Abstract
Neurons are usually regarded as postmitotic cells that undergo apoptosis in response to cell cycle reactivation. Nevertheless, recent evidence indicates the existence of a defined developmental program that induces DNA replication in specific populations of neurons, which remain in a tetraploid state for the rest of their adult life. Similarly, de novo neuronal tetraploidization has also been described in the adult brain as an early hallmark of neurodegeneration. The aim of this review is to integrate these recent developments in the context of cell cycle regulation and apoptotic cell death in neurons. We conclude that a variety of mechanisms exists in neuronal cells for G1/S and G2/M checkpoint regulation. These mechanisms, which are connected with the apoptotic machinery, can be modulated by environmental signals and the neuronal phenotype itself, thus resulting in a variety of outcomes ranging from cell death at the G1/S checkpoint to full proliferation of differentiated neurons.
Abbreviations
| AD | = | Alzheimer disease |
| BDNF | = | brain-derived neurotrophic factor |
| BrdU | = | 5-bromo-2′-deoxyuridine |
| Cdk | = | cyclin-dependent kinase |
| CKI | = | Cdk-inhibitor |
| Cip/Kip | = | cyclin inhibitor protein/kinase inhibitor protein |
| CNS | = | central nervous system |
| G0 | = | quiescent state |
| G1 | = | growth phase 1 |
| G2 | = | growth phase 2 |
| Ink | = | inhibitor of kinase |
| Mcm2 | = | minichromosome maintenance 2 |
| p38MAPK | = | p38 mitogen-activated protein kinase |
| p75NTR | = | neurotrophin receptor p75 |
| PCNA | = | proliferating cell nuclear antigen |
| PD | = | Parkinson disease |
| Rb | = | Retinoblastoma |
| RGCs | = | retinal ganglion cells |
| S-phase | = | synthesis phase. |
The Cell Cycle: A Rapid Overview
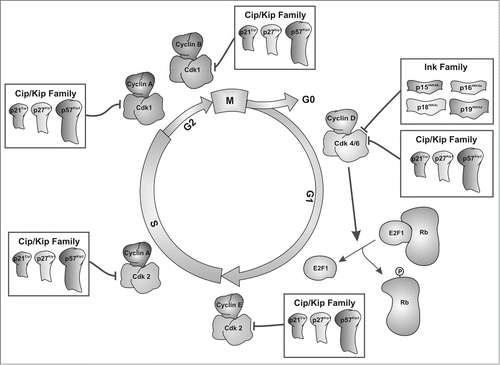
Mitosis represents a crucial event by which eukaryotic cells divide and equally segregate their genetic material into 2 daughter cells.Citation1,2 This process consists of 4 consecutive phases: prophase, when chromatin is condensed, nucleoli and nuclear membrane disappear, and the mitotic spindle is formed; metaphase, when chromosomes are arranged at the equatorial plane of the cell; anaphase, when chromatids separate toward the opposite sides of the mitotic spindle; and telophase, in which chromosomes decondense into diffuse chromatin, the nuclear membrane becomes generated, 2 new nuclei are formed, and cytokinesis begins to take place. Once the daughter cells have been produced, they go through an interphase period subdivided in 3 different stages: G1, when the proteins responsible for DNA replication are synthesized; S phase, when nuclear DNA is replicated; and G2, when the proteins responsible for cell division are synthesized. Cells can also be found in G0 when they have withdrawn from the cell cycle, as happens with most differentiated cells.Citation1,2 As shown in , the transitions between these stages are regulated by cyclins that bind to their specific Cdks, activating their kinase activity.Citation3 Cyclin D is synthesized at the beginning of G1, and it binds and activates Cdk4/6 when the cell leaves the quiescent state. Cdk4/6 phosphorylates Rb protein, inducing the release of the transcription factor E2F1, which in turn induces the synthesis of the proteins necessary for DNA replication.Citation4 G1/S progression is regulated by the association between cyclin E and Cdk2, which phosphorylates additional residues of Rb.Citation5 DNA synthesis is then driven by the association of cyclin A with Cdk2.Citation6 During late S-phase, the cyclin A/Cdk1 complex activates late replication origins and during late G2 phase, this complex initiates the condensation of chromosomes.Citation7-9 Finally, G2/M transition is regulated by the formation of the Cdk1/cyclin B complex.Citation1,3,Citation10-15
As shown in , cell cycle progression can also be regulated by CKIs from the Ink and Cip/Kip families, which inhibit the activity of the Cdk/cyclin complexes. In this regard, the members of the Ink family (p15Ink4b, p16Ink4a, p18Ink4c, and p19Ink4d) regulate the quiescent state by their specific binding to Cdk4/6, thus preventing the interaction of the latter with cyclin D.Citation16 In contrast to the Ink family members, whose inhibitory capacity is restricted to a specific cell cycle stage, the members of the Cip/Kip family (p21Cip1, p27Kip1, and p57Kip2) can bind and modulate the activity of specific complexes formed by Cdks and cyclins.Citation17 Interestingly, the interaction of p27Kip1 and p21Cip1 with cyclin D-dependent kinases relieves cyclin E/Cdk2 from Cip/Kip constraint, thereby facilitating cyclin E/Cdk2 activation later in G1 phase.Citation5
Progression through the different phases of the cell cycle is regulated by checkpoints that ensure that the cell has completed a phase before entering the next one.Citation15 These checkpoints result from the activation of different signaling pathways leading to the inhibition of Cdk/cyclin complexes.Citation18 If a defect is detected, these checkpoints induce the blockade of cell cycle until it has been repaired, thus impeding its transmission to the resulting daughter cells or inducing apoptosis if the defect cannot be corrected.Citation12,19 Different kinds of checkpoints are distributed along the cell cycle, especially in the transitions between the different cell cycle stages.Citation1,2,15 Two different checkpoints regulate G1: the first one at the beginning of this phase, when the cell senses the accessibility of growth factors responsible for the suppression of the quiescent signaling, and the other one during late G1, when the cell checks the availability of nutrients and also if the cell has reached the appropriate size to undergo DNA replication.Citation20 The intra-S-phase checkpoint is activated by DNA damage produced during DNA synthesis or by unrepaired DNA damage that has not been detected by the G1/S checkpoint.Citation18 Before undergoing mitosis, the cell must verify that the DNA is not damaged to avoid transmission of mutations to the daughter cells. In both of the latter cases, the tumor suppressor gene p53 is activated if DNA has been damaged, blocking the cell cycle until the damage has been repaired or inducing apoptosis if this damage is unrepairable.
