Over the last 5 decades, the seminal success story in pediatric oncology has been the implementation of cytotoxic chemotherapy through protocol-driven care in the context of cooperative group trials.Citation1,2 These advances have resulted in the expectation that 4 out of 5 children presenting with cancer will be cured of their disease. However, we have reached a therapeutic plateau with conventional chemotherapy. Further refinements in conventional chemotherapies alone have not rendered dramatic additional benefits in pediatric cancers, and the unfortunate subset of patients with relapsed, refractory disease continue to have poor outcomes. Of particular concern are high-grade brain tumors, advanced bone sarcomas, soft-tissue sarcomas and high-risk acute lymphoblastic leukemia and acute myeloid leukemia; these diseases all urgently need novel drug for salvage treatment of children who have disease progression on current therapies.
Pediatric drug development has always been the stepchild in novel drug development.Citation3 Industry-funded trials of new molecules rarely include patients aged 18 y or younger, even in the era of targeted therapy. Additionally, the challenges that plague adult oncology clinical trial research are magnified in the pediatric oncology research community: namely, (1) the lack of funding, (2) lower patient numbers for clinical trial participation, (3) a limited number of trained clinical investigators, (4) limited awareness of the benefit of clinical trials, (5) regulatory/industry unfamiliarity or misunderstanding for fear of retribution and (6) limited advocacy.Citation3 Most predominant of all, the cost of new drug development remains high enough that these rare cancers rarely become an economically viable priority in industry. Furthermore, the complex biology of therapy-refractory childhood cancers is such that there is rarely a single targetable aberration such as those seen in some adult cancers, e.g., BRAF mutations in melanoma or EML4-ALK rearrangements in lung cancer.Citation4
Given these challenges, out-of-the-box ideas and collaboration with the developers of adult oncology drugs are needed to fast-track novel developmental therapeutics to thwart the subterfuges of these aggressive pediatric cancers. Because of these cancers’ complexity, single-agent phase I trials may not yield satisfactory results; tackling these tumors calls for combinations of active agents, together with innovative statistically sound clinical trial design, in which molecular profiling is incorporated much earlier in the disease course.Citation5
One issue in question is whether a separate phase I clinical trial is needed for pediatric patients when an agent has been studied in adults. A comparative analysis of pediatric and adult dose-finding targeted therapy trials revealed that separate pediatric phase I studies may not be required for all molecularly targeted agents.Citation6 This analysis found that critical data for pharmacokinetics, pharmacodynamics and even efficacy in pediatric patients could be extrapolated from adult clinical trials.Citation6 Many such drugs are often used off label once the drug is approved by the US. Food and Drug Administration (FDA) in adults, and there is no formal process and safety data for pediatric malignancies. Furthermore, a few investigator-initiated trials of combinations of US. FDA-approved agents allow patients younger than 18 y. A recent publication analyzed the outcomes of pediatric patients enrolled in phase I studies that were primarily designed for adult cancer patients but also allowed enrollment of patients under 18 y.Citation7 It was safe, feasible and viable to conduct early-phase clinical trials in younger patients based on adult protocols. In addition, the Royal Marsden Hospital (RMH) and MD Anderson Cancer Center (MDACC) prognostic scores, which have been validated in adult phase I trials, were validated in the pediatric patients.Citation7
How do we incorporate all extrapolated data into personalized oncology or precision medicine for the individual pediatric patient? In parallel with the surge in the repertoire of novel targeted and immune therapeutics, increased high-throughput molecular profiling technologies have brought major advancements in our understanding of the underpinnings of the biology of childhood cancers. These genetic vulnerabilities can now be exploited for targeted therapy.
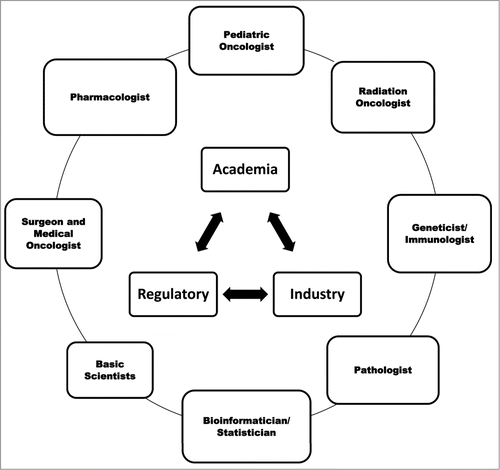
One translational concept that may be feasible in major academic centers in pediatric oncology is an N-of-1 precision-oncology design ().Citation5 Such a study may serve to build evidence, which may provide a springboard and a model for future drug development in pediatrics. Briefly, in this study design, tumor tissue from each patient with relapsed disease and high risk of death undergoes molecular profiling that includes whole genomic/proteomic and transcriptomic sequencing analyzed by robust bioinformatics algorithms. The results are discussed by a multidisciplinary “molecular tumor board.” The patient then receives molecularly matched therapy, which requires accessibility to a rich pipeline of drugs with data from adults that can be extrapolated for young patients. Patients are monitored for safety, efficacy, toxicity, and response; if acquired resistance develops, the tissues are biopsied and analyzed to identify further molecular targets to overcome resistance.
With more than 500 new agents, including targeted therapy, immunotherapy, antibody-drug conjugates, and radio-immunoconjugates, in different stages of clinical development, we are witnessing unprecedented advances in medical oncology. The prior model of drug development, wherein pediatric cancers are the last to undergo testing with novel agents only if an agent seems promising in adult cancers, may need to be revisited. Intractable aggressive-biology cancers continue to take the lives of young patients at the prime of their lives. Current strategies may not be quick enough. We need to accelerate precision medicine for the pediatric cancer patient. In addition to conducting prospective trials, the future role of cooperative groups should be to accelerate close collaboration among academia, industry, federal regulators, and third-party payers to “step up for the stepchild” and facilitate fast-tracking novel drugs for personalized therapy in pediatric oncology.
References
- Smith MA, Reaman GH. Pediat Clin N Am 2015; 62:301-12; PMID:25435124; http://dx.doi.org/10.1016/j.pcl.2014.09.018
- Pui CH, et al. Nat Rev Clin Oncol 2011; 8:540-9; PMID:21709698; http://dx.doi.org/10.1038/nrclinonc.2011.95
- Boklan J. Mol Cancer Therap 2006; 5:1905-8; PMID:16928809; http://dx.doi.org/10.1158/1535-7163.MCT-06-0179
- Egas-Bejar D, et al. Oncoscience 2014; 1:167-79; PMID:25126591.
- Subbiah V. Curr Oncol Rep 2014; 16:401; PMID:25030655; http://dx.doi.org/10.1007/s11912-014-0401-5
- Paoletti X, et al. Eur J Cancer 2013; 49:2392-402; PMID:23540589; http://dx.doi.org/10.1016/j.ejca.2013.02.028
- Corrales-Medina FF, et al. Oncoscience 2014;1(7):522-30; PMID: 25587555.