Abstract
Somatic cells can be reprogrammed into embryonic stem cells (ESCs) by nuclear transfer (NT-ESCs), or into induced pluripotent stem cells (iPSCs) by the “Yamanaka method.” However, recent studies have indicated that mouse and human iPSCs are prone to epigenetic and transcriptional aberrations, and that NT-ESCs correspond more closely to ESCs derived from in vitro fertilized embryos than iPSCs. In addition, the procedure of NT-ESCs does not involve gene modification. Demonstration of generation of NT-ESCs using an easily-accessible source of adult cell types would be very important. Adipose tissue is a source of readily accessible donor cells and can be isolated from both males and females at different ages. Here we report that NT-ESCs can be generated from adipose tissue-derived cells (ADCs). At morphological, mRNA and protein levels, these NT-ESCs show classic ESC colonies, exhibit alkaline phosphatase (AP) activity, and display normal diploid karyotypes. Importantly, these cells express pluripotent markers including Oct4, Sox2, Nanog and SSEA-1. Furthermore, they can differentiate in vivo into various types of cells from 3 germinal layers by teratoma formation assays. This study demonstrates for the first time that ESCs can be generated from the adipose tissue by somatic cell nuclear transfer (SCNT) and suggests that ADCs can be a new donor-cell type for potential therapeutic cloning.
Introduction
Somatic cell nuclear transfer (SCNT) and transcription factor transduction are both mature reprogramming methods for generating patient-matched nuclear transfer embryonic stem cells (NT-ESCs) or induced pluripotent stem cells (iPSCs) for studies of disease mechanisms and for developing specific therapies. However, it is now generally believed that iPSCs show a high frequency of genetic and epigenetic abnormalities, such as sub-chromosomal duplications and deletions detected as copy number variations,Citation1,2 protein-coding mutations3 and defects in DNA methylation and gene expression at regions subject to imprinting and X-chromosome inactivation.Citation4-7 Recently, human NT-ESCs have been obtained not only from normal fetalCitation8 and adult cellsCitation9 but also from patient skin cells with type 1 diabetes.Citation10 In addition, several recent studies indicated that not only mouse and human NT-ESCs are more similar to ESCs derived from in vitro fertilized embryos than iPSCs,Citation11-13 but also SCNT-mediated reprogramming mitigates telomere dysfunction and mitochondrial defects to a greater extent than iPSC-based reprogramming.Citation14 Furthermore, the procedure of reprogramming somatic cells to NT-ESCs does not involve gene modification. Therefore, somatic cells can be more faithfully reprogrammed to pluripotency by SCNT and are more desirable for cell replacement therapies. Toward that direction, demonstration of generation of NT-ESCs using additional easily-accessible source of adult cell types would be very important.
As compared to other adult somatic cells, such as foreskin fibroblasts or bone marrow-derived cells, adipose tissue is an attractive source of easily-accessible adult candidate cells for cell reprogramming and can be isolated from both males and females at different ages, as obesity is currently a common problem and liposuction is a relatively safe and popular procedure. Both the human and the mouse ADCs have been successfully reprogrammed into iPSCs by the “Yamanaka factors”.Citation15,16 In addition, we have recently reported that cloned mice can be produced from adipose tissue-derived lineage negative (Lin−) cells and revealed that these cells possess good genetic stability.Citation17 However, whether the ADCs can be reprogrammed into NT-ESCs via SCNT has so far not been demonstrated.
In this study, we first purified and characterized the Lin− cells which expressed expected specific mesenchymal stem cell (MSC) markers and possessed osteogenic, chondrogenic and adipogenic differentiation potential. We showed clearly that by performing SCNT, cloned blastocysts could be efficiently obtained and NT-ESCs were successfully generated. These NT-ESCs showed classic ESC colonies, exhibited alkaline phosphatase (AP) activity, and displayed normal diploid karyotypes. RT-PCR and immunostaining analyses revealed that they expressed pluripotent markers including Oct4, Sox2, Nanog and SSEA-1. In addition, the Lin− cells-derived NT-ESCs displayed the ability to differentiate into 3 germinal layer cells in vivo by a teratoma formation assay. Therefore the adiposed-derived cells can be a new alternative adult somatic cell type for therapeutic cloning.
