Abstract
Mesenchymal stem cells (MSCs) play an important role in chemoresistance. Exosomes have been reported to modify cellular phenotype and function by mediating cell-cell communication. In this study, we aimed to investigate whether exosomes derived from MSCs (MSC-exosomes) are involved in mediating the resistance to chemotherapy in gastric cancer and to explore the underlying molecular mechanism. We found that MSC-exosomes significantly induced the resistance of gastric cancer cells to 5-fluorouracil both in vivo and ex vivo. MSC-exosomes antagonized 5-fluorouracil-induced apoptosis and enhanced the expression of multi-drug resistance associated proteins, including MDR, MRP and LRP. Mechanistically, MSC-exosomes triggered the activation of calcium/calmodulin-dependent protein kinases (CaM-Ks) and Raf/MEK/ERK kinase cascade in gastric cancer cells. Blocking the CaM-Ks/Raf/MEK/ERK pathway inhibited the promoting role of MSC-exosomes in chemoresistance. Collectively, MSC-exosomes could induce drug resistance in gastric cancer cells by activating CaM-Ks/Raf/MEK/ERK pathway. Our findings suggest that MSC-exosomes have profound effects on modifying gastric cancer cells in the development of drug resistance. Targeting the interaction between MSC-exosomes and cancer cells may help improve the efficacy of chemotherapy in gastric cancer.
Introduction
Mesenchymal stem cells (MSCs) are a distinct population of cells with self-renewal and multi-lineage differentiation abilities. They can home to injured areas or tumors in response to the induction of local inflammatory factors. MSCs may participate in the formation of tumor microenvironment where they modulate the immune system and facilitate tumor growth. Recent studies have shown that MSCs also play an important role in mediating the resistance of cancer cells to various anti-cancer drugs.Citation1 They exert their growth stimulating effects either via paracrine secretion of growth factors and anti-apoptotic factors or by differentiating into tumor-associated fibroblasts, which can enhance tumor growth, metastasis formation and therapy resistance.Citation2-4
Exosomes are major players in inter- and intra-cellular communication. They have been reported to deliver diverse biological molecules ranging from mRNAs, miRNAs, to proteins. Exosomes from tumor microenvironment cells modulate epithelial to mesenchymal transition, cancer stemness, angiogenesis and metastasis.Citation7,8 Recent studies have shown that chemotherapy enhanced the secretion of exosomes in tumor cells, leading to the transfer of chemoresistance related miRNAs and mRNAs to neighboring cells to alter their sensitivity to chemotherapy, suggesting a critical role of exosomes in the cellular response to chemotherapy.Citation9-11
Gastric cancer is the fourth most common malignant tumor worldwide and the second most frequent cause of cancer death after lung cancer.Citation12,13 Pre- and post-operative chemotherapy with 5-fluorouracil (5-FU) and cisplatin (DDP) have improved the survival rates of gastric cancer patients.Citation12-14 But the development of resistance is one of the most significant obstacles to effective gastric cancer therapy.Citation1 However, whether MSC-exosomes are also involved in chemoresistance in gastric cancer and the potential mechanisms remain unknown.
We have previously shown that MSCs could treat liver and kidney injuries, enhance the growth of gastric cancer in mouse models, and MSC-exosomes had similar effects with MSCs.Citation3-6 Since tumor microenvironment is emerging as a significant determinant of a tumor's response to chemotherapyCitation15,16 and MSCs has been considered as an important component of the tumor microenvironment, we hypothesized that MSC-exosomes might contribute to the development of resistance to chemotherapy in gastric cancer. In this study, we found that MSC-exosomes potentiated chemoresistance in gastric cancer cells in vivo and ex vivo. MSC-exosomes exerted this role at least in part through the activation of CaM-Ks (predominantly CaM-KII and CaM-KIV) and the downstream Raf/MEK/ERK pathway in gastric cancer cells. These findings not only reveal the profound effects of MSC-exosomes on modifying gastric cancer cells in the development of drug resistance, but also provide a new target for improving the efficacy of chemotherapy in gastric cancer.
Results
MSC-exosomes induce the resistance of gastric cancer cells to 5-FU in vivo
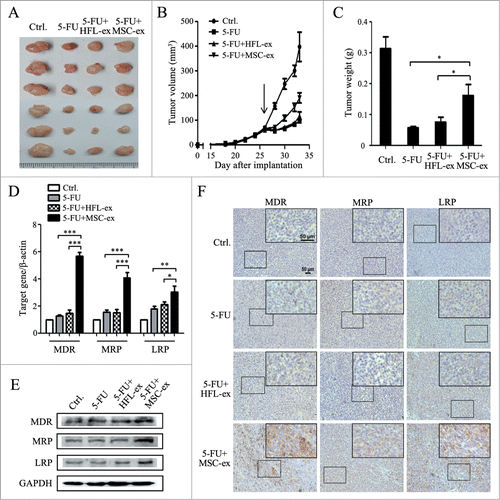
Exosomes derived from human MSCs and HEL1 cells were small round vesicles with a diameter ranging from 40 to 100 nm (Fig. S1A), expressed the exosomal markers CD9 and CD63 (Fig. S1B), and could be internalized by HGC-27 after exposure for 4 hours while exosomes controls showed no effect (Fig. S1C). To test whether MSC-exosomes are involved in inducing the resistance to chemotherapy, we established a subcutaneous xenograft tumor model and co-injected MSC-exosomes to tumor-bearing mice with 5-FU. As shown in , MSC-exosomes significantly inhibited the chemotherapeutic effect of 5-FU, whereas HFL1-exosomes had minimal effect. Tumor growth in MSC-exosome group was faster than that in 5-FU alone and HFL1-exosome groups. The mean tumor size in MSC-exosome group was about 200 mm3 at the seventh day after chemotherapy, almost 2 times larger than that in 5-FU alone and HFL1-exosome groups (). The mean tumor weight in MSC-exosome group was approximately 3 times heavier than that in 5-FU alone group, while there was no statistically significant difference in the mean tumor weight between HFL1-exosome and 5-FU alone groups ().The results of real-time RT-PCR analyses showed that MSC-exosomes co-treatment enhanced the expression of MDR (5.6 ± 0.6-fold), MRP (4.7 ± 0.9-fold), and LRP (3.0 ± 0.8-fold) compared to 5-FU alone group (). In support of this data, the results of Western blot showed that co-treatment with MSC-exosomes increased MDR, MRP, and LRP protein levels in gastric cancer tissues (). Immunohistochemical analysis showed that tumor tissues from MSC-exosome group displayed more positivity for MDR, MRP, and LRP than that in other groups (). Taken together, these data indicated that MSC-exosomes prompted the development of resistance to chemotherapy in gastric cancer in vivo.