G2/M transition is the last step before mitosis, so it is tightly controlled to avoid that either incomplete DNA synthesis or aberrant distribution of chromosomes could eventually lead to cancer.Citation2,12 Therefore, this transition is subjected to a number of checkpoints to control that the previous steps have been correctly completed.Citation15,21 Although the detailed sequence of events that lead to the activation of G2/M transition is not fully understood, it includes the activatory phosphorylation of the Thr160/Thr161 motif of Cdk1, catalyzed by the Cdk-activating kinase complex.Citation22-26 In addition, Cdk1 is also subjected to inhibitory phosphorylation, catalyzed by both Wee1, which phosphorylates this kinase in Tyr15,Citation27-30 and Myt1, which phosphorylates Cdk1 in Thr14.Citation31 Both phosphorylation events can be reverted by the phosphatase Cdc25, thus fully activating the Cdk1 complex.Citation32,33 The regulation of the subcellular location of every protein and their regulators, as well as the absence or inactivation of the cyclin kinase inhibitors also plays a role in the activation of the Cdk1/Cyclin B complex.Citation34 Finally, other checkpoints monitor the correct position of the mitotic spindle, separation of the chromosomes and cell cycle exit.Citation1,2,15
Cell Cycle in Neurons
Cell cycle re-entry in neurons and apoptosis
Unlike most cell types, neurons are believed to have permanently blocked their capacity to proliferate once they are differentiated, being typically found in a quiescent state in the adult nervous system. However, a number of genes that encode for regulators of G1/S transition, including cyclin D1, Cdk4, Rb proteins, E2Fs, and CKIs, can be detected in different structures of the normal adult brain (see ). Most of these transcripts are actually translated, as evidenced by the detection of the proteins they encode in normal adult neurons.Citation35-40 Traditionally, the presence of core cell cycle regulators in adult neurons has been explained as these molecules may fulfill differentiative functions, including neuronal migration, neuronal maturation, and synaptic plasticity.Citation41,42 Nevertheless, it remains plausible that, potentially, these proteins could also lead to cell cycle re-entry provided that specific conditions are met. In this regard, there are examples in which specific neuronal types, including sympathetic and cortical neurons, upregulate the expression of cell cycle markers and try to reactivate the cell cycle when subjected to acute insults such as neurotrophic factor deprivation, activity withdrawal, DNA damage, oxidative stress, and excitotoxicity. Under these conditions, they usually die at the G1/S checkpoint before any sign of DNA synthesis can be observed (for a review see refs. 43,44). This process, classically referred to as “abortive cell cycle re-entry,” is characterized by upregulation of cyclin D-Cdk4/6 activity and deregulation of E2F transcription factors,Citation45-51 followed by cell death. In this regard, E2F1 can be a trigger of neuronal apoptosis,Citation52,53 and 2 proapoptotic signaling pathways have been shown to be activated by this transcription factor in cerebellar granule cells and cortical neurons. These pathways include the activation of Bax/caspase-3 in a p53-independent mannerCitation54,55 and the induction of the Cdk1/FOXO1/Bad pathway.Citation56-58 In addition, deregulation of p130/E2F4, a repressive complex that maintains the postmitotic state of neurons, has also been shown to participate in the induction of neuronal apoptosis through the upregulation of B-myb and C-myb.Citation59,60 Overall, these observations indicate that a number of signaling pathways triggered by different environmental conditions can elicit cell cycle reactivation and cell death in specific neuronal phenotypes.
Table 1. Expression of cell cycle genes in the adult mouse brain. In situ hybridization raw expression values as defined by Allen Brain Atlas (http://mouse.brain-map.org). Average values above 1.00 from all described experiments are shown. CX: isocortex, OL: olfactory areas, HP: Hippocampal formation, CS: cortical subplate, ST: Striatum, PA: Pallidum, TH: Thalamus, HY: Hypothalamus, MB: Midbrain, PO: Pons, ME: Medulla, CB: Cerebellum.
Cell cycle re-entry in neurons and tetraploidy
There are cases in which neuronal injury results in G1/S transition and DNA synthesis. Examples of this situation are cerebellar granule neurons subjected to excitotoxic stimuliCitation61 and cortical and hippocampal neurons subjected to hypoxia/reperfusion.Citation62 Although terminally differentiated neurons that replicate their DNA are typically fated to die,Citation63,64 this is not always the case,Citation65 and these neurons may remain alive with double amount of DNA content. For instance, sensory and sympathetic neurons are able to replicate their DNA without any apoptotic response,Citation66,67 and Rb-deficient brain neurons have been shown to undergo cell cycle re-entry and remain alive with 4C DNA content.Citation68 These observations are consistent with the capacity for DNA replication of a population of differentiating RGCs in the developing chick retina.Citation69 Evidence from our laboratory indicates that these newly formed neurons, defined by the expression of specific differentiation markers,Citation69 re-enter into the cell cycle during their migration to the ganglion cell layer in response to the activation of the receptor p75NTR by nerve growth factor, and then they remain with 4C DNA content during adulthood.Citation69 This process, which participates in the normal development of the nervous system, is not generalized. Instead, tetraploid neurons in the chick retina constitute a specific population of large RGCs that innervate defined layers of their target tissue.Citation69 Therefore, duplication of the DNA content in neurons during development constitutes a mechanism for neuronal diversification in vertebrates. As these neurons cannot proliferate it is not possible to determine the number of chromosomes they contain, therefore they are referred to as somatic tetraploid neurons in a broad sense. Heteroploidy in the retina does not seem to be exclusive of the RGCs. Indeed, a recent study suggests that other newly formed retinal neurons, constituting a subpopulation of horizontal cells, may also become tetraploid.Citation70 This observation fits with the increase in ploidy observed in horizontal cells from mice with retina-specific knock-out of the Rb1 gene.Citation71 Like in the chick, the mouse retina also contains tetraploid RGCs,Citation69 an observation consistent with the maintenance of proteins involved in cell cycle progression in differentiated mouse RGCs.Citation72 The presence of neuronal markers in 6–7% of the Ki67+ cells located in the proliferating layer of the mouse retinaCitation72 suggests that, like in the chick, a population of migrating RGCs undergo cell cycle re-entry and tetraploidization in this species.
The mechanism used by p75NTR to induce cell cycle re-entry in newly formed chick RGCs is not dependent on the activity of Cdk4/6,Citation73,74 an observation consistent with the absence of cyclin D1 in a subpopulation of Ki67+/BrdU+ cells located in the developing mouse retina,Citation72 as well as the lack of Rb in differentiating chick tetraploid neurons.Citation69 Therefore, cell cycle re-entry in these neurons seems to differ from the canonic mechanism used by quiescent cells when they reactivate the cell cycle, based on Cdk4/6-dependent phosphorylation of Rb and subsequent release of E2F1.Citation4 In newly formed RGCs, p75NTR induces a novel signaling pathway for cell cycle re-entry, mediated by p38MAPK, which leads to the phosphorylation of E2F4 in a conserved Thr-containing motif.Citation73 The capacity of phospho-E2F4 to lead to cell cycle progression in differentiating retinal neurons contrasts with the role of E2F4 as a cell cycle repressor that participate in neuronal differentiation.Citation75 E2F1, which is also expressed in newly formed RGCs that become tetraploid,Citation69 might cooperate with phospho-E2F4 in the production of tetraploid RGCs.
The presence of tetraploid neurons in the vertebrate nervous system is not restricted to the neural retina. In fact, around 10% of human cortical neurons have DNA content higher than 2C, and 2% of them are tetraploid.Citation76 Tetraploid neurons have also been found in the mouse cerebral cortex, where most of them constitute a subpopulation of long-projection neurons,Citation77 as well as in different regions from the chick nervous system, including the optic lobes, cerebellum, spinal cord and dorsal root ganglia.Citation78 Cortical tetraploid neurons in the mouse are generated through a p75NTR-dependent mechanism that differs from that observed in the chick retina, since these neurons do actually express Rb as they migrate through the neuroepithelium to the differentiated layers.Citation77 Therefore, different mechanisms for regulating G1/S during neuronal tetraploidization seem to exist, depending on the neuronal phenotype.
The reason why the E2F1-dependent mechanisms that induce apoptosis when neurons trespass the G1/S checkpoint are not active in the examples described above remains unknown, but this may be related to their specific phenotype and/or environmental conditions they are subjected to.