Results
Isolation and characterization of Lin− cells from adipose tissue
Adipose tissue is composed of heterogeneous cell populations, containing multipotent procusor cells and differentiated cells. On the basis of cell lineage markers, adipose tissue-derived cells (ADCs) can be separated into a lineage-positive (Lin+) cell population that includes endothelial cells (CD31+), erythrocytes (Ter119+), haematopoietic cells (CD45+), and a lineage-negative (Lin−) cell population which represents the remaining cells primarily composed of precusor cells that are enriched mesenchymal stem cells (MSCs).Citation18 Previously, we have used Lin− cells to successfully generate cloned mice via SCNT.Citation17 We found that the rate of in vitro development of reconstructed oocytes into blastocysts was significantly higher from Lin− cells than from Lin+ cells. In addition, while Lin− cells can derive cloned mice via SCNT, the Lin+ cells fail to do so.Citation17 Therefore, in the present study we used Lin− cells for generation of NT-ESCs.
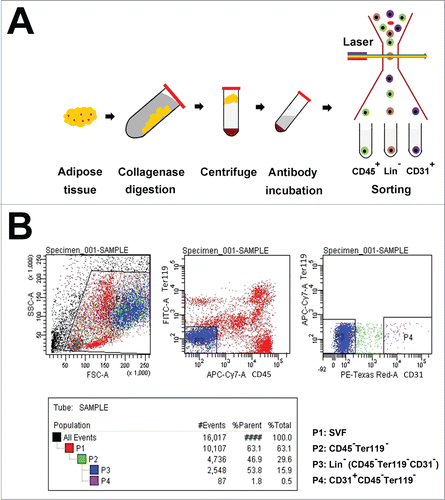
Adult male B6D2F1 mice were used for the isolation of Lin− cells by fluorescence-activated cell sorting (FACS). First, the adipose tissue collected from inguinal fat pads was digested with collagenase and then centrifuged. The supernatant fractions contained mature adipocytes, and the bottom consisted of the stromal vascular fraction (SVF) (). The SVF was re-suspended and incubated with fluorochrome-conjugated antibodies against various cell-surface markers expressed by Lin+ cells, including CD31, CD45, and Ter119, and then sorted by FACS (Fig. 1B). Lin− cells were separated by removing Lin+ cells, based on the staining for CD31, Ter119, and CD45, respectively ().
Figure 1. Isolation and characterization of Lin− cells from the adipose tissue. (A) Schematic drawings for isolating Lin− cells from the adipose tissue. After adipose tissue was digested with collagenase and centrifuged, the supernatant fractions were mature adipocytes, and the wine-colored bottom masses were SVF. The SVF were incubated with primary antibodies including CD45-APC-Cy7 and Ter119-FITC or CD31-Biotin and PE-Texas Red. The stained cells were then sorted by FACS and Lin− cells were obtained. (B) Sorting Lin− cells by FACS. P1 zone showed the SVF. P2 zone represented Ter119+ and CD45− cells, which were removed from P1 zone. P3 zone displayed Lin− cells which were obtained by discarding CD31+ cells from the P2 zone.
To confirm that the sorted Lin− cell population is purified mesenchymal stem cells (MSCs), we used several specific surface markers to characterize these cells, including MSC marker - CD140a, CD140b, CD146, CD105, CD13, CD73, CD44, Sca-1; mouse MSC marker - CD34; adipocyte progenitor marker - CD24; haematopoietic cell marker - CD45 and mouse pluripotent stem cell marker - SSEA-1. As expected, flow cytometry analyses revealed that these cells highly expressed MSC markers, including CD140a (96%), CD140b (92.4%), CD146 (91.2%), CD105 (72.9%), CD13 (97.1%), CD73 (76.2%), CD44 (90.4%), CD34 (90.7%) and Sca-1 (99%), but were negative for CD45 and SSEA-1 ().
Figure 2. Characterization of Lin− cells. (A) Flow cytometry analysis indicated that Lin− cells highly expressed MSC markers, including CD140a (96%), CD140b (92.4%), CD146 (91.2%), CD105 (72.9%), CD13 (97.1%), CD73 (76.2%), CD44 (90.4%), CD34 (90.7%) and Sca-1 (99%), but were negative for CD45 and SSEA-1. (B) Alizarin Red staining revealed Lin− cells could differentiate into osteoblasts; Alcian Blue staining showed that Lin− cells could differentiate into chondrocytes; Oil Red O staining indicated that Lin− cells had the capability to differentiate into adipocytes.
