Cancer cell migration is a prerequisite for cancer metastasis. Cell migration involves multiple steps, including cell polarization/protrusion, adhesion and de-adhesion.Citation1 Thus, disturbance of any of these steps may intervene cancer metastasis.
The mammalian target of rapamycin (mTOR), a serine/threonine (Ser/Thr) kinase, is a central controller of cell growth/proliferation and survival.Citation2 While extensive data have highlighted the importance of mTOR in cell growth, proliferation and survival, research of mTOR in cell motility, particularly in cell adhesion, is still in its infancy. Previous studies have shown that mTOR regulates cell adhesion in colon cancer cells (HCT116) or in renal carcinoma cells (A498, Caki-1 and KTC-26) by using rapamycin (a specific mTORC1 inhibitor) or RAD001 (a rapamycin analog).Citation3,4 Recently our group has further observed that rapamycin inhibits the basal and IGF-1 stimulated adhesion of tumor cells derived from human rhabdomyosarcoma (Rh30), Ewing sarcoma (Rh1), colon carcinoma (HT29) and cervical adenocarcinoma (HeLa).Citation5 Collectively, these data indicate that mTOR plays a pivotal role in cell adhesion.
To uncover how mTOR regulates cell adhesion, we employed pharmacological and genetic approaches. As rapamycin inhibits cell differentiation by an mTOR kinase independent manner,Citation6 we first asked whether rapamycin inhibits cell adhesion by inhibiting the kinase activity of mTOR. Using recombinant adenoviruses expressing a rapamycin-resistant but kinase-active mTOR mutant (S2035T, mTOR-T) or kinase-dead mTOR-T mutant (S2035T/D2357E, mTOR-TE), we found that expression of mTOR-T, but not mTOR-TE, prevented rapamycin from inhibiting IGF-1-stimulated cell adhesion in Rh30 cells, revealing that mTOR kinase activity is essential for cell adhesion.Citation5 This is further supported by the results that the adhesion of Rh30 and HeLa cells treated with mTOR shRNA or PP242 (an mTORC1/2 kinase inhibitor) was substantially inhibited.Citation5 Our new data further underline a critical role of mTOR in cell adhesion.
In mammalian cells, mTOR functions at least as 2 complexes, mTORC1 and mTORC2, whose activities are greatly impacted by the complex integrity, especially their associations with raptor and rictor, respectively.Citation2 Both mTORC1 and mTORC2 have been found to be involved in the regulation of cell motility.Citation6 Next, we investigated whether mTOR regulates cell adhesion through mTORC1 and mTORC2-dependent mechanisms. For this, mTORC1 and mTORC2 were disrupted by raptor shRNA and rictor shRNA, respectively. Of interest, silencing raptor or rictor suppressed the basal and IGF-1-stimulated adhesion of Rh30 and HeLa cells.Citation5 Since rictor also has mTORC2-independent functions related to regulation of cytoskeleton and cell migration, to validate the role of mTORC2 in the regulation of cell adhesion, PP242, an mTOR kinase inhibitor that inhibits both mTORC1 and mTORC2, was utilized. We found that PP242 inhibited cell adhesion more potently than rapamycin (mTORC1 inhibitor).Citation5 Therefore, the results strongly suggest that both mTORC1 and mTORC2 are involved in the regulation of cell adhesion ().
Figure 1. Schematic diagram of mTOR signaling in cell adhesion. Both mTORC1 and mTORC2 participate in IGF-1-stimulted cell adhesion in cancer cells. mTORC1 regulates cell adhesion through S6K1 and 4E-BP1/eIF4E pathways, but mTORC2 mediates cell adhesion independently of Akt. PP242 (an mTORC1/2 kinase inhibitor) suppresses cell adhesion more potently than rapamycin (an allosteric mTORC1 inhibitor).