Neuronal cell cycle re-entry in neurodegenerative diseases and other injuries of the nervous system
Cell cycle reactivation in adult neurons is an early hallmark of neurodegenerationCitation79 and CNS injury.Citation80-82 Although cell cycle reactivation has been classically linked to apoptosis (see above), cumulative evidence indicates that neurons can actively re-enter cell cycle, replicate its DNA, and survive as tetraploid neurons during the course of different neurodegenerative diseases. Nevertheless, these neurons seem to be much more vulnerable to die than diploid neurons, and therefore they may directly participate in the etiology of the disease.Citation83,84 In the next lines, we will summarize what is currently known about cell cycle re-entry and de novo tetraploidization of neurons in different diseases and injuries affecting the nervous system.
AD is likely the best documented example of a neurodegenerative disease where affected neurons may undergo DNA replication, as evidenced by Mcm2 phosporylation,Citation85 and de novo tetraploidization.Citation76,83,Citation84,86-88 DNA replication in AD neurons is consistent with the presence in these cells of proliferation markers such as PCNA and the Ki-67 antigen, as well as a number of regulators of G1/S transition, including Cyclin D, Cdk4, hyperphophorylated Rb, E2F1, and cyclin E.Citation89-94 Importantly, the presence of cell cycle events in the affected neurons is likely to be involved in the development of the disease. In this regard, transgenic mice expressing oncogenes in postmitotic cortical neurons to force these cells to re-enter the cell cycle show a phenotype that is reminiscent of AD, which includes intracellular tau hyperphosphorylationCitation64,94 and extracellular accumulation of β-amyloid peptide.Citation64 In contrast to the idea that cell cycle re-entry causes rapid neuronal death by apoptosis,Citation92 tetraploid neurons observed in the AD brain survive for years, as expected from the slow progression of this neurodegenerative condition. In this regard, the percentage of hyperploid neurons (i.e. those with a more than diploid content) is doubled in brains from AD patients as compared to that from non-affected individuals,Citation76 being these neurons much more susceptible to degenerate only at final stages of the disease.Citation83
Not so much information is available about the mechanism that lead to cell cycle reactivation and de novo-generated tetraploidy in the diseased brain. In this regard, a recent study has reported that cell cycle re-entry in AD may be regulated by MiR-26b, a microRNA whose levels are elevated in relevant pathological areas from early stages of the disease.Citation95 MiR-26b induces cell cycle re-entry through an Rb1/E2F dependent mechanism that leads to upregulation of cyclin E1 and downregulation of p27Kip1.Citation95 It is also worth to note that p75NTR becomes upregulated in response to stress in AD-affected neurons.Citation96 Moreover, p38MAPK has been linked with AD as wellCitation97 and its active form can be detected in the brain of AD patients from the very early stages of the disease, becoming increased with age.Citation98,99 In addition, E2F4 can also be detected in the normal brainCitation100 (), suggesting that the p75NTR/p38MAPK/E2F4 pathway may also participate in this neurodegenerative process.
Cell cycle markers in neurons can also be found in neural tissue subjected to ischemia/hypoxia,Citation101-103 suggesting the existence of DNA replication in these cells. In this regard, Burns et al.Citation104 have demonstrated this notion since the vast majority of neurons that incorporate BrdU in response to ischemia/hypoxia do it once they are differentiated, indicating that they had not been generated by adult neurogenesis. Importantly, those neurons that incorporate BrdU remain alive 7 d after stroke.Citation104
Cell cycle reactivation, evidenced by BrdU incorporation and FISH, has also been observed in the affected neurons of patients suffering PD.Citation105 In this regard, different cell cycle markers including pRb, E2F1 and PCNA, associated with DNA replication, can be detected in affected neurons from the PD brain.Citation106 Cell cycle markers, including E2F1, were also found by immunohistochemistry in animal models of PD.Citation105,107 Furthermore, inhibition of Cdk4 by flavopiridol and removal of E2F1 have neuroprotective effects for PD as have been demonstrated by in vivo and in vitro studies.Citation105,108 These studies suggest that in PD, post-mitotic dopaminergic neurons can re-enter the cell cycle, cross the G1/S checkpoint, and then become blocked in G2/M transition. This suggests that tetraploid neurons could be generated during the course of PD, as occurs in AD.
G2/M transition in neurons and neuronal death
Although AD-affected neurons can re-enter into the cell cycle, mitosis is rarely observed in these cells.Citation109 Therefore, neurons that undergo S-phase block cell cycle progression at the G2/M transition, acquire a tetraploid condition, and survive for long time in the affected brain. This situation is reminiscent of what occurs in neurons becoming tetraploid during normal development, which block the cell cycle at G2/M, and die if they trespass this checkpoint.Citation69,74,110 This suggests that in neurodegenerative diseases where neurons become tetraploid, G2/M transition blockade likely plays a key role for the survival of the affected neurons.
Blockage of G2/M transition in newly formed, tetraploid neurons seems to be independent of DNA damage response,Citation111 the canonical cause for cell cycle arrest at this stage of the cell cycle.Citation18 Indeed, the mechanism that prevents G2/M transition in differentiating tetraploid RGCs is based on the capacity of BDNF to block this particular stage.Citation69,110 This process is crucial for the removal of tetraploid RGCs from the central retina.Citation69,112 Indeed, the use of BDNF blockers results in a significant increase of ectopic mitoses and cell death in the differentiating chick retina.Citation69 A similar mechanism is likely to occur in the developing mouse retina, whose neuroepithelium also contains ectopic mitoses in a small proportion of β3 tubulin-positive cells,Citation72 likely those fated to p75NTR-dependent death.Citation113 BDNF prevents G2/M transition in tetraploid neurons through its neurotrophic receptor TrkB, due to its capacity to decrease the expression of both cyclin B and Cdk1 in differentiating retinal neurons.Citation74,110 In addition, BDNF leads to a further decrease of Cdk1 activity triggered by the phosphorylation of this kinase in Tyr15,Citation110 thus blocking G2/M transition in tetraploid neurons.
In AD-affected neurons G2/M transition seems to be also blocked. Indeed, the Cdk1/cyclin B complex can be detected in neurofibrilary tangle-containing neurons.Citation89,92,Citation114-117 However, this complex is not translocated to the nucleus, thus likely contributing to the arrest at the G2/M transition.Citation116 The mislocation of this complex likely facilitates the aberrant phosphorylation of proteins such as tau or other cytoskeletal proteins, which display many features of the mitotic phase and contribute to AD pathology.Citation118,119
The molecular mechanism used to block G2/M transition in the tetraploid neurons that are generated during the course of neuropathological conditions could derive from the DNA damage response.Citation18 Alternatively, it could be reminiscent of the inactivation of Cdk1 induced by BDNF through TrkB.Citation110 In the absence of BDNF, differentiating tetraploid neurons try to divide and then they die.Citation74,110 A similar situation might occur in the AD brain. In AD, neurons show markers of deregulated G2/M transition. For instance, the Cdk1 activators Cdc25A and Cdc25B show higher activity in degenerating neurons in vivo,Citation120,121 while a lower activity of the Cdk1 inhibitor Wee1 can be observed in these neurons.Citation122 Moreover, pH3 phosphorylation, a marker of the G2/M transition, can be found in AD hippocampal neurons, but aberrantly localized in the cytoplasm, suggesting a mitotic catastrophe that leads to apoptosis.Citation123 This latter notion is consistent with the lack of chromatin condensation and spindle formation in AD-affected neurons, suggesting that mitosis cannot be completed.Citation115 BDNF increases neuronal survival in different neurodegenerative diseases.Citation124-126 Therefore, the decrease of both TrkB and BDNF, observed in the late stages of AD,Citation127 could participate in the induction of neuronal degeneration.Citation84
Although the mechanisms leading to apoptosis in adult neurons that undergo G2/M transition are not fully understood, what is widely accepted is that Cdk1 is involved in different signaling pathways that lead to cell death. In this sense, Cdk1 can induce FOXO1 phosphorylation in Ser249, which disrupts its interaction with 14–3–3 proteins. This leads to its nuclear accumulation, where it triggers the expression of cell death genes in neurons.Citation58,128,129 Cdk1 also induces the phosphorylation and the activation of the pro-apoptotic protein Bad by inducing its phosphorylation in Ser128, which blocks the interaction of the Ser136-phosphorylated Bad, induced by growth factors, with 14–3–3 proteins.Citation57 Alternatively, apoptosis can be derived from unknown mechanisms induced by mitosis in postmitotic neurons.