Figure 1. MSC-exosomes induce resistance of gastric cancer cells to 5-FU in vivo. (A) The size of tumors at the end of the experiment from mice treated with PBS (Ctrl.), 5-FU, 5-FU+HFL1-exosomes, 5-FU+MSC-exosomes. (B) Tumor growth curves in mice treated with PBS, 5-FU, 5-FU+HFL1-exosomes, or 5-FU+MSC-exosomes (n=6). Treatment was initiated when tumors reached a volume of 50–100 mm3. (C) The mean weight of tumors at the end of the experiment (at day 7 after chemotherapy treatment) from mice treated with PBS, 5-FU, 5-FU+HFL1-exosomes, or 5-FU+MSC-exosomes (n=6). (* P<0.05). (D) Relative quantitative PCR analyses of MDR, MRP, and LRP gene expression in tumor tissues from mice treated with PBS, 5-FU, 5-FU+HFL1-exosomes, or 5-FU+MSC-exosomes. (* P<0.05, ** P<0.01, *** P<0.001.) (E) The expression levels of MDR, MRP, and LRP proteins were examined by using western blot. (F) Immunohistochemical analyses of MDR, MRP and LRP protein expression in tumor tissues from mice treated with PBS, 5-FU, 5-FU+HFL1-exosomes, or 5-FU+MSC-exosomes. Original magnification, × 100, smaller one at top right corner, × 200. Scale bar = 50 μm.
MSC-exosomes induce the resistance of gastric cancer cells to chemotherapy ex vivo
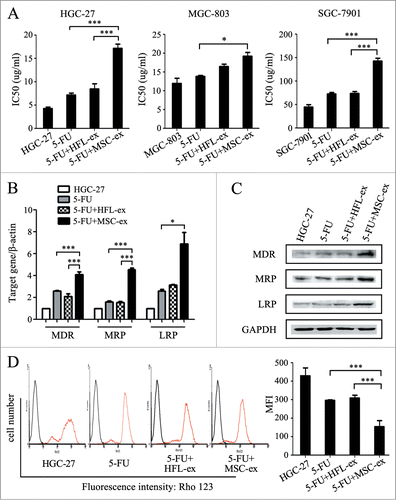
To determine the role of MSC-exosomes in the development of resistance to chemotherapy ex vivo, we established drug-resistant cell models by repeated exposure of gastric cancer cells to increasing concentrations of chemotherapeutic agents in the presence or absence of MSC-exosomes. MTT assay showed that the half maximal inhibitory concentration (IC50) of HGC-27 to 5-FU in MSC-exosome group was notably elevated compared to the 5-FU alone group and the resistance index was 2.8 ± 1.0 (). To confirm the chemoresistance-inducing effect of MSC-exosomes, we tested different gastric cancer cell lines for their responses to chemotherapy by using the same procedure. The resistance index of MSC-exosome group to 5-FU alone group was 1.3 ± 0.4 for MGC-803 cells and 2.0 ± 1.9 for SGC-7901 cells, respectively (). In consistent with this observation, MSC-exosomes also aggravated the resistance of gastric cancer cells to cisplatin (DDP). The resistance index of MSC-exosome to 5-FU alone group was 1.3 ± 0.3 for HGC-27 cells, 1.4 ± 0.4 for MGC-803 cells, and 1.9 ± 0.3 for SGC-7901 cells, respectively (Fig. S2). The presence of MSC-exosomes increased the expression of multi-drug resistance associated genes including MDR, MRP, and LRP (), which was further confirmed by Western blot analyses (). The function of MDR was investigated by using the efflux of the fluorescent substrate Rho-123. Flow cytometric analyses showed that the mean fluorescence intensity (MFI) in MSC-exosome group was less than half of that in 5-FU alone group, suggesting there was more Rho-123 accumulation in 5-FU alone group than that in MSC-exosome group (). In summary, MSC-exosomes increased the expression of multi-drug resistant proteins in gastric cancer cells, resulting in the decrease of sensitivity to chemotherapy.
Figure 2. MSC-exosomes induce resistance of gastric cancer cells to 5-FU ex vivo. (A) MTT assay for IC50 of parental and chemoresistant HGC-27, MGC-803, and SGC-7901 cells in response to 5-FU. The cells were treated with 5-FU for 24 h, then changed to normal medium until cell recovery. Exosomes from MSCs and HFL1 cells were added at the start of treatment for 72 h. The control cells were cultured in normal medium without any treatment. (* P < 0.05, *** P < 0.001). (B) The expression of MDR, MRP, and LRP genes in parental and chemoresistant HGC-27 cells was determined by using relative quantitative PCR. (* P < 0.05, *** P < 0.001). (C) Western blot assays for MDR, MRP and LRP protein expression in parental and chemoresistant HGC-27 cells. (D) Fluorescent intensity of Rho-123 in parental and chemoresistant HGC-27 cells. The cells were labeled with Rho-123 after exposure to 5-FU for 6 h (red line). The cells without Rho-123 labeling were used as control (black line). For each assay, 10,000 cells were analyzed. The x-axis corresponds to the fluorescence intensity, and the y-axis, to the number of cells per channel. The quantitative data are presented as the mean ± SD of triplicate experiments. MFI: the mean fluorescent intensity. (*** P < 0.001).