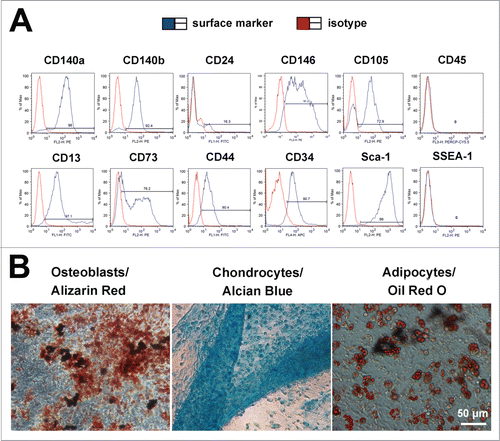
Furthermore, to verify the lineage differentiation potentials of the Lin− cells in vitro, we used specific inducing media to induce them to differentiate down osteogenic, chondrogenic, adipogenic pathways in vitro. Osteogenic differentiation was assessed by Alizarin Red staining. Chondrogenic differentiation was assessed by Alcian Blue staining. Adipogenic differentiation was assessed by Oil Red O staining. As shown in Figure 2B, the Lin− cells could efficiently differentiate into osteoblasts, chondrocytes, and adipocytes. These data support that the purified Lin− cells are MSCs.
Development of cloned embryos from Lin− cells and derivation of NT-ESCs
In order to obtain NT-ESCs from the ADCs, we made use of our previously established method, that is, development of cloned blastocysts via NT. The expanded or hatched blastocysts were treated with acid Tyrode's solution to remove the zona pellucida (ZP), and each blastocyst was then transferred into individual wells in a 96-well plate seeded with feeders in ESC medium supplemented with 20% KnockOut™ Serum Replacement (KSR) after 5–7 d in culture, proliferating outgrowths were trypsinized and re-plated on the feeder layer in fresh ESC medium until stable cell lines grew out (as schematically represented in ).
Figure 3. Derivation of NT-ESCs. (A) Strategy for derivation of NT-ESCs. By injecting the nuclei of Lin− cells into enucleated oocytes, reconstructed oocytes were obtained. When the cloned embryos developed to the stage of expanded or hatched blastocysts, the ZP was removed and each of the blastocysts was transferred into individual wells in a 96-well plate. After 5–7 d in culture, proliferating, outgrown colonies were trypsinized and re-plated on the feeder layer in fresh ES medium until stable cell lines grew out. (B) Intact oocytes. The spindle site is pointed by an arrow. (C) Enucleated oocyte. (D and E) The procedure of injecting the nuclei of Lin− cells into enucleated oocytes. (F) 2-cell embryo. (G) 4-cell embryo. (H) Morulae embryo. (I) Expanded blastocyst. (J) Proliferating outgrowths from one hatched blastocyst on the feeders. (K) Lin− cells-derived NT-ESCs. (L) AP positive staining in the amplified colonies. (M) Karyotype of NT-ESCs showing the normal diploid complement of 40 chromosomes.
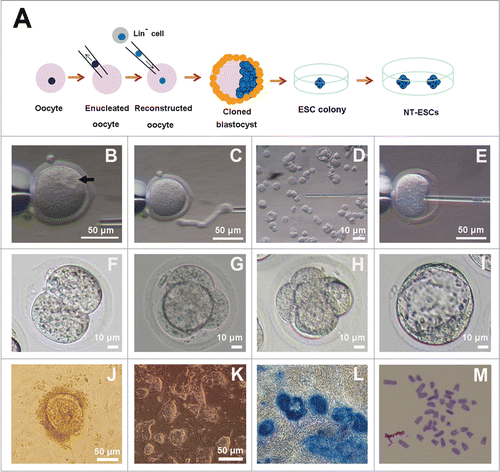
In this study, by injecting the nuclei of the purified Lin− cells into enucleated oocytes, we obtained 60 reconstructed embryos (). Of them, 57 embryos developed to 2-cell stage (95%, % of 1-cell), and 28 embryos developed to the blastocyst stage (46.7%, % of 1-cell) (). Then we placed 4 expanded or hatched blastocysts in which the ZP was removed on feeders in ESC medium supplemented with 20% KSR, 1,500 U/ml leukemia inhibitory factor (LIF), 3 M CHIR99021, and 1 M PD0325901. After 6 additional days in culture, one colony was generated from one of 4 blastocysts (). The proliferating outgrowths were typsinized and seeded to a 96-well plate with a fresh feeder layer in fresh medium. When the colony was passaged from 48-well plates to 6-well plates with feeder cells and then to 6-well plates for routine culture, and the cells displayed classic ESC colony morphology (). In addition, these amplified colonies exhibited AP activities as evidenced by the AP kit staining (). In this way, we successfully established one NT-ES cell line (25%) which could be maintained for more than 20 passages in cultures or successfully cropreserved and thawed to regrow. The efficiency of establishment of NT-ESCs is shown in .