Mitosis and proliferating neurons
Interestingly, mitosis in neurons does not necessarily represent a synonym of apoptosis. Indeed, there are examples of neurons capable of dividing without undergoing cell death. This is the case of retinal horizontal cells, which can proliferate in absence of Rb and p130 while maintaining its differentiated state.Citation130 Therefore, under certain circumstances horizontal cells, which have capacity to become tetraploid,Citation70 can undergo full cell cycle progression as other proliferating cells. This astonishing observation indicates that neurons can no longer be considered as pure postmitotic cells, and that they can potentially proliferate provided that specific conditions are met. It can therefore be concluded that it is only the particular phenotype and the environmental signals what determines whether neurons can overcome G1/S and G2/M checkpoints with or without dying.
Perspectives and Future Directions
Evidence described throughout this Review indicates that, under defined situations, neurons can activate the cell cycle and progress to the G1/S transition. If the proapoptotic signals associated with this stage are then prevented, they can undergo full DNA replication and remain in a G2-like state, or even divide without dying (). Several questions about the molecular mechanisms regulating this complex behavior remain to be responded, a constraint even stricter if one consider that most of our current knowledge about cell cycle regulation comes from studies performed just in a few cell systems, including yeast, oocytes, fibroblasts, and cancer cell lines, and that little is known about the specific regulation of the cell cycle and its different checkpoints in most vertebrate tissues,Citation11 especially in neurons. Therefore, further studies are required to deeply understand the complexity of the G1/S and G2/M checkpoints in neurons, their connection with the apoptotic machinery, as well as the molecular mechanisms leading to these cells to reactivate the cell cycle. This will facilitate our understanding of why some postmitotic neurons re-enter the cell cycle and survive as tetraploid neurons while other neurons die by apoptosis as soon as they reach the S-phase. The mechanism used by the adult brain to generate tetraploid neurons during the course of different neurodegenerative conditions, as well as the mechanism employed to prevent G2/M transition in these neurons during early stages of the disease has not yet been determined. Moreover, little is known about the proapoptotic pathways that are activated in the tetraploid neurons that are raised de novo in the diseased brain once they presumably cross the line defined by the G2/M checkpoint. Finally, nothing is known about the mechanism that prevents cell death in horizontal neurons that proliferate in the absence of Rb and p130 expression.Citation130 The answer to all these questions could help designing specific drugs for therapy against neurodegeneration. In this regard, different cell cycle modulators have been proposed as therapeutic strategies for neurodegenerative conditions such a stroke,Citation82,131 excitotoxicity,Citation52 Alzheimer disease,Citation52 and brain trauma.Citation80 Preclinical experiments using cell cycle protein inhibitors such as flavopiridol, olomoucine or roscovitine demonstrated improved behavioral outcomes and increased neuronal survival in a series of CNS disease models such as AD,Citation79 PD,Citation108 and stroke.Citation82,131,132 We propose that modulation of G2/M transition can be a therapeutic approach to avoid neuronal apoptosis in advanced stages of neurodegeneration, to prevent the death of de novo-generated tetraploid neurons.
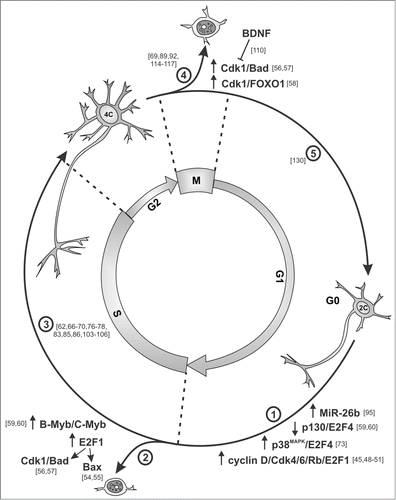
Figure 2. Scheme showing the evidence for cell cycle progression in neurons. Postmitotic neurons can reactivate the cell cycle (1) using the referred pathways. In many instances, this process is aborted at the G1/S transition and the neuron die through a number of pathways initiated by either E2F1 or Myb proteins (2). There are also examples of neurons that replicate their nuclear DNA and become tetraploid (3). These neurons die if they try to undergo mitosis, and evidence exists indicating that Cdk1 is involved in this process (4). In some instances, these neurons can divide and proliferate (5). References supporting the different steps are shown in brackets.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from “Ministerio de Economía y Competitividad” (SAF2012–38316) and “Fundación Ramón Areces.”
References
- Fisher RP. The CDK network: linking cycles of cell division and gene expression. Genes Cancer 2012; 3:731-8; PMID:23634260; http://dx.doi.org/10.1177/1947601912473308
- Williams GH, Stoeber K. The cell cycle and cancer. J Pathol 2012; 226:352-64; PMID:21990031; http://dx.doi.org/10.1002/path.3022
- Hochegger H, Takeda S, Hunt T. Cyclin-dependent kinases and cell-cycle transitions: does one fit all? Nat Rev Mol Cell Biol 2008; 9:910-6; PMID:18813291; http://dx.doi.org/10.1038/nrm2510
- Liu N, Lucibello FC, Engeland K, Müller R. A new model of cell cycle-regulated transcription: repression of the cyclin A promoter by CDF-1 and anti-repression by E2F. Oncogene 1998; 16:2957-63; PMID:9662327
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 1999; 13:1501-12; PMID:10385618
- Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J 1992; 11:961-71; PMID:1312467
- Gong D, Ferrell JE Jr. The roles of cyclin A2, B1, and B2 in early and late mitotic events. Mol Biol Cell 2010; 21:3149-61; PMID:20660152; http://dx.doi.org/10.1091/mbc.E10-05-0393
- Katsuno Y, Suzuki A, Sugimura K, Okumura K, Zineldeen DH, Shimada M, Niida H, Mizuno T, Hanaoka F, Nakanishi M. Cyclin A-Cdk1 regulates the origin firing program in mammalian cells. Proc Natl Acad Sci USA 2009; 106:3184-9; PMID:19221029; http://dx.doi.org/10.1073/pnas.0809350106
- Merrick KA, Fisher RP. Why minimal is not optimal: driving the mammalian cell cycle–and drug discovery–with a physiologic CDK control network. Cell Cycle 2012; 11:2600-5; PMID:22732498; http://dx.doi.org/10.4161/cc.20758