MSC-exosomes enhance the anti-apoptotic ability of gastric cancer cells
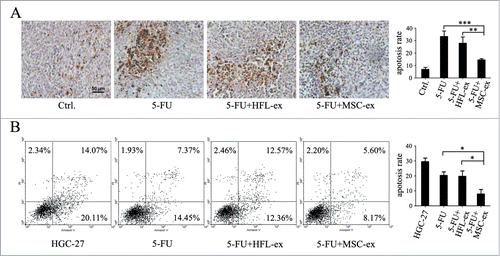
There is accumulating evidence that resistance to apoptosis is a hallmark of cancer and can cause resistance to drug treatment.Citation18 To further investigate the functional roles of MSC-exosomes in the resistance of gastric cancer cells to chemotherapy, we determined chemotherapy-induced apoptosis in gastric cancer cells in the presence or absence of MSC-exosomes. TUNEL staining demonstrated that the number of apoptotic cells in the tumor tissues increased after treatment with 5-FU. However, co-treatment with MSC-exosomes reduced the apoptotic rate (). To further demonstrate the effect of MSC-exosomes on apoptosis, the parental and drug-resistant HGC-27 cells were exposed to 5-FU for 48 h and the percentage of apoptotic cells was analyzed by using Annexin V-FITC/PI apoptosis staining. The apoptotic rate in MSC-exosome group was 7.95 ± 5.82%, which was significantly lower than that in 5-FU (20.25 ± 3.92%) and HFL1-exosome groups (19.78 ± 6.04%) (). Taken together, these results suggested that MSC-exosomes could prevent the induction of apoptosis by chemotherapy in gastric cancer cells.
Figure 3. MSC-exosomes protect gastric cancer cells from chemotherapy-induced apoptosis. (A) Tumors from mice treated with PBS (Ctrl.), 5-FU, 5-FU+HFL1-exosomes, 5-FU+MSC-exosomes were paraffin-embedded and sectioned, followed by staining of apoptotic cell by using TUNEL assay. The number of TUNEL-positive cells notably increased in the 5-FU and 5-FU+HFL1-exosome groups compared to the 5-FU+MSC-exosome group, while the control group treated with PBS had few apoptotic cells. Original magnification, × 200. Scale bar = 50 μm. The quantitative analyses of apoptosis (TUNEL) indices were calculated by counting the number of positive cells in 10 random fields. (** P < 0.01, *** P < 0.001). (B) Flow cytometric analyses of apoptotic cells ex vivo. The parental and chemoresistant HGC-27 cells were exposed to 5-FU for 48 h, collected and subjected to Annexin V/PI double staining, followed by FACS analyses. For each assay, 10,000 cells were analyzed. The quantitative data are presented as the mean ± SD of triplicate experiments. (* P < 0.05).
MSC-exosomes promote the activation of CaM-Ks in gastric cancer cells
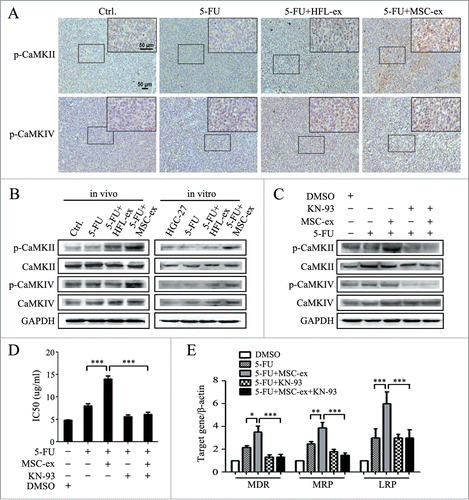
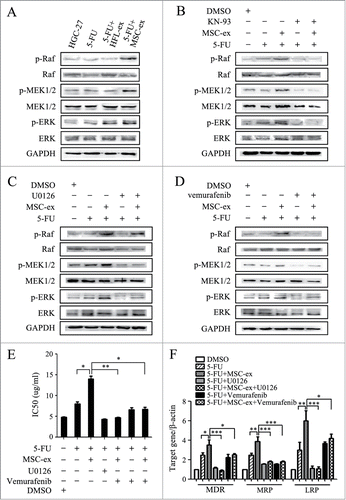
To determine the mechanisms by which MSC-exosomes conferred chemoresistance in gastric cancer, we examined the expression of membrane pump P-glycoprotein (P-gp) in MSC-exosomes. We found that P-gp/MDR was expressed in MSC-exosomes by using Western bolt (Fig. S1B). The increased expression of membrane pump P-glycoprotein in cancer cells resulted in the influx of intracellular calcium, the formation of calcium/calmodulin complexes and the subsequent activation of the CaM-kinases (CaM-Ks).Citation19 We next determined the expression of phosphorylated CaM-Ks in chemoresistant HGC-27 cells in vivo and ex vivo. As shown in , the positive staining of p-CaM-KII in tumor tissues from MSC-exosome group was stronger than that in other groups by immunohistochemistry. However, only a slight increase in p-CaM-KIV staining was detected in tumor tissues from MSC-exosome group. The increased expression of p-CaM-KII in MSC-exosome group was further confirmed by Western blot (). In addition, the expression of p-CaM-KIV had minimal change while no difference was observed in the expression of p-CaM-KI (data not shown). To determine the role of CaM-Ks activation in chemoresistance induced by MSC-exosomes, we treated the chemoresistant cells with KN-93, an effective inhibitor of CaM-Ks phosphorylation. We found that the addition of KN-93 almost fully prevented MSC-exosomes-induced CaM-Ks activation (), drug resistance (), and multi-drug resistant associated genes expression (), suggesting that the activation of CaM-Ks activation was critical for the chemoresistance induced by MSC-exosomes.
