stress response (ISR) leading to DR5 activation.Citation11 In endometrial tissue, studies have shown that malignant transformation is associated with reduced expression of death receptors DR4/DR5Citation18 and TRAIL has been shown to have a proapoptotic effect on endometrial cancer cell lines in vitro.Citation19 We demonstrate that in endometrial cancer cell lines ONC201 activates the integrated stress response and induces protein expression of DR5 and TRAIL at the cell surface consistent with other model systems.Citation7,Citation11 This suggests that ONC201 is able to prime the cell for TRAIL-mediated apoptosis in endometrial cancer. Similar to the breast cancer modelCitation12 when endometrial cancer cells were treated with TRAIL after being primed with ONC201, we were able to push the cells into apoptosis. In a pilot study of AN3CA cell line, pretreatment with ONC201 resulted in decreased cell viability following treatment with a DR5 agonist (data not shown). Our data supports the hypothesis that ONC201 upregulation of DR5 sensitizes tumor cells to TRAIL, leading to conversion from growth arrest to cell death in ONC201/TRAIL-treated cells. Further exploration of this combination therapy would need to be verified in a clinical trial.
ONC201 advanced from preclinical to clinical trials in 2014Citation5 and is now the focus of a number of clinical trials in a variety of solid tumors including three phase II clinical trials in advanced endometrial cancer (NCT03099499, NCT03485729, NCT03394027), which have been accruing. Although ONC201 had promising findings as a single agent,Citation5 it has been accepted in the field of GYN oncology that the future of adjuvant therapy for GYN cancers lies in combination therapy. We are now seeing clinical trials for ONC201 being tested in combination including an upcoming trial of ONC201 with paclitaxel in platinum-resistant refractory or recurrent ovarian cancer (NCT04055649). The data presented here provide preclinical support and rationale for an ONC201/TRAIL combination therapy trial for endometrial cancer.
Current investigative trials for endometrial cancer therapies have been limited by their modest efficacy or toxicity profile.Citation20 For example, current trials with combined MEK and AKT inhibitors (Trametinib with Uprosertib) or combined mTOR and antiangiogenic agents (temsirolimus with bevacizumab) were discontinued due to toxicity.Citation21,Citation22 We demonstrate in this paper that both ONC201 alone or in combination with recombinant TRAIL had little toxicity in vivo. This is consistent with our preclinical data that established ONC201 does not affect non-neoplastic cellsCitation7 and with data from the first in human phase I trial that showed ONC201 to be to be well tolerated with no drug related > grade 1 adverse events when given as a single agent.Citation5 Similarly, a recent study by Ralff et al. demonstrated little toxicity of the combination treatment of ONC201 with recombinant TRAIL in a mouse model of breast cancer.Citation12 TRAIL-based therapies have historically been associated with liver toxicity.Citation23 Given the low levels of recombinant TRAIL required for efficacy when given in combination, there was little toxicity seen in our in vivo studies. Furthermore, less toxic forms of recombinant trail and trail receptor antagonists are in development, which may further expand their use clinically.
Although we were able to achieve efficacy with the combination treatment of ONC201 and TRAIL in four of the five endometrial cell lines tested, the HEC1A endometrial cells were only minimally impacted. This suggests that an underlying resistance mechanism exists in a subset of endometrial cancer cells. Cancer cells have developed numerous mechanisms to overcome cell death. One mechanism includes dysregulating the equilibrium between anti- and proapoptotic family members. However, our testing of a number of antiapoptotic family members showed no differences in expression following drug delivery between the AN3CA and HEC1A cell line, suggesting this was not the resistance mechanism displayed by HEC1A. Additionally, no differences were found in their ability to activate the integrated stress response or upregulate TRAIL expression. Resistance mechanisms toward ONC201 would be clinically relevant and warrant further investigation.
In conclusion we were able to demonstrate that ONC201 decreases the cell viability of endometrial cancer cells at clinically achievable μM concentrations and that when given in combination with recombinant TRAIL it promotes cell death in vitro and in vivo. These preclinical data provide rationale for future human clinical trials.