- Gopinathan L, Ratnacaram CK, Kaldis P. Established and novel Cdk/cyclin complexes regulating the cell cycle and development. Results Probl Cell Differ 2011; 53:365-89. PMID:21630153; http://dx.doi.org/10.1007/978-3-642-19065-0_16
- Harashima H, Dissmeyer N, Schnittger A. Cell cycle control across the eukaryotic kingdom. Trends Cell Biol 2013; 23:345-56; PMID:23566594; http://dx.doi.org/10.1016/j.tcb.2013.03.002
- Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 2009; 9:153-66; PMID:19238148; http://dx.doi.org/10.1038/nrc2602
- Merrick KA, Fisher RP. Putting one step before the other: distinct activation pathways for Cdk1 and Cdk2 bring order to the mammalian cell cycle. Cell Cycle 2010; 9:706-14; PMID:20139727
- Santamaria D, Ortega S. Cyclins and CDKS in development and cancer: lessons from genetically modified mice. Front Biosci 2006; 11:1164-88; PMID:16146805
- Yasutis KM, Kozminski KG. Cell cycle checkpoint regulators reach a zillion. Cell Cycle 2013; 12:1501-9; PMID:23598718; http://dx.doi.org/10.4161/cc.24637
- Cánepa ET, Scassa ME, Ceruti JM, Marazita MC, Carcagno AL, Sirkin PF, Ogara MF. INK4 proteins, a family of mammalian CDK inhibitors with novel biological functions. IUBMB Life 2007; 59:419-26; PMID:17654117
- Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell 2008; 14:159-69; PMID:18267085; http://dx.doi.org/10.1016/j.devcel.2008.01.013
- Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem 2004; 73:39-85; PMID:15189136
- Rieder CL. Mitosis in vertebrates: the G2/M and M/A transitions and their associated checkpoints. Chromosome Res 2011; 19:291-306; PMID:21194009; http://dx.doi.org/10.1007/s10577-010-9178-z
- Foster DA, Yellen P, Xu L, Saqcena M. Regulation of G1 cell cycle progression: distinguishing the restriction point from a nutrient-sensing cell growth checkpoint(s). Genes Cancer 2010; 1:1124-31; PMID:21779436; http://dx.doi.org/10.1177/1947601910392989
- Recolin B, van der Laan S, Tsanov N, Maiorano D. Molecular mechanisms of DNA replication checkpoint activation. Genes (Basel) 2014; 5:147-75; PMID:24705291; http://dx.doi.org/10.3390/genes5010147
- Fesquet D, Labbé JC, Derancourt J, Capony JP, Galas S, Girard F, Lorca T, Shuttleworth J, Dorée M, Cavadore JC. The MO15 gene encodes the catalytic subunit of a protein kinase that activates cdc2 and other cyclin-dependent kinases (CDKs) through phosphorylation of Thr161 and its homologues. EMBO J. 1993; 12:3111-21; PMID:8344251
- Fisher RP, Morgan DO. A novel cyclin associates with MO15/CDK7 to form the CDK-activating kinase. Cell 1994; 78:713-24; PMID:8069918
- Poon RY, Yamashita K, Adamczewski JP, Hunt T, Shuttleworth J. The cdc2-related protein p40MO15 is the catalytic subunit of a protein kinase that can activate p33cdk2 and p34cdc2. EMBO J 1993; 12:3123-32; PMID:8393783
- Solomon MJ, Lee T, Kirschner MW. Role of phosphorylation in p34cdc2 activation: identification of an activating kinase. Mol Biol Cell 1992; 3:13-27; PMID:1532335
- Labbé JC, Martinez AM, Fesquet D, Capony JP, Darbon JM, Derancourt J, Devault A, Morin N, Cavadore JC, Dorée M. p40MO15 associates with a p36 subunit and requires both nuclear translocation and Thr176 phosphorylation to generate cdk-activating kinase activity in Xenopus oocytes. EMBO J 1994; 13:5155-64; PMID:7957080
- Gould KL, Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature 1989; 342:39-45; PMID:2682257
- Meijer L, Azzi L, Wang JY. Cyclin B targets p34cdc2 for tyrosine phosphorylation. EMBO J 1991; 10:1545-54; PMID:1709096
- Parker LL, Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science. 1992 Sep 25;257:1955-7; PMID:1384126
- Parker LL, Atherton-Fessler S, Lee MS, Ogg S, Falk JL, Swenson KI, Piwnica-Worms H. Cyclin promotes the tyrosine phosphorylation of p34cdc2 in a wee1+ dependent manner. EMBO J 1991; 10:1255-63; PMID:1850698
- Liu F, Stanton JJ, Wu Z, Piwnica-Worms H. The human Myt1 kinase preferentially phosphorylates Cdc2 on threonine 14 and localizes to the endoplasmic reticulum and Golgi complex. Mol Cell Biol 1997; 17:571-83; PMID:9001210
- Boutros R, Dozier C, Ducommun B. The when and wheres of CDC25 phosphatases. Curr Opin Cell Biol 2006; 18:185-91; PMID:16488126
- Draetta G, Eckstein J. Cdc25 protein phosphatases in cell proliferation. Biochim Biophys Acta 1997; 1332:M53-63; PMID:9141461
- Johnson ES, Kornbluth S. Phosphatases driving mitosis: pushing the gas and lifting the brakes. Prog Mol Biol Transl Sci 2012; 106:327-41; PMID:22340723; http://dx.doi.org/10.1016/B978-0-12-396456-4.00008-0
- Koeller HB, Ross ME, Glickstein SB. Cyclin D1 in excitatory neurons of the adult brain enhances kainate-induced neurotoxicity. Neurobiol Dis 2008; 31:230-41; PMID:18585919; http://dx.doi.org/10.1016/j.nbd.2008.04.010
- Müller M, Lutter D, Püschel AW. Persistence of the cell-cycle checkpoint kinase Wee1 in SadA- and SadB-deficient neurons disrupts neuronal polarity. J Cell Sci 2010; 123:286-94; PMID:20026642; http://dx.doi.org/10.1242/jcs.058230
- Okano HJ, Pfaff DW, Gibbs RB. RB and Cdc2 expression in brain: correlations with 3H-thymidine incorporation and neurogenesis. J Neurosci 1993; 13:2930-8; PMID:8331381
- Schmetsdorf S, Gärtner U, Arendt T. Constitutive expression of functionally active cyclin-dependent kinases and their binding partners suggests noncanonical functions of cell cycle regulators in differentiated neurons. Cereb Cortex 2007; 17:1821-9; PMID:17050646
- Ting JH, Marks DR, Schleidt SS, Wu JN, Zyskind JW, Lindl KA, Blendy JA, Pierce RC, Jordan-Sciutto KL. Targeted gene mutation of E2F1 evokes age-dependent synaptic disruption and behavioral deficits. J Neurochem 2014; 129:850-63; PMID:24460902; http://dx.doi.org/10.1111/jnc.12655
- Cunningham JJ, Levine EM, Zindy F, Goloubeva O, Roussel MF, Smeyne RJ. The cyclin-dependent kinase inhibitors p19Ink4d and p27Kip1 are coexpressed in select retinal cells and act cooperatively to control cell cycle exit. Mol Cell Neurosci 2002; 19:359-74; PMID:11906209
- Frank CL, Tsai LH. Alternative functions of core cell cycle regulators in neuronal migration, neuronal maturation, and synaptic plasticity. Neuron 2009; 62:312-26; PMID:19447088; http://dx.doi.org/10.1016/j.neuron.2009.03.029
- Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development 2013; 140:3079-93; PMID:23861057; http://dx.doi.org/10.1242/dev.091744