Figure 4. MSC-exosomes activate CaM-KII and CaM-KIV in gastric cancer cells. (A) Tumors from mice treated with PBS (Ctrl.), 5-FU, 5-FU+HFL1-exosomes, 5-FU+MSC-exosomes were paraffin-embedded and sectioned, followed by immunohistochemical staining of p-CaM-KII and p-CaM-KIV. Original magnification, × 100, smaller one at top right corner, × 200. Scale bar = 50 μm. (B) Western blot analyses of CaM-KII, CaM-KIV, and their phosphorylated forms in tumor tissues and cells. (C) HGC-27 cells were treated with MSC-exosomes in the presence or absence of KN-93 (10 μM). The protein levels of p-CaM-KII and p-CaM-KIV were examined by using protein gel blot. (D, E) HGC-27 cells were treated with MSC-exosomes in the presence or absence of KN-93 (10 μM). The IC50 of HGC-27 cells in response to 5-FU were determined by using MTT assay (D). The expression of MDR, MRP, and LRP genes was determined by using relative quantitative PCR (E). (* P < 0.05, ** P < 0.01, *** P < 0.001).
MSC-exosomes activate the Raf/MEK/ERK signaling pathway
The Raf/MEK/ERK kinase cascade is one of the downstream of the CaM-Ks. As shown in , higher levels of activated Raf1, MEK1/2 and ERK1/2 were detected in MSC-exosome group. In contrary, the inhibition of CaM-Ks by KN-93 blocked the activation of Raf/MEK/ERK kinase by MSC-exosomes (). The specific inhibitor U0126 could restrain the activation of MEK1/2 and ERK1/2 while not affect p-Raf1 expression induced by MSC-exosomes (). We further demonstrated that Raf1 kinase inhibitor Vemurafenib also blocked the activation of MEK1/2 and ERK1/2 induced by MSC-exosomes (), suggesting that MSC-exosomes sequentially activate the CaM-Ks/Raf/MEK/ERK kinase cascade in gastric cancer cells. MTT assay showed that the increase of half maximal inhibitory concentration by MSC-exosomes in gastric cancer cells was inhibited by simultaneous treatment with U0126 and Vemurafenib (). The increased expression of MDR, MRP, and LRP by MSC-exosomes was also inhibited after treatment with U0126 or Vemurafenib (). In brief, MSC-exosomes conferred drug resistance in gastric cancer cells through the activation of CaM-Ks/Raf/MEK/ERK signaling pathway.
Figure 5. Activation of the CaM-Ks/Raf/MEK/ERK pathway is critical for chemoresistance induced by MSC-exosomes. (A) The expression of p-Raf, p-MEK, and p-ERK in the parental and chemoresistant HGC-27 cells was detected by using western blot. (B) Western blot assays for the expression of p-Raf, p-MEK, and p-ERK in HGC-27 cells treated with MSC-exosomes in the presence or absence of KN-93 (10 μM). (C) HGC-27 cells were treated with MSC-exosomes in the presence or absence of U0126 (10 μM). The levels of phosphorylated Raf, MEK, and ERK were examined by using protein gel blot. (D) HGC-27 cells were treated with MSC-exosomes together with or without vemurafenib (20 μM). The expression of p-Raf, p-MEK, and p-ERK was examined by using western blot. (E, F) HGC-27 cells were treated with MSC-exosomes in the presence or absence of U0126 (10 μM) or vemurafenib (20 μM). The IC50 of HGC-27 cells in response to 5-FU was determined by using MTT assay (E). The expression of MDR, MRP, and LRP genes was determined by using relative quantitative PCR (F). (* P < 0.05, ** P < 0.01, *** P < 0.001).
MSC-exosomes trigger the activation of CaM-Ks/Raf/MEK/ERK pathway mainly through proteins
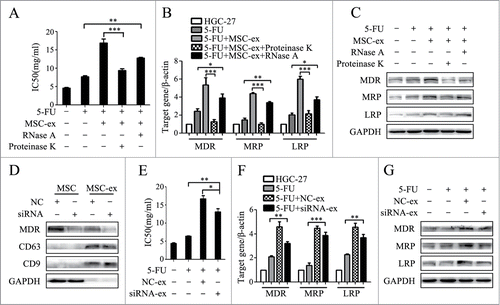
Exosomes act on the target cells through multiple modes, including coupling of the ligand with the receptor in target cells, membrane fusion, and endocytosis to transit signaling molecules. In order to look into the molecules that were delivered to gastric cancer cells to induce drug resistance, MSC-exosomes were pre-treated with nucleic acid-hydrolyzing enzyme (RNase A) and proteolytic enzyme (Proteinase K). The IC50 of RNase A group increased statistically compared to 5-FU alone group, while the pre-treatment with Proteinase K almost completely abrogated MSC-exosomes mediated chemoresistance (). In consistent with the MTT data, the similar changes were also observed in the expression of multi-drug resistance associated genes and proteins including MDR, MRP and LRP (). These results suggested that proteins of MSC-exosomes might play a major role in the promotion of drug resistance. Since we have observed the expression of P-gp/MDR in MSC-exosomes and a significant change in MDR expression after treatment with MSC-exosomes, we then knocked down MDR with siRNA and prepared MSC-exosomes (siRNA-ex). The control cells were transfected with a non-targeting siRNA (NC-ex) (). As shown in , knockdown of MDR slightly reversed the induced chemoresistance of HGC-27 cells to 5-FU by MSC-exosomes. Though the IC50 of siRNA-exosome group was lower than that of NC-exosome group, it was still significantly higher than that of 5-FU alone group. The expression of MDR, MRP and LRP genes and proteins remained higher in siRNA-exosome group than that in 5-FU alone group (). Taken together, our results indicated that the proteins but not P-gp/MDR delivered by MSC-exosomes play a dominant role in activating CaM-Ks/Raf/MEK/ERK signaling pathway to confer drug resistance in gastric cancer cells.