Table 1. Efficiency of generation of NT-ESCs from Lin- cells
To determine chromosomal stability of NT-ESCs, we did the karyotype analysis for both early and late passaged cells. As expected, the numbers of chromosomes were all 40 without any noticeable abornormality based on the analysis of 10 metaphase spreads. An example of the karotyping for the cells at passage 20 is shown in . These results suggested that NT-ESCs from adipose tissue displayed normal diploid karyotypes and maintained chromosomal stabilility in vitro.
Pluripotency of NT-ESCs
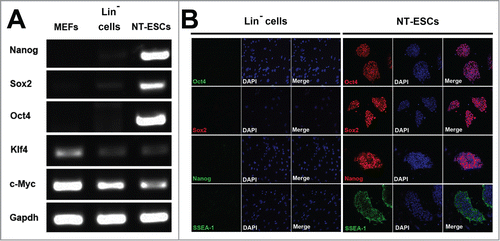
To verify whether the NT-ESCs have pluripotency, we performed RT-PCR analysis to measure expression levels of pluripotent genes including Nanog, Sox2, Oct4, Klf4 and c-Myc. As shown in , while the Lin− cell-derived NT-ESCs expressed these 5 pluripotent genes, the control cells, either the naïve Lin− cells without NT or the MEFs, did not express Nanog, Sox2, and Oct4. As expected, the latter 2 cells express Klf4 and c-Myc. Consistently, immunostaining also confirmed that the Lin− cell-derived NT-ESCS also expressed Oct4, Sox2, Nanog and SSEA-1 proteins (). In sharp contrast, the naïve Lin− cells without NT did not express these 4 markers (). Thus, the Lin− cells-derived NT-ESCs express the essential pluripotent markers at both mRNA and protein levels in vitro.
Figure 4. Pluripotency of NT-ESCs in vitro. (A) RT-PCR analysis showed NT-ESCs expressed Nanog, Sox2, Oct4, Klf4 and c-Myc. The naïve Lin− cells without NT or the MEFs did not express Nanog, Sox2, and Oct4, but the 2 cells express Klf4 and c-Myc. (B) Immunostaining displayed that NT-ESCs also expressed Oct4, Sox2, Nanog and SSEA-1 proteins. In sharp contrast, the naïve Lin− cells without NT did not express these 4 markers.
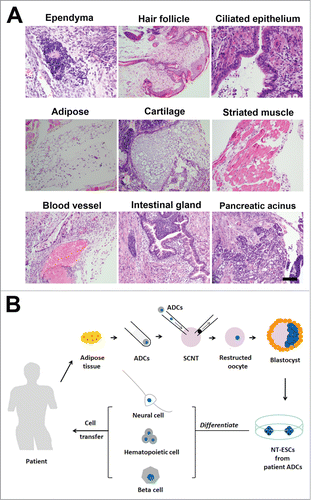
To further determine the pluripotency of the NT-ESCs in vivo, we performed teratoma formation assays. After the NT-ESCs were injected under the flank region of the skin in BALB/c nude mice, we indeed observed formation of teratoma including 3 germinal layer cells. As shown in , at 3 weeks following cell transplantation under the skin, the NT-ESCs differentiated into epidermal, mesodermal and endodermal layer cells. These epidermal layer cells included ependymal cells in brain ventricle-like tissue, hair follicle in skin-like tissue and ciliated epithelial cells in respiratory epithelial-like tissue. The mesodermal layer cells contained adipocytes in adipose-like tissue, chondrocytes in cartilage-like tissue, myocytes in striated muscle-like tissue. The endodermal layer cells included endothelial cells in blood vessels, glandular cells in intestinal glands and islet cells in pancreatic glands.
