Hypoxia inducible factors (HIFs) are essential for adapting the cell´s oxygen homeostasis to hypoxia, physiologically as well as pathologically, especially in fast growing tumors. Hypoxia, the lack of sufficient oxygen, causes accumulation of hypoxia inducible factor α subunits (HIF-1α and −2α) which after heterodimerisation with the constitutively expressed HIF-1β-subunit bind to hypoxia responsive elements (HREs) in their target genes. To overcome insufficient oxygen and nutrient supply as well as acidosis genes for vascular endothelial growth factor, adrenomedullin, erythropoietin, glucose transporter 1 (GLUT1) or carbonic anhydrase IX (CA9) are induced by HIFs. HIF-heterodimers of HIF-1α/-1β or HIF-2α/-1β bind to the same HRE and can activate target genes redundant for HIF-1α or HIF-2α or - depending on cell type - α-subunit specific genes.Citation1 The mechanisms how HIF-1α or HIF-2α can differentiate between HREs in different promotors or enhancers still remain elusive. Certain co-factors appear to bind specifically to one HIF-subunit, for example NF-κB essential modulator (NEMO) which supports HIF-2α mediated transcriptional activityCitation2 or apurinic/apyrimidinic endonuclease/redox factor-1 (Ref-1) for HIF-1α transcriptional activity.Citation3 Still, the specificity issue of HIF-α-subunits has not been settled, in part due to effects knocking down one subunit has on the abundance of the other subunit.
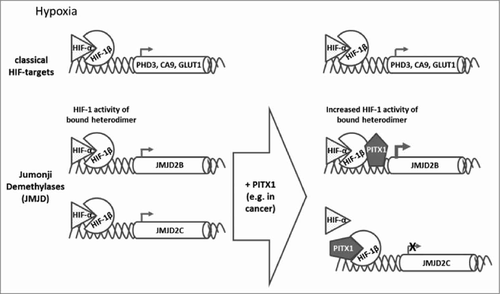
In this issue now Mudie et al.Citation4 report PITX1 as a new interacting partner for HIF-1β under normoxic conditions that would even allow distinguishing between different HIF-1α/-1β dependent genes. Depletion of PITX1 affected HIF-1 regulated Jumonji domain-containing demethylases (JMJDs) under hypoxia but with no effect on classical hypoxic target genes like prolyl hydroxylase 3 (PHD3), CA9 or GLUT1 (Fig. 1). In particular JMJD2B was sensitive to PITX1 depletion which caused significantly lower expression of this demethylase under hypoxia. JMJD2B demethylates trimethylated lysine 9 of histone 3 (H3K9me3), critically controlling the activity of different promotors by repressing the formation of heterochromatin. JMJD2B overexpression is found in cancer where methylation is at least partially lost, giving tumor cells the possibility to dedifferentiate.Citation5 In contrast, hypoxic expression of JMJD2C was reduced with PITX1 knock-down. JMJD2C has already been linked with HIF-1 targets to demethylate H3K9me3 and induce HIF-1 binding to HREs of classical HIF-1 targets like GLUT1 or lactate dehydrogenase A (LDHA) but not to HREs of HIF-2 targets.Citation6 This opens another level of regulation for PITX1 in the transcription of HIF target genes.
So far, the present work by Mudie et al. elegantly shows the effects of deregulated PITX1 levels in tumor cells affecting hypoxic JMJD regulation via the combination of PITX1 and HIF-1 and how the amount of PITX1 is crucial for the outcome of HIF-1 activity. It is most likely that PITX1 also affects transcriptional activity of HIF-2α, but this remains to be studied. Further insights may open new therapeutic avenues particularly for tumor with dysregulated PITX1 expression.
Figure 1. Hypoxia induced HIF-1 heterodimer complexes regulate the expression of HIF-1 target genes under hypoxia. In PITX1 overexpressing cells only a certain subset of HIF-1 dependent genes shows increased expression, here JMJD 2B, while classical HIF-1 target genes are not affected (PHD3, CA9, GLUT1) or even reduced (JMJD2C).
References
- Fandrey J, et al. Cardiovasc Res. 2006; 71(4):642-51; PMID:16780822; https://doi.org/10.1016/j.cardiores.2006.05.005
- Bracken CP, et al. J Biol Chem 2005; 280(14):14240-51; PMID:15653678; https://doi.org/10.1074/jbc.M409987200
- Ziel KA, et al. FASEB J 2004; 18(9):986-8; PMID:15084519
- Mudie S et al. Cell Cycle 2014; 13(24):3878-91; PMID:25558831; https://doi.org/10.4161/15384101.2014.972889
- Kim J, Kim H. ILAR J. 2012; 53(3–4): 232-239; PMID:23744963; https://doi.org/10.1093/ilar.53.3-4.232
- Luo W, et al. Proc Natl Acad Sci U S A 2012; 109(49):E3367-76; PMID:23129632; https://doi.org/10.1073/pnas.1217394109