Figure 6. MSC-exosomes induce chemoresistance in gastric cells mainly though proteins. (A) MTT assay for IC50 of HGC-27 cells in response to 5-FU. MSC-exosomes were pre-incubated with nucleic acid-hydrolyzing enzyme (RNase A) and proteolytic enzyme (Proteinase K). HGC-27 were treated with 5-FU for 24 h, then changed to normal medium until cell recovery. The pre-treated and untreated MSC-exosomes were added at the start of 5-FU treatment for 72 h. The control cells were cultured in normal medium without any treatment. (** P < 0.01, *** P < 0.001). (B) The expression of MDR, MRP, and LRP genes in HGC-27 was determined by using relative quantitative PCR. (* P < 0.05, ** P < 0.01, *** P < 0.001). (C) Western blot assays for MDR, MRP and LRP proteins expression in HGC-27 cells. (D) MSCs were transfected with negative control (NC) and siRNA-MDR by using Lipofectin. The expression of MDR in MSCs and MSC-exosomes were determined by using protein gel blot. (E) MTT assay for IC50 of parental and chemoresistant HGC-27 cells to 5-FU. (* P < 0.05, ** P < 0.01). (F) Relative quantitative PCR analyses of MDR, MRP, and LRP genes expression. (** P<0.01, *** P<0.001). (G) The expression levels of MDR, MRP, and LRP proteins were examined by using western blot.
Discussion
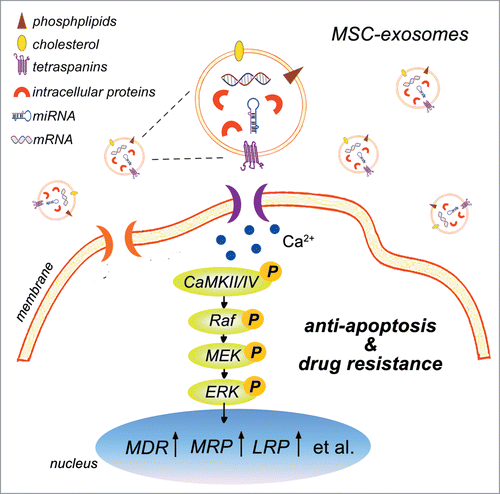
Resistance to therapy is one of the major obstacles in the treatment of cancer. Drug resistance can arise within tumor cells because of genetic changes causing increased drug efflux (intrinsic resistance), or it can be the result of the tumor microenvironment protecting tumor cells against treatment (extrinsic resistance).Citation20 We showed here for the first time that MSC-exosomes could robustly confer drug resistance in gastric cancer. MSC-exosomes increased the expression of multi-drug resistance associated genes and proteins in gastric cancer cells, enhanced the functionality of P-gp/MDR to discharge more intracellular drug, thus adjusted the sensitivity of gastric cancer cells to chemotherapy. We demonstrated that MSC-exosomes protected gastric cancer cells from chemotherapy-induced apoptosis, which might be one of the mechanisms responsible for the effects of MSC-exosomes on gastric cancer chemoresistance. We further confirmed that MSC-exosomes activated the CaM-Ks/Raf/MEK/ERK signaling cascade by proteins rather than directly transferring multi-drug resistance associated proteins or mRNAs to gastric cancer cells. Consequently, MSC-exosomes induced the up-regulation of multi-drug resistance associated genes and proteins in gastric cancer cells and conferred drug resistance ().
Figure 7. Schematic model for the role of MSC-exosomes in the development of drug resistance in gastric cancer. MSC-exosomes contains a variety of bioactive molecules ranging from mRNAs, proteins, to miRNAs. MSC-exosomes incorporated into the gastric cancer cells stimulate the activation of CaM-Ks (predominantly CaM-KII and CaM-KIV). Activation of CaM-KII and CaM-KIV trigger the activation of downstream Raf/MEK/ERK signaling cascade. Consequently, the expression of multi-drug resistant proteins is up-regulated in gastric cancer cells, resulting in the resistance to chemotherapy-induced apoptosis and the development of drug resistance.
Exosomes from cancer cells have been suggested to be critically involved in the development of drug resistance in cancer. Corcoran et al. have shown that prostate cancer cells exosomes participate in mediating docetaxel-resistance.Citation9 Xiao X et al. have demonstrated that A549 cells exosomes are involved in the decrease of the sensitivity of A549 cells to DDP.Citation10 Our studies further demonstrated that exosomes from MSCs could elicit drug resistance to 5-FU and DDP, which indicate that in addition to tumor cells, exosomes from tumor stromal cells such as MSCs may also mediate the resistance to chemotherapy.
Evidence is now arising that MSCs are associated with microenvironment mediated-drug resistance by producing a variety of factors, recycling macromolecule, activating certain signaling cascades to protect tumor cells against chemotherapy drugs.Citation21-24 We have previously demonstrated that conditioned medium and exosomes derived from MSCs could promote tumor growth comparable to that of MSCs.Citation4 Here we demonstrated that treatment of gastric cancer cells with MSC-exosomes was sufficient to confer the chemoresistance ex vivo and in vivo, suggesting that MSC-exosomes is one of the active factors that potentiate chemoresistance and the presence of MSCs itself may not be required for the induction of drug resistance in gastric cancer. MSCs may induce the change of gastric cancer cells to a more aggressive phonotype by secreting exosomes instead of physical contact in the tumor microenvironment.
One of the main mechanisms for chemoresistance is the overexpression of the membrane pump P-glycoprotein (P-gp) in cancer cells, which have been correlated with increased intracellular calcium concentration. The influx of intracellular calcium will result in the formation of calcium/calmodulin complexes and the subsequent activation of the CaM-kinases (CaM-Ks).Citation19,25 We demonstrated that gastric cancer cells treated with MSC-exosomes exhibited increased expression of phosphorylated CaM-KII. CaM-KII undergoes autophosphorylation whereas CaM-KI and CaM-KIV are phosphorylated by CaM-KK,Citation25 which may explain the stronger change of CaM-KII in our study. In consistent with the results from McCubrey et al,Citation26 we found in this study that the increase of CaM-KII led to the activation of Raf/MEK/ERK signaling pathway and the upregulation of MDR, MRP, and LRP. Although we found that MSC-exosomes did carry P-gp/MDR protein, knockdown of MDR gene in MSCs had minimal effect on MSC-exosomes induced chemoresistance. MSC-exosomes may mainly interact with gastric cancer cells by receptor ligation, leading to the activation of calcium channels and the influx of intracellular calcium, the formation of calcium/calmodulin complexes and the activation of Raf/MEK/ERK axis, resulting in the up-regulation of MDR, MRP, and LRP genes, and eventually inducing chemoresistance in gastric cancer. The release of P-gp/MDR from MSC-exosomes to gastric cancer cells may be also involved in the induction of chemoresistance but may play a minor role in our experimental setting.
