In 1889, Paget launched the ‘seed and soil’ hypothesis, which postulated that disseminated cancer cells—the ‘seeds’—can spread throughout the body but will preferentially metastasize to specific organs that provide congenial ‘soil’, the so-called metastatic niche.Citation1 For instance, breast cancer preferably metastasizes to the liver and the bone.Citation1 The transition from in situ to invasive breast cancer may show some likenesses with this ‘seed and soil’ hypothesis for metastatic breast cancer. Ductal carcinoma in situ (DCIS) is a clonal proliferation of transformed epithelial cells, confined to the ductal-lobular system without evidence for disruption of the basement membrane and invasion into the surrounding stroma.Citation2 DCIS is considered to be a non-obligate precursor of invasive ductal carcinoma. Invasion might occur when the surrounding breast stroma, i.e. the ‘soil’ for potentially invasive cancer cells, is prepared and cultivated by the pre-invasive lesion, like a farmer fertilizing his fields before seeding. The close reciprocal relation between stromal fibroblasts and transformed epithelial cells induces extensive changes in the microenvironment.Citation3 Stromal fibroblasts are converted into corruptive cancer-associated fibroblasts (CAFs) through cancer cell-induced modifications of fibroblast signaling pathways.Citation4 Hence CAFs contribute to the remodeling of the extracellular matrix (ECM), which is likely to be required for invasion since the normal breast stroma functions as a protective barrier.Citation3 Such an altered peritumoral stroma is not only presumed to play a role in breast cancer progression, but also in therapy response.Citation4
Alterations of the periductal stroma in DCIS are reflected in a myxoid stromal architecture, which is associated with an increased recurrence risk. The presence of myxoid periductal stroma strongly correlates with reduced periductal decorin expression in DCIS.Citation5 Decorin, a member of the small leucin-rich proteoglycan family, is abundantly present in the breast ECM and ‘decorates’ collagen. Decorin plays a major role in the assembly of collagen fibrils,Citation6 and reduced expression might contribute to the development of a myxoid stromal architecture. We attempted to clarify the pathogenesis of myxoid stroma and the role of decorin in this process, with an emphasis on the paracrine regulation of ECM protein expression. These studies revealed that transforming growth factor β1 (TGF-β1) and basic fibroblast growth factor (bFGF) are 2 growth factors capable of potently reducing decorin expression in CAFs. Simultaneously, both growth factors enhanced the expression of versican, biglycan and type I collagen in CAFs, albeit at different levels.Citation7 Despite having similar effects on the modulation of type I collagen and the aforementioned proteoglycans, TGF-β1 and bFGF differentially regulated the expression of α-smooth muscle actin (α-SMA), a hypothesized marker of CAFs. TGF-β1 caused a strong upregulation, whereas α-SMA was profoundly downregulated by bFGF.Citation7 This differential regulation might explain why the presence of myxoid stroma in DCIS is associated with stromal decorin expression but not with periductal α-SMA expression.Citation5
To explore whether breast cancer cell lines were able to induce similar alterations in ECM protein expression, CAFs were treated with cancer cell-secretome containing medium. Upon treatment with different cancer cell secretomes, a TGF-β1-like response was noted, in which CAFs presented downregulation of decorin expression and upregulation of α-SMA, type I collagen, biglycan and versican.Citation7 As a proof of concept, immunohistochemistry was performed on a series of 20 DCIS specimens. This analysis showed a trend toward periductal versican overexpression in DCIS with myxoid stroma, although there was no relation with stromal biglycan expression.Citation7 Comparable with Paget's ‘seed and soil’ hypothesis about metastatic niches for invasive breast cancers, we hypothesize that some pre-invasive DCIS lesions prepare the stroma for subsequent invasion. Transformed epithelial cells, provisionally confined to the ductal system, influence their neighboring stroma by secretion of growth factors (). Other mechanisms, such as the secretion of proteases, may further contribute to stromal remodeling by the degradation of ECM proteins that otherwise prevent invasion. Additionally, the differentiation of the myoepithelial cell layer is hampered and myoepithelial cells gradually disappear during breast cancer progression. Cancer-cell derived growth factors are likely to influence adjacent fibroblasts to prevent them from producing anti-invasive ECM proteins, and thus prevent them from acting hostile toward the pre-invasive lesion. Instead, pro-invasive growth factors turn fibroblasts into carcinoma-promoting allies that pave the path for invasion.
Figure 1. Ductal carcinoma in situ (DCIS) causes growth factor-induced transition of fibroblasts into cancer-associated fibroblasts (CAF). CAF-induced ECM remodeling turns sclerotic stroma into myxoid stroma. Decreased decorin expression and increased versican expression may contribute to this altered architecture. Alpha-smooth muscle actin (α-SMA) is differentially regulated by bFGF and TGF-β1. This CAF-induced altered ECM composition paves the path for invasion.
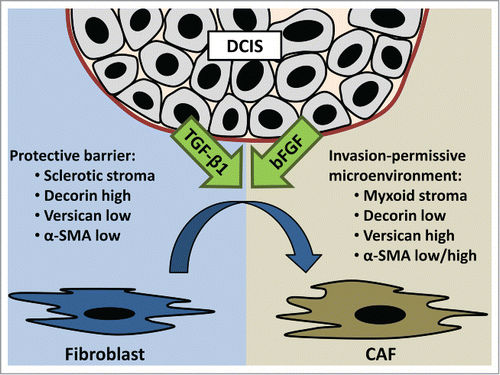
Our hypothesis was further supported by the anti-adhesive effects of decorin coatings on breast cancer cell adhesion.Citation7 The addition of type I collagen neutralized the inhibition of adhesion by decorin. Three-dimensional CAF-derived matrices were applied to mimic the peritumoral microenvironment.Citation3 Cancer cells presented significantly enhanced spreading when seeded on matrices derived from TGF-β1 treated CAFs.Citation7 Altogether these data indicate that preinvasive DCIS lesions might modulate the composition of the neighboring breast stroma through TGF-β1 release, to obtain an invasion-permissive and carcinoma-promoting microenvironment. This invasion-enabling microenvironment is probably reflected in myxoid stromal architecture. Breast cancer progression is likely to be accompanied by a tumor-induced imbalance in the ECM composition. Additional investigations on larger DCIS patient cohorts are warranted to elucidate the potential prognostic value of these stromal changes, such as increased stromal versican expression. Furthermore, TGF-β1 seems an attractive candidate for targeted therapy, and future research should explore the role of TGF-β1 blockade in cancer treatment.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Paget S. Lancet 1889; 133:571–3; http://dx.doi.org/10.1016/S0140-6736(00)49915-0
- Siziopikou KP. Arch Pathol Lab Med 2013; 137:462–6; PMID:23544935; http://dx.doi.org/10.5858/arpa.2012-0078-RA
- Castello-Cros R, Cukierman E. Methods Mol Biol 2009; 522:275-305; PMID:19247611; http://dx.doi.org/10.1007/978-1-59745-413-1_19
- De Wever O et al. Semin Cancer Biol 2014; 25:33-46; PMID:24406210; http://dx.doi.org/10.1016/j.semcancer.2013.12.009
- Van Bockstal M et al. Histopathology 2013; 63:520-33; PMID:23889174
- Danielson KG et al. J Cell Biol 1997; 136:729-43; PMID:9024701; http://dx.doi.org/10.1083/jcb.136.3.729
- Van Bockstal M, et al. Oncoscience 2014; 1:634-48; PMID:25593993