- Becker EB, Bonni A. Cell cycle regulation of neuronal apoptosis in development and disease. Prog Neurobiol 2004; 72:1-25; PMID:15019174
- Liu DX, Greene LA. Neuronal apoptosis at the G1/S cell cycle checkpoint. Cell Tissue Res 2001; 305:217-28; PMID:11545259
- Park DS, Morris EJ, Padmanabhan J, Shelanski ML, Geller HM, Greene LA. Cyclin-dependent kinases participate in death of neurons evoked by DNA-damaging agents. J Cell Biol 1998; 143:457-67; PMID:9786955
- Klein JA, Ackerman SL. Oxidative stress, cell cycle, and neurodegeneration. J Clin Invest 2003; 111:785-93; PMID:12639981
- Kruman II. Why do neurons enter the cell cycle? Cell Cycle 2004; 3:769-73; PMID:15136759
- Park DS, Levine B, Ferrari G, Greene LA. Cyclin dependent kinase inhibitors and dominant negative cyclin dependent kinase 4 and 6 promote survival of NGF-deprived sympathetic neurons. J Neurosci 1997; 17:8975-83; PMID:9364045
- Park DS, Morris EJ, Bremner R, Keramaris E, Padmanabhan J, Rosenbaum M, Shelanski ML, Geller HM, Greene LA. Involvement of retinoblastoma family members and E2F/DP complexes in the death of neurons evoked by DNA damage. J Neurosci 2000; 20:3104-14; PMID:10777774
- Chen MJ, Ng JM, Peng ZF, Manikandan J, Yap YW, Llanos RM, Beart PM, Cheung NS. Gene profiling identifies commonalities in neuronal pathways in excitotoxicity: evidence favouring cell cycle re-activation in concert with oxidative stress. Neurochem Int 2013; 62:719-30; PMID:23291249; http://dx.doi.org/10.1016/j.neuint.2012.12.015
- Padmanabhan J, Park DS, Greene LA, Shelanski ML. Role of cell cycle regulatory proteins in cerebellar granule neuron apoptosis. J Neurosci 1999; 19:8747-56; PMID:10516294
- Woods J, Snape M, Smith MA. The cell cycle hypothesis of Alzheimer disease: suggestions for drug development. Biochim Biophys Acta 2007; 1772:503-8; PMID:17223322
- Verdaguer E, Susana Gde A, Clemens A, Pallàs M, Camins A. Implication of the transcription factor E2F-1 in the modulation of neuronal apoptosis. Biomed Pharmacother 2007; 61:390-9; PMID:17178208
- Giovanni A, Keramaris E, Morris EJ, Hou ST, O'Hare M, Dyson N, Robertson GS, Slack RS, Park DS. E2F1 mediates death of B-amyloid-treated cortical neurons in a manner independent of p53 and dependent on Bax and caspase 3. J Biol Chem 2000; 275:11553-60; PMID:10766769
- O'Hare MJ, Hou ST, Morris EJ, Cregan SP, Xu Q, Slack RS, Park DS. Induction and modulation of cerebellar granule neuron death by E2F-1. J Biol Chem 2000; 275:25358-64; PMID:10851232
- Konishi Y, Bonni A. The E2F-Cdc2 cell-cycle pathway specifically mediates activity deprivation-induced apoptosis of postmitotic neurons. J Neurosci 2003; 23:1649-58; PMID:12629169
- Konishi Y, Lehtinen M, Donovan N, Bonni A. Cdc2 phosphorylation of BAD links the cell cycle to the cell death machinery. Mol Cell 2002; 9:1005-16; PMID:12049737
- Yuan Z, Becker EB, Merlo P, Yamada T, DiBacco S, Konishi Y, Schaefer EM, Bonni A. Activation of FOXO1 by Cdk1 in cycling cells and postmitotic neurons. Science 2008; 319:1665-8; PMID:18356527; http://dx.doi.org/10.1126/science.1152337
- Iyirhiaro GO, Zhang Y, Estey C, O'Hare MJ, Safarpour F, Parsanejad M, Wang S, Abdel-Messih E, Callaghan SM, During MJ, et al. Regulation of ischemic neuronal death by E2F4-p130 protein complexes. J Biol Chem 2014; 289:18202-13; PMID:24828495 http://dx.doi.org/10.1074/jbc.M114.574145
- Liu DX, Nath N, Chellappan SP, Greene LA. Regulation of neuron survival and death by p130 and associated chromatin modifiers. Genes Dev 2005; 19:719-32; PMID:15769944
- Verdaguer E, García-Jordà E, Canudas AM, Domínguez E, Jiménez A, Pubill D, Escubedo E, Pallàs JC, Camins A. Kainic acid-induced apoptosis in cerebellar granule neurons: an attempt at cell cycle re-entry. Neuroreport 2002; 13:413-6; PMID:11930151
- Kuan CY, Schloemer AJ, Lu A, Burns KA, Weng WL, Williams MT, Strauss KI, Vorhees CV, Flavell RA, Davis RJ, et al. Hypoxia-ischemia induces DNA synthesis without cell proliferation in dying neurons in adult rodent brain. J Neurosci 2004; 24:10763-72; PMID:15564594
- Feddersen RM, Ehlenfeldt R, Yunis WS, Clark HB, Orr HT. Disrupted cerebellar cortical development and progressive degeneration of Purkinje cells in SV40 T antigen transgenic mice. Neuron 1992; 9:955-66; PMID:1419002
- Park KH, Hallows JL, Chakrabarty P, Davies P, Vincent I. Conditional neuronal simian virus 40 T antigen expression induces Alzheimer-like tau and amyloid pathology in mice. J Neurosci 2007; 27:2969-78; PMID:17360920
- Copani A, Uberti D, Sortino MA, Bruno V, Nicoletti F, Memo M. Activation of cell-cycle-associated proteins in neuronal death: a mandatory or dispensable path? Trends Neurosci 2001; 24:25-31; PMID:11163884
- Smith DS, Leone G, DeGregori J, Ahmed MN, Qumsiyeh MB, Nevins JR. Induction of DNA replication in adult rat neurons by deregulation of the retinoblastoma/E2F G1 cell cycle pathway. Cell Growth Differ 2000;11:625-33; PMID:11149597
- Rohrer H, Thoenen H. Relationship between differentiation and terminal mitosis: chick sensory and ciliary neurons differentiate after terminal mitosis of precursor cells, whereas sympathetic neurons continue to divide after differentiation. J Neurosci 1987; 7:3739-48; PMID:3681410
- Lipinski MM, Macleod KF, Williams BO, Mullaney TL, Crowley D, Jacks T. Cell-autonomous and non-cell-autonomous functions of the Rb tumor suppressor in developing central nervous system. EMBO J 2001; 20:3402-13; PMID:11432828
- Morillo SM, Escoll P, de la Hera A, Frade JM. Somatic tetraploidy in specific chick retinal ganglion cells induced by nerve growth factor. Proc Natl Acad Sci USA 2010; 107:109-14; PMID:20018664 http://dx.doi.org/10.1073/pnas.0906121107
- Shirazi Fard S, Jarrin M, Boije H, Fillon V, All-Eriksson C, Hallböök F. Heterogenic final cell cycle by chicken retinal Lim1 horizontal progenitor cells leads to heteroploid cells with a remaining replicated genome. PLoS One 2013; 8:e59133; PMID:23527113 http://dx.doi.org/10.1371/journal.pone.0059133
- Donovan SL, Corbo JC. Retinal horizontal cells lacking Rb1 sustain persistent DNA damage and survive as polyploid giant cells. Mol Biol Cell 2012; 23:4362-72; PMID:23015754; http://dx.doi.org/10.1091/mbc.E12-04-0293