Materials and Methods
Cell culture and reagents
The cell lines used to generate these data were obtained from the Fox Chase Cancer Center (FCCC) cell culture facility or directly from the American Type Culture Collection (ATCC). The Ishikawa cell line specifically was obtained from Sigma-Aldrich. All cell lines were authenticated and confirmed to be mycoplasma free. The cell lines were cultured in the media specified by ATCC or Sigma-Aldrich guidelines, supplemented with FBS (5 or 10% per guidelines) and 1% penicillin-streptomycin antibiotic. ONC201 was supplied by Oncoceutics, Inc. and was dissolved in DMSO. Recombinant human TRAIL (rhTRAIL) was generated as previously described.Citation12
Cell viability assays
Cell viability assays were performed using the CellTiter-Glo luminescent cell viability assay (Promega) according to the manufacturer’s instructions and captured using the in vivo imaging system (IVIS). Cells were plated in 96-well black bottom plates (5 × 103 cells/well) and allowed to adhere overnight. Wells were then treated in triplicate with a vehicle control or increasing concentrations of ONC201 for 72 hours. For the combination experiments, the media was removed after 72 hours and replaced with fresh media containing a vehicle control or rhTRAIL at varying doses. Cellular viability was quantitated after 4 hours of rhTRAIL treatment. GraphPad Prism software was used to generate dose-response curves, corrected to the control (DMSO alone).
Clonogenic colony formation assay
Cell were plated in 12-well plates (250–2000 cells/well depending on cell line) and allowed to adhere overnight. Wells were then treated in triplicate with a vehicle control or increasing concentrations of ONC201 for 72 hours. Doses around the G150 were selected as well as 2× and 4× the G150 were used to evaluate for trends. After 72 hours the media was exchanged for drug free media and changed every 3rd day with drug free media day for an additional 10 days of culture. The plates were then stained with 0.25% crystal violet solution and imaged using the IVIS system.
Flow cytometric PI staining
To assess the effects of ONC201 on the cell cycle and on the population of cell in the subG1, pre-treated cells were fixed in 70% ethanol and stained with propidium iodide in the presence of ribonuclease A (RNase A). The cells were then analyzed using an Elite Epics flow cytometer (Beckman Coulter). Cell cycle analysis was performed using FlowJo software. Samples were run in triplicate. For the subG1 and cell cycle experiments with ONC201 alone various doses of ONC201 were used to evaluate for trends. Doses around the GI50 were selected as well as 2× and 4× the GI50 were used. For the combination drug experiments, cells were treated with ONC201 at the GI50.
Immunoblotting
Cells were washed with PBS, and lysed with RIPA buffer (Sigma-Aldrich) supplemented with protease and phosphatase inhibitors (Roche). Protein concentration was then quantified using the Pierce BCA protein assay kit (Life Technologies) and equal amounts of protein were run on precast polyacrylamide gels (NuPAGE 4–12% Bis-Tris protein gels, Thermo Fischer Scientific) and transferred onto polyvinylidene difluoride (PVDF) membranes. Membranes were blocked for 1 hour at room temperature with a 5% nonfat milk powder in TBST and then incubated overnight at 4°C with primary antibodies diluted in TBST with 5% bovine serum albumin (BSA). Horseradish peroxidase (HRP) conjugated secondary antibodies (Thermo Fischer Scientific) diluted in TBST with 5% BSA were then applied and incubated for 1.5 hours. HRP activity was detected with ECL Western blotting substrate (Thermo Fischer Scientific) and chemiluminescent signal was measured using a gel documentation system (SYNGENE). Primary antibodies were purchased from Cell Signaling Technology (s.c) and include: Parp (c.s. 9542), total caspase 8 (c.s. 9746), total caspase 3 (c.s. 9662), cleaved caspase 3 (c.s. 9661), p27 (c.s. 3688), cyclin D1 (c.s. 2978), phosphor-Rb (c.s. 8516), DR5 (c.s. 3696), CIAP (c.s. 4952), FLIP (c.s. 56343), Mcl-1 (c.s. 4572), XIAP (c.s. 2042), Survivin (c.s. 2808), Bcl-XL (c.s. 2872). Actin was purchased from Sigma. Secondary antibodies were horseradish peroxidase conjugated and purchased from The Jackson Laboratory.