Figure 5. Pluripotency of the NT-ESCs in vivo. (A) Teratoma formation assays indicated NT-ESCs injected under the skin of nude mice could differentiate into epidermal, mesodermal and endodermal layer cells. These epidermal layer cells included ependymal cells in brain ventricle-like tissue, hair follicle in skin-like tissue and ciliated epithelial cells in respiratory epithelial-like tissue. The mesodermal layer cells contained adipocytes in adipose-like tissue, chondrocytes in cartilage-like tissue, myocytes in striated muscle-like tissue. The endodermal layer cells included endothelial cells in blood vessels, glandular cells in intestinal glands and islet cells in pancreatic glands. (B) Schematic drawing of generation of patient adipose tissue-derived NT-ESCs for therapeutic cloning in the future. By SCNT, patient ADCs can be reprogrammed into NT-ESCs, which can then be induced to any desired, specific type of cells such as neural cells, haematopoietic cells and β cells prior to transplantion in vivo for treatment of various types of diseases. Scale bar in (A), 100 micrometers.
Discussion
In this study, we demonstrate for the first time that the adipose cells can be successfully reprogrammed into ESCs by SCNT. At morphological, mRNA and protein levels, these NT-ESCs display classic ESC colonies, exhibit AP activity, display normal diploid karyotypes, and express pluripotent markers including Oct4, Sox2, Nanog and SSEA-1. Furthermore, they can differentiate in vivo into various types of cells from 3 germinal layers by teratoma formation assays. Therefore the ESCs can be generated from adipose tissue-derived cells via SCNT.
Although recent reports indicate that iPSCs can be generated from both human and mouse ADCs effectively using transcription factor-based reprogramming,Citation15,16 iPSCs show a high frequency of genetic and epigenetic abnormalities.Citation1-7 On the other hand, accumulating evidence indicate that mouse and human NT-ESCs are more similar to classic ESCsCitation11-13 and display much less telomere dysfunction and mitochondrial defects than iPSCs.Citation14 In addition, no gene modification is involved during the reprogramming of somatic cells to pluripotent stem cells by SCNT. Thus, NT-ESCs are more desirable for cell replacement therapies. Demonstration of generation of NT-ESCs from the adipose tissue provides a new donor-cell type for potential therapeutic cloning.
It is noted that several studies have recently reported that human NT-ESCs can be obtained from the skin cells in healthy peopleCitation8,9 and patient with type 1 diabetes.Citation10 However, the acquisition of skin cells has certain disadvantages such as pain and scar formation. Especially for those suffering from diabetes, the procedure may cause infection. As compared to skin cells, ADCs have advantages including easy acess as obesity affects health and liposuction is a relatively safe and popular procedure. In addition, adipose-derived multipotent stem cells possess not only higher efficiencies of reprogramming,Citation16,17 but also stronger proliferation and differentiation capabilities in vitro than skin fibroblasts.Citation21,22 The dipose tissue from patients can provide virtually unlimited cells for reprogramming.
It should be pointed out that owing to the difficulty to obtain human oocytes, in this study we used mouse ADCs and oocytes to perform SCNT and generated NT-ESCs. Generation of NT-ESCs from the human adipose cells is warranted in future studies. Ideally, the patients-derived NT-ESCs can be differentiated into related, specific types of cells such as neural cells, haematopoietic cells and β cells prior to transplantion to treat the corresponding diseases ().
Materials and Methods
Animal use and care
Adult (8–12 weeks of age) B6D2F1 and male BALB/c nude mice (6–8 weeks of age) were used. All animal procedures were performed under the ethical guidelines of Shanghai Jiaotong University and the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
Sorting and surface marker analyses by flow cytometry
Cell sorting was performed according to our previous report.Citation18 Briefly, the mouse inguinal fat pads were excised into very small pieces and then were digested with 0.1% collagenase (Sigma) for 50 minutes at 37°C. After the cell suspensions were centrifuged, the pelleted SVF cells were suspended in 200 μl PBS and were incubated on ice for 30 min with antibodies including CD45-APC-Cy7 (eBioscience), Ter119-FITC (eBioscience) and CD31-Biotin and PE-Texas Red (BD Biosciences). The cells were sorted on a BD FACSAria II cell sorter.