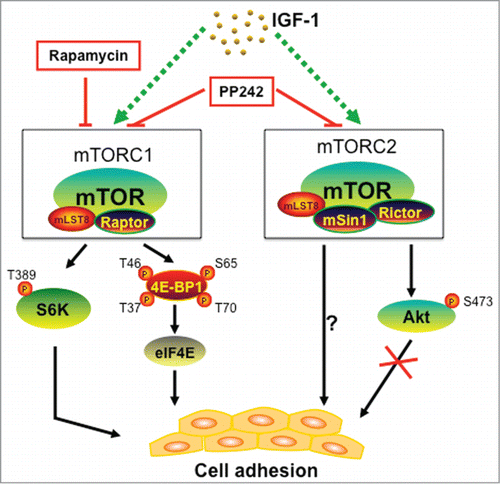
How do mTORC1 and mTORC2 regulate cell adhesion? As S6K1 and 4E-BP1 are 2 best-characterized downstream effector molecules of mTORC1,Citation2 we further examined whether these 2 downstream pathways participate in the regulation of cell adhesion. Of interest, ectopic expression of constitutively active and rapamycin-resistant mutant of S6K1 (S6K1-ca), but not wild-type S6K1 (S6K1-wt) or green fluorescence protein (GFP), conferred resistance to rapamycin inhibition of cell adhesion, whereas downregulation of S6K1 suppressed IGF-1-stimulated cell adhesion.Citation5 On the other hand, downregulation of 4E-BP1 attenuated the inhibitory effect of rapamycin on cell adhesion, while ectopic expression of constitutively hypophophorylated 4E-BP1 (4E-BP1-5A) inhibited IGF-1-stimulated cell adhesion.Citation5 The findings underscore that both mTORC1-mediated S6K1 and 4E-BP1/eIF4E pathways are required for cell adhesion ().
Akt is a best-characterized substrate of mTORC2.Citation2 Next, we determined whether the Akt pathway plays a role in mTORC2-mediated cell adhesion. To our surprise, inhibition of Akt with Akt inhibitor X did not suppress cell adhesion.Citation5 Furthermore, ectopic expression of constitutively active Akt (myr-Akt) or dominant negative Akt (dn-Akt) did not obviously stimulate or inhibit cell adhesion in Rh30 and HeLa cells either.Citation5 The results suggest that mTORC2-mediated cell adhesion is independent of Akt pathway. Since mTORC2 also regulates the phosphorylation of serum- and glucocorticoid-induced protein kinase 1 (SGK1), focal adhesion proteins (focal adhesion kinase, paxillin, and p130Cas) and protein kinase C-α, as well as the activities of small GTPases (RhoA, Cdc42 and Rac1), further studies are needed to address whether mTORC2 regulates cell adhesion by mediating any of these signaling molecules.
In conclusion, the studies from us Citation5 and others Citation3,4 have demonstrated that mTOR plays a critical role in cell adhesion. Our new data indicate that both mTORC1 and mTORC2 participate in the regulation of cell adhesion. However, mTORC1 regulates cell adhesion through S6K1 and 4E-BP1 pathways, but mTORC2 regulates cell adhesion via Akt-independent mechanism. The new finding highlights mTOR as a potential target to control cancer metastasis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Ridley AJ, et al. Science 2003; 302:1704–9; PMID: 14657486; http://dx.doi.org/10.1126/science.1092053
- Laplante M, et al. Cell 2012; 149:274–93; PMID: 22500797; http://dx.doi.org/10.1016/j.cell.2012.03.017
- Sawhney RS, et al. J Biol Chem 2004; 279:47379–90; PMID: 15304500; http://dx.doi.org/10.1074/jbc.M402031200
- Juengel E, et al. BMC Cancer 2009; 9:161; PMID:19473483; http://dx.doi.org/10.1186/1471-2407-9-161
- Chen L, et al. Oncotarget 2015; PMID: 25762619.
- Erbay E, et al. J Biol Chem 2001; 276:36079–82; PMID: 11500483; http://dx.doi.org/10.1074/jbc.C100406200
- Liu L, et al. J Biol Chem 2010; 285:38362–73; PMID: 20937815; http://dx.doi.org/10.1074/jbc.M110.141168