- Pacal M, Bremner R. Mapping differentiation kinetics in the mouse retina reveals an extensive period of cell cycle protein expression in post-mitotic newborn neurons. Dev Dyn 2012; 241:1525-44; PMID:22837015; http://dx.doi.org/10.1002/dvdy.23840
- Morillo SM, Abanto EP, Román MJ, Frade JM. Nerve growth factor-induced cell cycle reentry in newborn neurons is triggered by p38MAPK-dependent E2F4 phosphorylation. Mol Cell Biol 2012; 32:2722-37; PMID:22586272; http://dx.doi.org/10.1128/MCB.00239-12
- Frade JM. Unscheduled re-entry into the cell cycle induced by NGF precedes cell death in nascent retinal neurones. J Cell Sci 2000; 113:1139-48; PMID:10704365
- Persengiev SP, Kondova II, Kilpatrick DL. E2F4 actively promotes the initiation and maintenance of nerve growth factor-induced cell differentiation. Mol Cell Biol. 1999; 19:6048-56; PMID:10454552
- Mosch B, Morawski M, Mittag A, Lenz D, Tarnok A, Arendt T. Aneuploidy and DNA replication in the normal human brain and Alzheimer disease. J Neurosci 2007; 27:6859-67; PMID:17596434
- López-Sánchez N, Frade JM. Genetic evidence for p75NTR-dependent tetraploidy in cortical projection neurons from adult mice. J Neurosci 2013; 33:7488-500; PMID:23616554; http://dx.doi.org/10.1523/JNEUROSCI.3849-12.2013
- López-Sánchez N, Ovejero-Benito MC, Borreguero L, Frade JM. Control of neuronal ploidy during vertebrate development. Results Probl Cell Differ 2011; 53:547-63; PMID:21630159; http://dx.doi.org/10.1007/978-3-642-19065-0_22
- Wang W, Bu B, Xie M, Zhang M, Yu Z, Tao D. Neural cell cycle dysregulation and central nervous system diseases. Prog Neurobiol 2009;89:1-17; PMID:19619927; http://dx.doi.org/10.1016/j.pneurobio.2009.01.007
- Byrnes KR, Faden AI. Role of cell cycle proteins in CNS injury. Neurochem Res 2007; 32:1799-807; PMID:17404835
- Di Giovanni S, Movsesyan V, Ahmed F, Cernak I, Schinelli S, Stoica B, Faden AI. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc Natl Acad Sci USA 2005; 102:8333-8; PMID:15923260
- Osuga H, Osuga S, Wang F, Fetni R, Hogan MJ, Slack RS, Hakim AM, Ikeda JE, Park DS. Cyclin-dependent kinases as a therapeutic target for stroke. Proc Natl Acad Sci USA 2000; 97:10254-9; PMID:10944192
- Arendt T, Brückner MK, Mosch B, Lösche A. Selective cell death of hyperploid neurons in Alzheimer disease. Am J Pathol 2010; 177:15-20; PMID:20472889; http://dx.doi.org/10.2353/ajpath.2010.090955
- Frade JM, López-Sánchez N. A novel hypothesis for Alzheimer disease based on neuronal tetraploidy induced by p75NTR. Cell Cycle 2010; 9:1934-41; PMID:20436277
- Bonda DJ, Evans TA, Santocanale C, Llosá JC, Viña J, Bajic V, Castellani RJ, Siedlak SL, Perry G, Smith MA, et al.; Evidence for the progression through S-phase in the ectopic cell cycle re-entry of neurons in Alzheimer disease. Aging (Albany NY) 2009; 1:382-8; PMID:19946466
- Yang Y, Geldmacher DS, Herrup K. DNA replication precedes neuronal cell death in Alzheimer disease. J Neurosci 2001; 21:2661-8; PMID:1306619
- Arendt T. Cell cycle activation and aneuploid neurons in Alzheimer disease. Mol Neurobiol 2012; 46:125-35; PMID:22528601; http://dx.doi.org/10.1007/s12035-012-8262-0
- Yang Y, Varvel NH, Lamb BT, Herrup K. Ectopic cell cycle events link human Alzheimer disease and amyloid precursor protein transgenic mouse models. J Neurosci 2006; 26:775-84; PMID:16421297
- Busser J, Geldmacher DS, Herrup K. Ectopic cell cycle proteins predict the sites of neuronal cell death in Alzheimer disease brain. J Neurosci 1998; 18:2801-7; PMID:9525997
- Smith TW, Lippa CF. Ki-67 immunoreactivity in Alzheimer disease and other neurodegenerative disorders. J Neuropathol Exp Neurol 1995; 54:297-303; PMID:7745428
- Nagy Z, Vatter-Bittner B, Braak H, Braak E, Yilmazer DM, Schultz C, Hanke J. Staging of Alzheimer-type pathology: an interrater-intrarater study. Dement Geriatr Cogn Disord 1997; 8:248-51; PMID:9213071
- Yang Y, Mufson EJ, Herrup K. Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer disease. J Neurosci 2003; 23:2557-63; PMID:12684440
- Hoozemans JJ, Brückner MK, Rozemuller AJ, Veerhuis R, Eikelenboom P, Arendt T. Cyclin D1 and cyclin E are co-localized with cyclo-oxygenase 2 (COX-2) in pyramidal neurons in Alzheimer disease temporal cortex. J Neuropathol Exp Neurol 2002; 61:678-88; PMID:12152783
- McShea A, Lee HG, Petersen RB, Casadesus G, Vincent I, Linford NJ, Funk JO, Shapiro RA, Smith MA. Neuronal cell cycle re-entry mediates Alzheimer disease-type changes. Biochim Biophys Acta 2007; 1772:467-72; PMID:17095196
- Absalon S, Kochanek DM, Raghavan V, Krichevsky AM. MiR-26b, upregulated in Alzheimer disease, activates cell cycle entry, tau-phosphorylation, and apoptosis in postmitotic neurons. J Neurosci 2013; 33:14645-59; PMID:24027266; http://dx.doi.org/10.1523/JNEUROSCI.1327-13.2013
- Hu XY, Zhang HY, Qin S, Xu H, Swaab DF, Zhou JN. Increased p75NTR expression in hippocampal neurons containing hyperphosphorylated tau in Alzheimer patients. Exp Neurol 2002; 178:104-11; PMID:12460612
- Munoz L, Ammit AJ. Targeting p38 MAPK pathway for the treatment of Alzheimer disease. Neuropharmacology 2010; 58:561-8; PMID:19951717; http://dx.doi.org/10.1016/j.neuropharm.2009.11.010
- Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF. Localization of active forms of C-jun kinase (JNK) and p38 kinase in Alzheimer disease brains at different stages of neurofibrillary degeneration. J Alzheimers Dis 2001; 3:41-48; PMID:12214071
- Sun A, Liu M, Nguyen XV, Bing G. P38 MAP kinase is activated at early stages in Alzheimer disease brain. Exp Neurol 2003; 183:394-405; PMID:14552880
- Morgan KL, Chalovich EM, Strachan GD, Otis LL, Jordan-Sciutto KL. E2F4 expression patterns in SIV encephalitis. Neurosci Lett 2005; 382:259-64; PMID:15925101
- Love S. Neuronal expression of cell cycle-related proteins after brain ischaemia in man. Neurosci Lett 2003; 353:29-32; PMID:14642430
- Wen Y, Yang S, Liu R, Brun-Zinkernagel AM, Koulen P, Simpkins JW. Transient cerebral ischemia induces aberrant neuronal cell cycle re-entry and Alzheimer disease-like tauopathy in female rats. J Biol Chem 2004; 279:22684-92; PMID:14982935