Flow cytometric analysis of cell surface TRAIL and DR5 protein levels
AN3CA, HEC1A, and KLE cells were treated with a vehicle control or approximate GI50 doses of ONC201 for 48 hours and then stained with an anti-TRAIL or anti-DR5 antibody to assess cellular surface expression of TRAIL and DR5. Cells were harvested with enzyme-free cell dissociation buffer (Life Technologies), washed with PBS and stained with fluorophore conjugated primary or isotype control antibodies. The primary antibodies were obtained from BioLegend (DR5: cat # 307406, TRAIL: cat # 308206, mouse IgG1, k isotype control: cat # 400112) and were diluted in FACS buffer (PBS, 1% FBS, 0.1% sodium azide). Cells were analyzed using an LSR II flow cytometer (BD Biosciences). The geometric mean fluorescence intensity was determined using flow cytometric analysis.
In Vivo experiments
For subcutaneous xenografts, 9-week-old female athymic nu/nu mice (Taconic Biosciences) were inoculated subcutaneously with 0.75/2 × 106 AN3CA cells in each rear flank. Once tumors reached a volume of 150–250 mm3, treatment was initiated. Mice were treated with either a vehicle control (n = 10), 100 mg/kg ONC201 via oral gavage once per week (n = 10), recombinant TRAIL 5 mg/kg IV tail vein injection, once per week (n = 10), or a combination of ONC201 PO 100 mg/kg once per week followed by recombinant TRAIL 5 mg/kg IV 72 hours later (n = 10). This dosing scheme was repeated weekly, with caliper measurements of tumor volume taken every 2nd or 3rd day for the duration of the experiment. Mice were sacrificed when tumors reached the size limit specified by the protocol or after three cycles depending on experiment. n = the number of independent biological replicates.
Statistical analysis
All statistical analysis was performed using Prism 7 software (GraphPad). The data are presented as the mean -/+ SEM from three or more replicates. Two-way ANOVA was used to compare more than one group. Figures were annotated to indicate significance or lack thereof (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001, ns = nonsignificance).
Supplemental Material
Download MS Word (20.2 KB)Acknowledgments
This work was presented in an earlier form as part of the Annual Meeting of the American Association for Cancer Research (AACR) in April 2019. This work was supported in part by NCI grants CA173453 (W.S.E-D.), and P30CA006927. W.S.E-D. is an American Cancer Society Research Professor and is supported by the Mencoff Family University Professorship at Brown University. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute, the National Institutes of Health, or the American Cancer Society.
Disclosure statement
W.S.E-D. is a cofounder of Oncoceutics Inc., a subsidiary of Chimerix, and is fully compliant with NIH and institutional disclosure guidelines.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Society AC Key statistics for endometrial cancer.
- Lortet-Tieulent J, Ferlay J, Bray F, International Patterns JA. Trends in Endometrial Cancer Incidence, 1978-2013. J Natl Cancer Inst. 2018;110:354–361.
- Francis SR, Ager BJ, Do OA, Huang YJ, Soisson AP, Dodson MK, Werner TL, Sause WT, Grant JD, Gaffney DK. Recurrent early stage endometrial cancer: patterns of recurrence and results of salvage therapy. Gynecol Oncol. 2019;154:38–44.
- https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf NCCNCPGiOUNV.
- Stein MN, Bertino JR, Kaufman HL, Mayer T. First-in-Human Clinical Trial of Oral ONC201 in Patients with Refractory Solid Tumors. Clin Cancer Res. 2017;23:4163–4169.
- Allen JE, Crowder RN, El-Deiry WS. First-In-Class Small Molecule ONC201 Induces DR5 and Cell Death in Tumor but Not Normal Cells to Provide a Wide Therapeutic Index as an Anti-Cancer Agent. PLoS One. 2015;10:e0143082.