For surface marker analyses, at first, each set of Lin− cells was divided into 12 aliquots. Each aliquot contained approximately 500,000 cells and was labeled with one of the following antibodies: CD140a-PE (eBioscience, Clone: APA5), CD140b-PE (eBioscience, Clone: APB5), CD24-FITC (eBioscience, Clone: 30-F1), CD146-PE (eBioscience, Clone: P1H12), CD105-PE (eBioscience, Clone: MJ7/18), CD45-Percp-Cy5.5 (eBioscience, FClone: 30-F11), CD13-FITC (BD PharMingen, Clone: R3-242), CD73-PE (eBioscience, Clone: eBioTY/11.8), CD44-FITC (eBioscience, Clone: IM7), CD34-APC (BioLegend, Clone: MEC14.7), Sca-1-PE (eBioscience, Clone: D7), SSEA-1-PE (eBioscience, Clone: eBioMC-480) Flow cytometry was conducted on a Becton Dickinson FACSCalibur analyzer.
Adipogenic, osteogenic and chondrogenic differentiation
Briefly, the cells were plated into 24-well plates in DMEM supplemented with 10% FBS, allowed to grow to confluence and then induced medium was added. Osteogenic differentiation medium contained 10% FBS DMEM, 0.1 μM dexamethasone (Sigma), 10 mM β-glycerophosphate (Sigma) and 50 μg/mL ascorbic acid-2-phosphate (Sigma). Differentiated osteoblasts, adipocytes and chondrocyteswere stained with Alizarin red, Oil red O staining and Alcian blue staining, respectively.
Nuclear transfer
Nuclear transfer was performed as we described previously.Citation17 Briefly, by superovulation, metaphase II-arrested oocytes were obtained from adult female B6D2F1 mice, which were then enucleated in a droplet of HCZB medium containing 5 μg/ml cytochalasin B (CB) using a blunt Piezo-driven pipette. After enucleation, the oocytes were maintained in CZB medium up to 2 hours before nucleus injection. The Lin− cells were aspirated in and out of the injection pipette to remove the cytoplasmic material and then injected into enucleated oocytes. The reconstructed oocytes were cultured in CZB medium for 1 hour and then activated for 5–6 hours in activation medium containing 10 mM SrCl2, 5 ng/ml trichostatin A (TSA) and 5 μg/ml CB. The reconstructed embryos were cultured in KSOM medium supplemented with 5 ng/ml TSA for another 3–4 hours and maintained in KSOM medium with amino acids at 37°C under 5% CO2 in air.
Derivation of NT-ESCs
Derivation of NT-ESCs was achieved as described previously.Citation12 Briefly, the cloned embryos from Lin− cells were cultured in KSOM medium to reach the blastocyst stage. Expanded or hatched blastocysts were treated with acid Tyrode's solution to remove the zona pellucida, and each blastocyst was transferred into per well in 96-well plate seeded with feeders in ESC medium with KSR. After 5–7 d in culture, proliferating outgrowths were trypsinized and replated on feeder layer in fresh ESC medium until stable cell lines grew out.
Karyotype analysis
Karyotyping was analyzed as we described previously.Citation19 NT-ESCs were cultured in ES medium with 0.375 μg/ml demecolcine (Sigma) for 13 hours. After removing medium, the cells were added 1 ml 0.8% sodium citrate, incubated 1 hours at room temperature (RT), then fixed in methanol: acetic acid (3:1 in volume) for 30 min, these cells were dropped onto precooling slides and stained with Giemsa (GIBCO) for 15 min. More than 10 metaphase spreads were analyzed.