Recently, Boelens et al. demonstrated that stromal cells orchestrate an intricate crosstalk with breast cancer cells by utilizing exosomes to drive therapy resistance.Citation27 They found that stromal cells derived exosomes activated Notch3 pathway in breast cancer cells. Exosomes could activate various signaling pathways in target cells, suggesting the complexity of the interaction between exosomes and target cells. Furthermore, MSC-exosomes mediated drug resistance was also observed in other cancers. For instance, Wang et al. showed that bone marrow stromal cells derived exosomes served as a communicator in drug resistance in multiple myeloma cells.Citation28 Although we have shown that MSC-exosomes conferred chemoresistance in gastric cancer, we have not identified the exact molecule(s) in MSC-exosomes that mediate this effect. Future studies are warranted to provide additional information to further understand the role of MSC-exosomes in inducing the resistance to cancer therapy.
The results of the current study imply that MSCs may function remotely through exosomes rather in close proximity to cancer cells. The interaction between MSCs and gastric cancer cells by exosomes may provide further insight into the chemoresistance of gastric cancer and set the basis for the development of an exosome-based therapeutic strategy. Interventions against either the formation or release of exosomes from MSCs may provide opportunities to improve the efficacy of chemotherapy and prevent the development of resistance in gastric cancer. Our study also suggests that exosomes may be used as a potential tumor-targeting vehicle for delivering drugs or other cancer combating agents. Shedden et al. proved that anticancer drugs could be encapsulated in exosomes.Citation11 Therefore, exosomes have a great potential to be exploited to deliver anticancer drugs to extracellular space and alter the biological activities of cancer cells through releasing therapeutic agents.
In conclusion, we demonstrate in this study that MSC-exosomes confer drug resistance of gastric cancer cells ex vivo and in vivo. MSC-exosomes exert this role mainly through its proteins resulting in the activation of CaM-Ks/Raf/MEK/ERK signaling cascade, the upregulation of multi-drug resistance associated proteins, and the protection from chemotherapy-induced apoptosis in gastric cancer cells. Our findings suggest that exosomes is an important mediator in chemoresistance induced by MSCs and inhibiting the role of MSC-exosomes may help improve the therapy efficacy in gastric cancer.
Materials and Methods
Cell culture and exosomes extraction
MSCs were isolated from human umbilical cord and cultured as previously described.Citation17 Human foetal lung fibroblast HFL1 and gastric cancer cell lines HGC-27, MGC-803, and SGC-7901 were purchased from Cell Bank, Type Culture Collection Committee, Chinese Academy of Sciences. Gastric cancer cells were cultured in DMEM containing 10% FBS at 37°C in humidified air with 5% CO2. MSCs and HFL1 cells were cultured in serum-free medium. After 48 h, cell culture media were collected and exosomes were isolated using density gradient centrifugation method as previously described.Citation5 Exosomes were stored at −70°C until use. HEL-1 cells derived exosomes were used as an exosomal control.
Animal model
Twenty four male BALB/c nu/nu mice (Laboratory Animal Center of Shanghai, Academy of Science, China) aged 4–6 weeks were randomly divided into 4 groups (n=6 ). All groups received subcutaneous injections of HGC-27 cells (3×10 6 cells in 200 μl PBS per mouse). Five-fluorouracil (5-FU) was administered intraperitoneally (i.p.) at Maximum tolerated dose level (100 mg/kg) when tumors reached a volume of 50–100 mm3. MSC-exosomes and HFL-exosomes (100 μg/ml) were daily administered subcutaneously (s.c.) at the start of 5-FU treatment. The mice were examined every 2 d and sacrificed at 7 d after 5-FU treatment. Tumor volumes were calculated by the modified ellipsoidal formula: V= 1/2 (length×width2).
Ex vivo pretreatment of gastric cancer cells
Gastric cancer cells were seeded in 24-well plates at 1×10 5 cells per well and treated with 5-FU or DDP for 24 h, then changed to the complete medium for cell recovery. Exosomes (80 μg/ml) from MSCs and HFL1 cells were added at the start of chemotherapy for 72 h. The treatment was repeated for 3 cycles with the increase of drug concentration. The doses used were as follows: HGC-27/5-FU (1, 1.5, and 2 μg/ml), HGC-27/DDP (1, 2, and 3 μg/ml), MGC-803/5-FU (2, 3, and 4 μg/ml), MGC-803/DDP (0.25, 0.5, and 1 μg/ml), SGC-7901/5-FU (10, 15, and 20 μg/ml); SGC-7901/DDP (1, 2, and 3 μg/ml). For inhibitor studies, KN-93 (10 μM; Sigma, USA), U0126 (10 μM; Promega, USA), Vemurafenib (namely PLX4032; 20 μM; Santa Cruz, USA) were added together with exosomes. MSC-exosomes were pre-treated with nucleic acid-hydrolyzing enzyme (RNase A; Sigma, USA) and proteolytic enzyme (Proteinase K; Merck, USA) as previously described.Citation29 Human MDR1 siRNA and negative control were purchased from GenePharma (Shanghai, China) and were transfected into MSCs for 48 hours by using Lipofectin.
Cell viability assay
Cell viability was determined by using the MTT (3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide) assay. HGC-27, MGC-803, and SGC-7901 cells were seeded in 96-well plates at 3×10 3 cells per well and incubated with 5-FU for 48 h. MTT (5 mg/ml) was added to each well for the last 4 h of treatment. The reaction was stopped by the addition of dimethyl sulfoxide, and the optical density was determined at 490 nm on a multiwell plate reader (FLX800, Bio-TEK). Background absorbance of the medium in the absence of cells was subtracted. All samples were assayed in triplicate, and the mean for each experiment was calculated.
