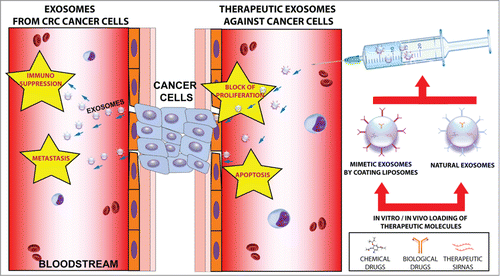
Exosomes are membrane-bound vesi-cles (50–100 nm) that are assembled and released by possibly all eukaryotic cells.Citation1 Their morpho-structural features and specific membrane protein profiles have been exploited to distinguish them from other vesicles.Citation1 Besides mRNAs, proteins and lipids, exosomes molecular cargoes also comprise non-coding RNAs (ncRNAs) as miRNAs: intercellular transfer of exosomes would determine their horizontal transfer as well.Citation2 Recently, researchers from Italy, Germany, Sweden reported that in vitro treatment of CRC cells with Cetuximab (an anti-EGFR antibody, which competes with physiological EGFR ligands and blocks downstream proliferative signaling) significantly increased the steady-state asimmetry of miRNAs and proteins distribution between whole CRC cells and their exosomes.Citation3 Exosomes from untreated CRC cells were highly enriched in miRNAs and proteins involved in blocking proliferation and immune escape: this was confirmed by functional data, which showed decreased proliferation of Caco-2 cells after transfection with exosomes from HCT-116 cells and viceversa (, left panel).Citation3 On the contrary, exosomes from Cetuximab-treated CRC cells were enriched in miRNAs and proteins involved in immune response, inflammation activation, cancer progression: in fact, viability of CRC cells increased after incubation with exosomes from treated cells (Fig. 1, left panel). To explain these data, we propose that selection and targeting of exosomal cargo (RNAs and proteins) is performed by a cellular machinery, which is comprised of proteins and ncRNAs regulating their expression; activity of this apparatus is regulated by Cetuximab (and possibly other drugs).Citation3 This Exosomal Cargo Selecting and Transporting Apparatus (ECSTA) could comprise the following components or their functional analogs: (i) proteins from ESCRT (Endosomal Sorting Complex Required for Transport) and others functionally associated, as Alix and TSC101;Citation4 (ii) Rab family members and other proteins involved in membrane trafficking, as GAPs and GEFs;Citation5 (iii) sortilin and functionally associated proteins, as TrkB and EGFR;Citation6 (iv) exosomal RNA-binding proteins (RBPs);Citation4 (v) miRNAs, long non-coding RNAs (lncRNAs), competing endogenous RNAs (ceRNAs), and other ncRNAs,Citation7 regulating synthesis, expression and activity of ECSTA protein members. Our data highlight questions that deserve to be experimentally explored. (a) Is Cetuximab-induced asimmetry also observed in vivo in laboratory animals and patients following treatment with Cetuximab ? If so, it should be possible to imagine ways to exploit this phenomenon for clinical therapy and biotechnological applications: for instance, procedures for exosomes removal could be developed and added to traditional drug treatments in CRC patients, when appropriate. (b) Do exosomes carry other ncRNAs, as lncRNAs, circular RNAs (circRNAs), and competing endogenous RNAs (ceRNAs)? (c) Does Cetuximab-induced asimmetry affect them as well? (d) What are the molecular mechanisms through which Cetuximab alters exosomal cargoes? (e) Beyond Cetuximab, are there other molecules capable of producing analogous effects and potentially useful for therapeutic and biotechnological applications? (f) How much does the biomolecular structure of exosomes (membranes and cargoes) depend on the specific phenotype of cells and their physiological state, including ECSTA activity? How much heterogeneous can be structure and cargoes of exosomes synthesized by the same cell type, when exposed to different cues in different biological niches? Incidentally, these 2 latter questions suggest that exosomal cargoes should be exploited with caution for diagnostic purposes. For translational applications, it will be important to improve our knowledge on the mechanics of exosomes interaction with target cells and its biomolecular effects. Exosomes-based drug delivery provides several attractive advantages: (a) low immunogenicity, when self-derived exosomes are used; (b) exosomes great stability in blood; (c) specific cargoes delivery to target cells (Fig. 1, right panel). RNAs and proteins with therapeutic properties can be engineered to be incorporated into natural exosomes: (1) RNA molecules can be modified by adding short sequence motifs, which would target them to exosomes; (2) proteins may be modified by adding domains from known exosomal proteins (e.g., the C1C2 domain of lactadherin). By engineering donor cells to express transmembrane domains of exosomal proteins fused to specific peptides (e.g., ligands of specific membrane receptors), it should be possible to address therapeutic exosomes to specific target cells. Finally, our present knowledge on nanovesicle molecular biology can be effectively exploited for developing artificial exosomes starting by liposomes. Liposome composition (i.e., sphingomyelins, cholesterol, proteins) can be modified ad hoc to mimic exosomal features, which could enhance uptake and delivery of cargo molecules to target cells. Switching to a wider scientific horizon, it will be interesting to verify whether exosomes could be used to horizontally transfer important biologic features among phenotypically different cells from the same organism, the same kingdom, or even across different life kingdoms. A final warning: for the exosome field to hold to the great expectations surrounding it, it will be critically important to homogenize technological methodology and nomenclature.
Figure 1. Exosomes in cancer: biological role and therapeutic applications. (Left panel) Exosomes from CRC cancer cells deliver metastatic signals to other cells and affect immune system. (Right panel) Therapeutic exosomes (both natural and mimetic exosomes) can be loaded with therapeutic molecules and injected into the blood as specific nanovectors of anticancer signals.
References
- Taylor DD, Gercel-Taylor C. Front Genet 2013 Jul 30; 4:142; PMID:23908664; http://dx.doi.org/10.3389/fgene.2013.00142
- Valadi H,et al. Nat Cell Biol 2007 Jun; 9(6):654-9; PMID:17486113; http://dx.doi.org/10.1038/ncb1596
- Ragusa M, et al. Oncoscience 2014; 1:132-57.
- Villarroya-Beltri C,et al. Semin Cancer Biol 2014 Oct; 28C:3-13; PMID: 24769058; http://dx.doi.org/10.1016/j.semcancer.2014.04.009
- Barr F, Lambright DG. Curr Opin Cell Biol 2010 Aug; 22(4):461-70; PMID:20466531; http://dx.doi.org/10.1016/j.ceb.2010.04.007
- Wilson CM,et al. J Cell Sci 2014, Sep 15; 127(Pt 18):3983-97; PMID: 25037567; http://dx.doi.org/10.1242/jcs.149336
- Tay Y,et al. Nature 2014 Jan 16; 505(7483):344-52; PMID: 24429633; http://dx.doi.org/10.1038/nature12986