RT-PCR
RT-PCR was performed according toour previous report.Citation17 Total RNA from the cells was extracted using the Absolutely RNA Nanoprep Kit (Stratagene). One microgram of total RNA was reverse transcribed using a First Strand cDNA Synthesis Kit (TOYOBO). PCR was performed for 30 cycles with an annealing temperature of 60ºC with Taq polymerase (Invitrogen), and PCR products were electrophoresed on 2% agarose gels. Primer sequences as follows:
Nanog Forward: AGGGTCTGCTACTGAGATGCTCTG
Reverse: CAACCACTGGTTTTTCTGCCACCG
Sox2: Forward: CACCATCCGGGATGAAAGTGAGAT
Reverse: ACCAGAAAATGTCGCTTTAGTTTC
Oct4 Forward: CTGAGGGCCAGGCAGGAGCACGAG
Reverse: CTGTAGGGAGGGCTTCGGGCACTT
Klf4 Forward: GTGCCCCGACTAACCGTTG
Reverse: GTCGTTGAACTCCTCGGTCT
c-Myc Forward: ATGCCCCTCAACGTGAACTTC
Reverse: CGCAACATAGGATGGAGAGCA
GAPDH: Forward: TGCCCAGAACATCATCCCT
Reverse: ATGCCTGCTTCACCACCTT
Cell immunostaining
Immunostaining was performed as previously described.Citation20 NT-ESCs were fixed in 4% paraformaldehyde solution for 10 min at RT. After being permeabilized using 0.1% Triton X-100 in PBS for 15 min at RT, The cells were blocked for 1 hour in 5% donkey serum in PBS. Then the cells were incubated with primary antibodies Oct4 (Cell signal), Nanog (Abcam), SSEA-1 (Millipore), and Sox2 (Abcam) overnight at 4°C. The cells were treated with a fluorescently coupled secondary antibody and then incubated for 1 hour at RT. The nuclei were stained with DAPI (Sigma) for 5 min at RT.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The study is supported by funds to W-Q Gao from the Chinese Ministry of Science and Technology (2012CB966800, 2013CB945600 and 2012CB967903), the National Natural Science Foundation of China (81130038 and 81372189), the Science and Technology Commission of Shanghai Municipality (Pujiang program), the Shanghai Education Committee Key Discipline and Specialty Foundation (J50208), the Shanghai Health Bureau Key Discipline and Specialty Foundation and the KC Wong foundation, and to Y Qin from the Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (2010KIP502).
References
- Hussein SM, Batada NN, Vuoristo S, Ching RW, Autio R, Närvä E, Ng S, Sourour M, Hämäläinen R, Olsson C, et al. Copy number variation and selection during reprogramming to pluripotency. Nature 2011; 471(7336):58-62; PMID:21368824; http://dx.doi.org/10.1038/nature09871
- Laurent LC, Ulitsky I, Slavin I, Tran H, Schork A, Morey R, Lynch C, Harness JV, Lee S, Barrero MJ, et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell 2011; 8(1):106-18; PMID:21211785; http://dx.doi.org/10.1016/j.stem.2010.12.003
- Ruiz S, Gore A, Li Z, Panopoulos AD, Montserrat N, Fung HL, Giorgetti A, Bilic J, Batchelder EM, Zaehres H, et al. Analysis of protein-coding mutations in hiPSCs and their possible role during somatic cell reprogramming. Nat Commun 2013; 4:1382; PMID:23340422; http://dx.doi.org/10.1038/ncomms2381
- Nazor KL, Altun G, Lynch C, Tran H, Harness JV, Slavin I, Garitaonandia I, Müller FJ, Wang YC, Boscolo FS, et al. Recurrent variations in DNA methylation in human pluripotent stem cells and their differentiated derivatives. Cell Stem Cell 2012; 10(5):620-34; PMID:22560082; http://dx.doi.org/10.1016/j.stem.2012.02.013
- Lister R, Pelizzola M, Kida YS, Hawkins RD, Nery JR, Hon G, Antosiewicz-Bourget J, O'Malley R, Castanon R, Klugman S, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature 2011; 471(7336):68-73.