- Rashidian J, Iyirhiaro G, Aleyasin H, Rios M, Vincent I, Callaghan S, Bland RJ, Slack RS, During MJ, Park DS. Multiple cyclin-dependent kinases signals are critical mediators of ischemia/hypoxic neuronal death in vitro and in vivo. Proc Natl Acad Sci USA 2005; 102:14080-5; PMID:16166266
- Burns KA, Ayoub AE, Breunig JJ, Adhami F, Weng WL, Colbert MC, Rakic P, Kuan CY. Nestin-CreER mice reveal DNA synthesis by nonapoptotic neurons following cerebral ischemia hypoxia. Cereb Cortex 2007; 17:2585-92; PMID:17259645
- Höglinger GU, Breunig JJ, Depboylu C, Rouaux C, Michel PP, Alvarez-Fischer D, Boutillier AL, Degregori J, Oertel WH, Rakic P, et al.; The pRb/E2F cell-cycle pathway mediates cell death in Parkinson disease. Proc Natl Acad Sci USA 2007; 104:3585-90; PMID:17360686
- Jordan-Sciutto KL, Dorsey R, Chalovich EM, Hammond RR, Achim CL. Expression patterns of retinoblastoma protein in Parkinson disease. J Neuropathol Exp Neurol 2003; 62:68-74; PMID:12528819
- El-Khodor BF, Oo TF, Kholodilov N, Burke RE. Ectopic expression of cell cycle markers in models of induced programmed cell death in dopamine neurons of the rat substantia nigra pars compacta. Exp Neurol 2003; 179:17-27; PMID:12504864
- Alvira D, Tajes M, Verdaguer E, de Arriba SG, Allgaier C, Matute C, Trullas R, Jiménez A, Pallàs M, Camins A. Inhibition of cyclin-dependent kinases is neuroprotective in 1-methyl-4-phenylpyridinium-induced apoptosis in neurons. Neuroscience 2007; 146:350-65; PMID:17343987
- Bowser R, Smith MA. Cell cycle proteins in Alzheimer disease: plenty of wheels but no cycle. J Alzheimers Dis 2002; 4:249-54; PMID:12226545
- Ovejero-Benito MC, Frade JM. Brain-derived neurotrophic factor-dependent cdk1 inhibition prevents G2/M progression in differentiating tetraploid neurons. PLoS One 2013; 8:e64890; PMID:23741412; http://dx.doi.org/10.1371/journal.pone.0064890
- Shirazi Fard S, All-Ericsson C, Hallböök F. The heterogenic final cell cycle of chicken retinal Lim1 horizontal cells is not regulated by the DNA damage response pathway. Cell Cycle 2014; 13:408-17; PMID:24247150; http://dx.doi.org/10.4161/cc.27200
- Frade JM, Bovolenta P, Martínez-Morales JR, Arribas A, Barbas JA, Rodríguez-Tébar A. Control of early cell death by BDNF in the chick retina. Development 1997; 124:3313-20; PMID:9310326
- Frade JM, Barde YA. Genetic evidence for cell death mediated by nerve growth factor and the neurotrophin receptor p75 in the developing mouse retina and spinal cord. Development 1999; 126:683-90; PMID:9895316
- Nagy Z, Esiri MM, Cato AM, Smith AD. Cell cycle markers in the hippocampus in Alzheimer disease. Acta Neuropathol 1997; 94:6-15; PMID:9224524
- Tsujioka Y, Takahashi M, Tsuboi Y, Yamamoto T, Yamada T. Localization and expression of cdc2 and cdk4 in Alzheimer brain tissue. Dement Geriatr Cogn Disord 1999; 10:192-8; PMID:10325446
- Vincent I, Jicha G, Rosado M, Dickson DW. Aberrant expression of mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer disease brain. J Neurosci 1997; 17:3588-98; PMID:9133382
- Pei JJ, Braak H, Gong CX, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF. Up-regulation of cell division cycle (cdc) 2 kinase in neurons with early stage Alzheimer disease neurofibrillary degeneration. Acta Neuropathol 2002; 104:369-76; PMID:12200623
- Dranovsky A, Vincent I, Gregori L, Schwarzman A, Colflesh D, Enghild J, Strittmatter W, Davies P, Goldgaber D. Cdc2 phosphorylation of nucleolin demarcates mitotic stages and Alzheimer disease pathology. Neurobiol Aging 2001; 22:517-28; PMID:11445251
- Husseman JW, Nochlin D, Vincent I. Mitotic activation: a convergent mechanism for a cohort of neurodegenerative diseases. Neurobiol Aging 2000; 21:815-28; PMID:11124425
- Vincent I, Bu B, Hudson K, Husseman J, Nochlin D, Jin L. Constitutive Cdc25B tyrosine phosphatase activity in adult brain neurons with M phase-type alterations in Alzheimer disease. Neuroscience 2001; 105:639-50; PMID:11516829
- Ding XL, Husseman J, Tomashevski A, Nochlin D, Jin LW, Vincent I. The cell cycle Cdc25A tyrosine phosphatase is activated in degenerating postmitotic neurons in Alzheimer disease. Am J Pathol 2000; 157:1983-90; PMID:11106571
- Tomashevski A, Husseman J, Jin LW, Nochlin D, Vincent I. Constitutive Wee1 activity in adult brain neurons with M phase-type alterations in Alzheimer neurodegeneration. J Alzheimers Dis 2001; 3:195-207; PMID:12214061
- Ogawa O, Zhu X, Lee HG, Raina A, Obrenovich ME, Bowser R, Ghanbari HA, Castellani RJ, Perry G, Smith MA. Ectopic localization of phosphorylated histone H3 in Alzheimer disease: a mitotic catastrophe? Acta Neuropathol 2003; 105:524-8; PMID:12677454
- Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther 2013; 138:155-75; PMID:23348013; http://dx.doi.org/10.1016/j.pharmthera.2013.01.004
- Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol 2009; 5:311-22; PMID:19498435; http://dx.doi.org/10.1038/nrneurol.2009.54
- Skaper SD. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol Disord Drug Targets 2008; 7:46-62; PMID:18289031
- Ferrer I, Marín C, Rey MJ, Ribalta T, Goutan E, Blanco R, Tolosa E, Martí E. BDNF and full-length and truncated TrkB expression in Alzheimer disease. Implications in therapeutic strategies. J Neuropathol Exp Neurol 1999; 58:729-39; PMID:10411343
- Kim AH, Bonni A. Cdk1-FOXO1: a mitotic signal takes center stage in post-mitotic neurons. Cell Cycle 2008; 7:3819-22; PMID:19124971
- Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol 2013; 14:83-97; PMID:23325358 http://dx.doi.org/10.1038/nrm3507
- Ajioka I, Martins RA, Bayazitov IT, Donovan S, Johnson DA, Frase S, Cicero SA, Boyd K, Zakharenko SS, Dyer MA. Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice. Cell 2007; 131:378-90; PMID:17956737
- Wang F, Corbett D, Osuga H, Osuga S, Ikeda JE, Slack RS, Hogan MJ, Hakim AM, Park DS. Inhibition of cyclin-dependent kinases improves CA1 neuronal survival and behavioral performance after global ischemia in the rat. J Cereb Blood Flow Metab 2002; 22:171-82; PMID:11823715
- Verdaguer E, Jiménez A, Canudas AM, Jordà EG, Sureda FX, Pallàs M, Camins A. Inhibition of cell cycle pathway by flavopiridol promotes survival of cerebellar granule cells after an excitotoxic treatment. J Pharmacol Exp Ther 2004; 308:609-16; PMID:14610234