- Allen JE, Krigsfeld G, Mayes Pa, Patel L, Dicker DT, Patel AS. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci Transl Med. 2013;5:171ra17.
- Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113.
- Wu GS, Burns TF, McDonald ER, 3rd, Jiang W. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet. 1997;17:141–143.
- ONC 201 in uterine cancer 8. 2018.pdf.
- Kline CL, Van den Heuvel AP, Allen JE, Prabhu VV, Dicker DT, El-Deiry WS. ONC201 kills solid tumor cells by triggering an integrated stress response dependent on ATF4 activation by specific eIF2alpha kinases. Sci Signal. 2016;9:ra18.
- Ralff MD, Jhaveri A, Ray JE, Zhou L, Lev A, Campbell KS, Dicker DT, Ross EA, El-Deiry WS. TRAIL receptor agonists convert the response of breast cancer cells to ONC201 from anti-proliferative to apoptotic. Oncotarget. 2020; 3753–3769.
- Fang Z, Wang J, Clark LH, Sun W. ONC201 demonstrates anti-tumorigenic and anti-metastatic activity in uterine serous carcinoma in vitro. Am J Cancer Res. 2018;8:1551–1563.
- Tsuda H, Yamamoto K, Inoue T, Uchiyama I, Umesaki N. The role of p16-cyclin d/CDK-pRb pathway in the tumorigenesis of endometrioid-type endometrial carcinoma. Br J Cancer. 2000;82:675–682.
- Liang S, Mu K, Wang Y, Zhou Z, Zhang J, Sheng Y, Zhang T. CyclinD1, a prominent prognostic marker for endometrial diseases. Diagn Pathol. 2013;8:138.
- Tanaka T, Terai Y, Ashihara K, Fujiwara S, Tanaka Y, Sasaki H, Tsunetoh S, Ohmichi M. The efficacy of the cyclin-dependent kinase 4/6 inhibitor in endometrial cancer. PLoS One. 2017;12:e0177019.
- Ralff MD, Kline CLB, Kucukkase OC, Wagner J, Lim B, Dicker DT, Prabhu VV, Oster W, El-Deiry WS. ONC201 Demonstrates Antitumor Effects in Both Triple-Negative and Non-Triple-Negative Breast Cancers through TRAIL-Dependent and TRAIL-Independent Mechanisms. Mol Cancer Ther. 2017;16:1290–1298.
- Gottwald L, Szwalski J, Piekarski J, Pasz-Walczak G, Kubiak R, Spych M, Suzin J, Tylinski W, Sek P, Jeziorski A. Membrane expression of the death ligand trail receptors DR4 and DR5 in the normal endometrium, endometrial atypical hyperplasia and endometrioid endometrial cancer. J Obstet Gynaecol. 2013;33:512–518.
- Sadarangani A, Kato S, Espinoza N, Lange S, Llados C, Espinosa M, Villalon M, Lipkowitz S, Cuello M, Owen GI. TRAIL mediates apoptosis in cancerous but not normal primary cultured cells of the human reproductive tract. Apoptosis. 2007;12:73–85.
- MacKay HJ, Freixinos VR, Fleming GF. Therapeutic Targets and. Opportunities in Endometrial Cancer: update on Endocrine Therapy and Nonimmunotherapy Targeted Options. Am Soc Clin Oncol Educ Book. 2020;40:1–11.
- Alvarez EA, Brady WE, Walker JL, Rotmensch J. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2013;129:22–27.
- Westin SN, Sill MW, Coleman RL, Waggoner S. Safety lead-in of the MEK inhibitor trametinib in combination with GSK2141795, an AKT inhibitor, in patients with recurrent endometrial cancer: an NRG Oncology/GOG study. Gynecol Oncol. 2019;155:420–428.
- Zuch de Zafra CL, Ashkenazi A, Darbonne WC, Cheu M. Antitherapeutic antibody-mediated hepatotoxicity of recombinant human Apo2L/TRAIL in the cynomolgus monkey. Cell Death Dis. 2016;7:e2338.