- Ohi Y, Qin H, Hong C, Blouin L, Polo JM, Guo T, Qi Z, Downey SL, Manos PD, Rossi DJ, et al. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nat Cell Biol 2011; 13(5):541-49; PMID:21499256; http://dx.doi.org/10.1038/ncb2239
- Ruiz S1, Diep D, Gore A, Panopoulos AD, Montserrat N, Plongthongkum N, Kumar S, Fung HL, Giorgetti A, Bilic J, et al. Identification of a specific reprogramming-associated epigenetic signature in human induced pluripotent stem cells. Proc Natl Acad Sci USA 2012; 109(40):16196-201; PMID:22991473; http://dx.doi.org/10.1073/pnas.1202352109
- Tachibana M1, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, Ma H, Kang E, Fulati A, Lee HS, Sritanaudomchai H, et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell 2013; 153(6):1228-38; PMID:23683578; http://dx.doi.org/10.1016/j.cell.2013.05.006
- Chung YG, Eum JH, Lee JE, Shim SH, Sepilian V, Hong SW, Lee Y, Treff NR, Choi YH, Kimbrel EA, et al. Human somatic cell nuclear transfer using adult cells. Cell Stem Cell 2014; 14(6):777-80; PMID:24746675; http://dx.doi.org/10.1016/j.stem.2014.03.015
- Yamada M, Johannesson B, Sagi I, Burnett LC, Kort DH, Prosser RW, Paull D, Nestor MW, Freeby M, Greenberg E, et al. Human oocytes reprogram adult somatic nuclei of a type 1 diabetic to diploid pluripotent stem cells. Nature 2014; 510(7506):533-6; PMID:24776804; http://dx.doi.org/10.1038/nature13287
- Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010; 467(7313):285-90; PMID:20644535; http://dx.doi.org/10.1038/nature09342
- Jiang J, Ding G, Lin J, Zhang M, Shi L, Lv W, Yang H, Xiao H, Pei G, Li Y, et al. Different developmental potential of pluripotent stem cells generated by different reprogramming strategies. J Mol Cell Biol 2011; 3(3):197-9; PMID:21624975; http://dx.doi.org/10.1093/jmcb/mjr012
- Ma H, Morey R, O'Neil RC, He Y, Daughtry B, Schultz MD, Hariharan M, Nery JR, Castanon R5, Sabatini K, Mitalipov Set al. Abnormalities in human pluripotent cells due to reprogramming mechanisms. Nature 2014; 511(7508):177-83; PMID:25008523; http://dx.doi.org/10.1038/nature13551
- Le R, Kou Z, Jiang Y, Li M, Huang B, Liu W, Li H, Kou X, He W, Rudolph KL, et al. Enhanced telomere rejuvenation in pluripotent cells reprogrammed via nuclear transfer relative to induced pluripotent stem cells. Cell Stem Cell 2014; 14(1):27-39; PMID:24268696; http://dx.doi.org/10.1016/j.stem.2013.11.005
- Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, Jia F, Hu S, Cherry AM, Robbins RC, Longaker MT, et al. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci U S A 2009; 106(37):15720-5; PMID:19805220; http://dx.doi.org/10.1073/pnas.0908450106
- Sugii S, Kida Y, Kawamura T, Suzuki J, Vassena R, Yin YQ, Lutz MK, Berggren WT, Izpisúa Belmonte JC, Evans RM. Human and mouse adipose-derived cells support feeder-independent induction of pluripotent stem cells. Proc Natl Acad Sci U S A 2010; 107(8):3558-63; PMID:20133714; http://dx.doi.org/10.1073/pnas.0910172106
- Qin Y, Lin J, Zhou C, Yin Q, Xie Z, Zhang X, Liu XY, Gao W, Li J. Mice cloned from white adipose tissue-derived cells. J Mol Cell Biol 2013; 5(5):348-50; PMID:23757368; http://dx.doi.org/10.1093/jmcb/mjt019
- Qin Y, Zhou P, Zhou C, Li J, Gao WQ. The adipose-derived lineage-negative cells are enriched mesenchymal stem cells and promote limb ischemia recovery in mice. Stem Cells Dev 2014; 23(4):363-71; PMID:24083854; http://dx.doi.org/10.1089/scd.2013.0212
- Qin Y, Ji H, Wu Y, Liu H. Chromosomal instability of murine adipose tissue-derived mesenchymal stem cells in long-term culture and development of cloned embryos. Cloning Stem Cells 2009; 11(3):445-52; PMID:19594392; http://dx.doi.org/10.1089/clo.2009.0006
- Yang H, Shi L, Wang BA, Liang D, Zhong C, Liu W, Nie Y, Liu J, Zhao J, Gao X, et al. Generation of genetically modified mice by oocyte injection of androgenetic haploid embryonic stem cells. Cell 2012; 149(3):605-17; PMID:22541431; http://dx.doi.org/10.1016/j.cell.2012.04.002
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001; 7(2):211-28; PMID:11304456; http://dx.doi.org/10.1089/107632701300062859
- Rodeheffer MS1, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell 2008; 135(2):240-9; PMID:18835024; http://dx.doi.org/10.1016/j.cell.2008.09.036