Rho-123 accumulation assay
Cells were seeded in 6-well plates at 3×10 5 per well and exposed to 5-FU for 6 h prior to analysis. For the rhodamine-123 (Rho-123; 2-[6-amino-3-imino-3H-xanthen-9-yl] benzoic acid methyl ester; Sigma, USA) accumulation assay, Rho-123 (200 ng/ml) was added to equal number of cells and incubated for 40 min at 37°C. After incubation, cells were washed twice with PBS and resuspended in ice-cold DMEM. The accumulation of Rho-123 in cells was analyzed with flow cytometry. HGC-27 which had not been exposed to 5-FU and Rho-123, were used to determine the background of autofluorescence.
Cell apoptosis assay
Cells in early and late stages of apoptosis were detected by using an Annexin V-FITC apoptosis detection kit from Bio-Vision (Mountain View, USA), according to the manufacturer's protocol. In brief, 3×10 5 cells were exposed to 5-FU for 48 h, collected and analyzed in a Becton Dickinson FACS Calibur instrument. Cells that were positive for Annexin V-FITC alone (early apoptosis) and Annexin V-FITC and PtdIns (late apoptosis) were counted. All samples were assayed in triplicate.
Western blot
Tissue samples and gastric cancer cells were homogenized and lysed in RIPA buffer supplemented with proteinase inhibitors. Equal amount of proteins were loaded and separated on a 10% SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis). The proteins were transferred to a PVDF (polyvinylidene difluoride) membrane, blocked in 5% (w/v) non-fat milk and incubated with the primary antibodies. Sources of primary antibodies were: anti-MDR1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA); anti-MRP, anti-LRP, anti-(phosphor)-CaM-KII, anti-(phosphor)-CaM-KIV (all from Bioworld Technology, Louis Park, MN, USA); anti-GAPDH (Cwbio, China); and anti-(phosphor)-Raf, anti-(phosphor)-MEK1/2, anti-(phosphor)-ERK1/2 (all from Signalway Antibody, USA).
Immunohistochemistry
Formalin-fixed paraffin-embedded tissue sections were deparaffinized in xylene, rehydrated through graded ethanol and then boiled for 10 min in citrate buffer (10 mM, pH 6.0) for antigen retrieval. Endogenous peroxidase activity was suppressed by exposure to 3% hydrogen peroxide for 10 min. Slides were then blocked with 5% BSA (bovine serum albumin; Boster Bioengineering, Wuhan, China), incubated with diluted MDR, MRP, LRP, p-CaM-KII, p-CaM-KIV primary antibody for 1 h at 37°C and then incubated with secondary antibody for 20 min. Slides were visualized with DAB (3,3'-diaminobenzidine) and counterstained with hematoxylin for microscopic examination.
TUNEL staining
Apoptotic cells were visualized by using the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay. The TUNEL procedure was performed by using an in situ cell death detection kit (Boster, China) according to the manufacturer's instructions.
RNA extraction and real-time RT-PCR
Total RNA was extracted from cells and tissues using TRIzol Reagent (Life technologies, Carlsbad, CA, USA), and equal amount of RNA was used for real-time RT-PCR analyses. The cDNAs were synthesized by using a reverse transcription kit according to the manufacturer's instruction (Vazyme, Nanjing, China). β-actin was used as an internal control. The sequences of specific primers are listed in Table S1.
Statistical analysis
The experimental values were expressed as mean ± SD. and the significance of differences was analyzed by ANOVA for t-test. All reported P-values are 2 tailed, and P<0.05 were considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
1005530_Supplementary_Materials.zip
Download Zip (2.1 MB)Funding
This work was supported by the Major Research Plan of the National Natural Science Foundation of China (Grant no. 91129718), the National Natural Science Foundation of China (Grant no. 81272481), Jiangsu Province's Project of Scientific and Technological Innovation and Achievements Transformation (Grant no.BL2012055), Jiangsu Province for Outstanding Sci-tech Innovation Team in Colleges and Universities(Grant no. SJK2013–10), Jiangsu Province's Outstanding Medical Academic Leader and Sci-tech Innovation Team Program (Grant no. LJ201117),Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Roodhart JM, Daenen LG, Stigter EC, Prins HJ, Gerrits J, Houthuijzen JM, Gerritsen MG, Schipper HS, Backer MJ, van Amersfoort M, et al. Mesenchymal stem cells induce resistance to chemotherapy through the release of platinum-induced fatty acids. Cancer Cell 2011; 20(3):370-83; PMID:21907927; http://dx.doi.org/10.1016/j.ccr.2011.08.010
- Cukierman E, Bassi DE. The mesenchymal tumor microenvironment: a drug-resistant niche. Cell Adh Migr 2012; 6(3):285-96; PMID:22568991; http://dx.doi.org/10.4161/cam.20210
- Zhu W, Huang L, Li Y, Qian H, Shan X, Yan Y, Mao F, Wu X, Xu WR. Mesenchymal stem cell-secreted soluble signaling molecules potentiate tumor growth. Cell Cycle 2011; 10(18):3198-207; PMID:21900753; http://dx.doi.org/10.4161/cc.10.18.17638
- Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y, Xu X, Wang M, Qian H, Xu W. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett 2012; 315(1):28-37; PMID:22055459; http://dx.doi.org/10.1016/j.canlet.2011.10.002
- Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev 2013; 22(6):845-54; PMID:23002959; http://dx.doi.org/10.1089/scd.2012.0395
- Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther 2013 Apr; 4(2):34; http://dx.doi.org/10.1186/scrt194
- Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev 2013 Dec; 32(3-4): 623-42; http://dx.doi.org/10.1007/s10555-013-9441-9
- Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013; 501(7467):346-54; PMID:24048067; http://dx.doi.org/10.1038/nature12626
- Corcoran C, Rani S, O'Brien K, O'Neill A, Prencipe M, Sheikh R, Webb G, McDermott R, Watson W, Crown J, et al. Docetaxelresistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS One 2012; 7(12):e50999; http://dx.doi.org/10.1371/journal.pone.0050999
- Xiao X, Yu S, Li S, Wu J, Ma R, Cao H, Zhu Y, Feng J. Exosomes: decreased sensitivity of lung cancer A549 cells to cisplatin. PLoS One 2014; 9(2):e89534; PMID:24586853; http://dx.doi.org/10.1371/journal.pone.0089534
- Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res 2003; 63(15):4331-7; PMID:12907600
- Sun J, Song Y, Wang Z, Chen X, Gao P, Xu Y, Zhou B, Xu H. Clinical significance of palliative gastrectomy on the survival of patients with incurable advanced gastric cancer: a systematic review and meta-analysis. BMC Cancer 2013; 13:577; PMID:24304886; http://dx.doi.org/10.1186/1471-2407-13-577
- Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J, Savastano B, Mabilia A, et al. Treatment of gastric cancer. World J Gastroenterol 2014; 20(7):1635-49; PMID:24587643; http://dx.doi.org/10.3748/wjg.v20.i7.1635
- Kwee RM, Kwee TC. Role of imaging in predicting response to neoadjuvant chemotherapy in gastric cancer. World J Gastroenterol 2014; 20(7):1650-56; PMID:24587644; http://dx.doi.org/10.3748/wjg.v20.i7.1650
- Chien J, Kuang R, Landen C, Shridhar V. Platinum-sensitive recurrence in ovarian cancer: the role of tumor microenvironment. Front Oncol 2013; 3:251; PMID:24069583; http://dx.doi.org/10.3389/fonc.2013.00251
- Mao Y, Keller ET, Garfield DH, Shen K, Wang J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev 2013; 32(1-2):303-15; PMID:23114846; http://dx.doi.org/10.1007/s10555-012-9415-3
- Qiao C, Xu W, Zhu W, Hu J, Qian H, Yin Q, Jiang R, Yan Y, Mao F, Yang H, et al. Human mesenchymal stem cells isolated from the umbilical cord. Cell Biol Int 2008; 32(1):8-15; PMID:17904875; http://dx.doi.org/10.1016/j.cellbi.2007.08.002
- Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 2013 Oct; 13(10):714-26; http://dx.doi.org/10.1038/nrc3599
- Riganti C, Doublier S, Viarisio D, Miraglia E, Pescarmona G, Ghigo D, Bosia A. Artemisinin induces doxorubicin resistance in human colon cancer cells via calcium-dependent activation of HIF-1aand P-glycoprotein overexpression. Br J Pharmacol 2009; 156(7):1054-66; PMID:19298255; http://dx.doi.org/10.1111/j.1476-5381.2009.00117.x
- Guan J, Chen J. Mesenchymal stem cells in the tumor microenvironment. Biomed Rep 2013; 1(4):517-21; PMID:24648978
- Houthuijzen JM, Daenen LG, Roodhart JM, Voest EE. The role of mesenchymal stem cells in anti-cancer drug resistance and tumor progression. Br J Cancer 2012; 106(12):1901-6; PMID:22596239; http://dx.doi.org/10.1038/bjc.2012.201
- Sanchez CG, Penfornis P, Oskowitz AZ, Boonjindasup AG, Cai DZ, Dhule SS, Rowan BG, Kelekar A, Krause DS, Pochampally RR. Activation of autophagy in mesenchymal stem cells provides tumor stromal support. Carcinogenesis 2011; 32:964-72; PMID:21317300; http://dx.doi.org/10.1093/carcin/bgr029
- De Boeck A, Pauwels P, Hensen K, Rummens JL, Westbroek W, Hendrix A, Maynard D, Denys H, Lambein K, Braems G, et al. Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression through paracrine neuregulin 1/HER3 signalling. Gut 2013; 62:550-60; PMID:22535374; http://dx.doi.org/10.1136/gutjnl-2011-301393
- Vianello F, Villanova F, Tisato V, Lymperi S, Ho KK, Gomes AR, Marin D, Bonnet D, Apperley J, Lam EW, et al. Bone marrow mesenchymal stromal cells non-selectively protect chronic myeloid leukemia cells from imatinib-induced apoptosis via the CXCR4/CXCL12 axis. Haematologica 2010; 95(7):1081-9; PMID:20179085; http://dx.doi.org/10.3324/haematol.2009.017178
- Rodriguez-Mora O, LaHair MM, Howe CJ, McCubrey JA, Franklin RA. Calcium/calmodulin-dependent protein kinases as potential targets in cancer therapy. Expert Opin Ther Targets 2005; 9(4):791-808; PMID:16083343; http://dx.doi.org/10.1517/14728222.9.4.791
- McCubrey JA, Abrams SL, Stadelman K, Chappell WH, Lahair M, Ferland RA, Steelman LS. Targeting signal transduction pathways to eliminate chemotherapeutic drug resistance and cancer stem cells. Adv Enzyme Regul 2010; 50(1):285-307; PMID:19895837; http://dx.doi.org/10.1016/j.advenzreg.2009.10.016
- Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, Azzam DJ, Victor CTS, Wiemann BZ, Ishwaran H, et al. Exosome Transfer from Stromal to Breast Cancer Cells Regulates Therapy Resistance Pathways. Cell 2014; 159(3):499-513; PMID:25417103; http://dx.doi.org/10.1016/j.cell.2014.09.051
- Wang J, Hendrix A, Hernot S, Lemaire M, Bruyne ED, Valckenborgh EV, Lahoutte T, Wever OD, Vanderkerken K, Menu E. Bone marrow stromal cell-derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood 2014; 124(4):555-66; PMID:24928860; http://dx.doi.org/10.1182/blood-2014-03-562439
- Shelke GV, Lässer C, Gho YS, Lötvall J. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J Extracell Vesicles 2014; 3:24783.
