Abstract
We have previously revealed that exogenously added lithocholic bile acid (LCA) extends the chronological lifespan of the yeast Saccharomyces cerevisiae, accumulates in mitochondria and alters mitochondrial membrane lipidome. Here, we use quantitative mass spectrometry to show that LCA alters the age-related dynamics of changes in levels of many mitochondrial proteins, as well as numerous proteins in cellular locations outside of mitochondria. These proteins belong to 2 regulons, each modulated by a different mitochondrial dysfunction; we call them a partial mitochondrial dysfunction regulon and an oxidative stress regulon. We found that proteins constituting these regulons (1) can be divided into several “clusters”, each of which denotes a distinct type of partial mitochondrial dysfunction that elicits a different signaling pathway mediated by a discrete set of transcription factors; (2) exhibit 3 different patterns of the age-related dynamics of changes in their cellular levels; and (3) are encoded by genes whose expression is regulated by the transcription factors Rtg1p/Rtg2p/Rtg3p, Sfp1p, Aft1p, Yap1p, Msn2p/Msn4p, Skn7p and Hog1p, each of which is essential for longevity extension by LCA. Our findings suggest that LCA-driven changes in mitochondrial lipidome alter mitochondrial proteome and functionality, thereby enabling mitochondria to operate as signaling organelles that orchestrate an establishment of an anti-aging transcriptional program for many longevity-defining nuclear genes. Based on these findings, we propose a model for how such LCA-driven changes early and late in life of chronologically aging yeast cause a stepwise development of an anti-aging cellular pattern and its maintenance throughout lifespan.
Abbreviations
| D | = | diauxic growth phase |
| DMSO | = | dimethyl sulfoxide |
| ER | = | endoplasmic reticulum |
| ETC | = | electron transport chain |
| ISC | = | iron-sulfur clusters |
| LCA | = | lithocholic acid |
| MAM | = | mitochondria-associated membrane |
| OS | = | oxidative stress |
| PD | = | post-diauxic growth phase |
| PMD | = | partial mitochondrial dysfunction |
| ROS | = | reactive oxygen species |
| ST | = | stationary growth phase |
| TCA | = | tricarboxylic acid |
| WT | = | wild type |
Introduction
The composition, morphology and functional state of mitochondria have been implicated in cell growth, division, differentiation, homeostasis, metabolism, stress response, signaling, immune response, aging, survival and death in evolutionarily distant eukaryotes; these cellular organelles are therefore central to the physiology, health and disease of eukaryotic organisms across phyla.Citation1–7 Mitochondria are indispensable for many vital cellular processes, including the following: (1) the synthesis of most cellular ATP via oxidative phosphorylation coupled to the electron transfer chain (ETC) in the inner mitochondrial membrane;Citation2,3 (2) the generation of the tricarboxylic acid (TCA) cycle intermediates, some of which are used for the synthesis of amino acids, lipids and heme in mitochondria;Citation3-5 (3) the maintenance of a metabolic status-specific NAD+/NADH ratio, AMP/ATP ratio, level of acetyl-CoA and level of S-Adenosylmethionine; these mitochondria-derived metabolites modulate activities of several protein sensors governing energy-producing cellular metabolism and are also used for acetylation and methylation of numerous non-mitochondrial proteins involved in many cellular processes;Citation3,57,8 (4) the synthesis and assembly of iron-sulfur clusters (ISC), inorganic cofactors of many mitochondrial, nuclear and cytosolic proteins playing essential roles in vital cellular processes;Citation9 (5) the formation of reactive oxygen species (ROS); these by-products of mitochondrial respiration play critical roles in regulating cell proliferation, differentiation, metabolism, signaling, immune response, aging, survival and death;Citation4,5,10-14 (6) the proteolytic degradation of unfolded proteins accumulated in mitochondria above a toxic threshold; the efflux of the resulting peptides from the mitochondria elicits a specific transcriptional response in the nucleus, thus reducing the number of unfolded proteins in mitochondria below the toxic threshold;Citation4,14-17 (7) the efflux of cytochrome c and other pro-apoptotic proteins from mitochondria to initiate a programmed form of apoptotic cell death as well as to modulate various non-apoptotic cellular processes, including cell cycle progression, differentiation, metabolism, autophagy, inflammation, immunity and regulated necrotic death;Citation1-3,7,18-20 (8) the assembly and disassembly of various multi-protein complexes on the outer mitochondrial surface; these dynamic multi-protein complexes have been implicated in cell differentiation, signaling, metabolism, immune response, hypoxic response and death, as well as in mitochondrial fusion, fission, motility, inheritance, DNA maintenance and autophagic degradation;Citation1,3,6,20,21-27 (9) the establishment of zones of close apposition between the outer mitochondrial membrane and the mitochondria-associated membrane (MAM) domains of the endoplasmic reticulum (ER), plasma membrane, peroxisomes, vacuoles and autophagosomes; the MAM domains have been implicated in maintaining the homeostasis of intracellular Ca2+, sustaining membrane phospholipid homeostasis, controlling mitochondrial biogenesis and morphology, regulating mitochondrial division and movement, controlling ER stress, influencing ROS and ATP production, orchestrating autophagosome biogenesis during non-selective and mitochondria-selective forms of autophagy, impinging on immune signaling and inflammation, and defining cell susceptibility to a programmed form of apoptotic death;Citation3,6,20,25,28–33 and (10) the communication of mitochondria with lysosomes and peroxisomes via small mitochondria-derived vesicles, which contribute to mitochondrial quality control and peroxisome biogenesis (respectively).Citation34,35
All these findings support the view that mitochondria operate as signaling organelles that are intimately integrated with other cellular compartments in orchestrating many vital processes within eukaryotic cells.Citation3,6,20,25,31,33 A challenge is to uncover molecular mechanisms through which certain changes in the molecular composition of mitochondria influence their role as a signaling compartment that integrates cellular responses to various physiological conditions. Our recent demonstration that exogenously added lithocholic bile acid (LCA) extends yeast chronological lifespan, accumulates in mitochondria and alters mitochondrial membrane lipidomeCitation36 provided us with an opportunity to explore how LCA-driven, longevity-extending changes in the composition of mitochondrial membrane phospholipids impact the ability of mitochondria to function as signaling organelles in aging. As a first step toward attaining our objective, in this study we used quantitative mass spectrometry to investigate the effect of LCA on the age-related dynamics of alterations in levels of mitochondrial proteins and proteins in cellular locations outside of mitochondria. Yeast cells for these experiments were cultured under caloric restriction (CR) conditions on 0.2% glucose rather than under non-CR conditions on 2% glucose. We chose CR conditions for the following reasons: (1) mitochondria, the organelles whose LCA-driven ability to operate as signaling compartments in yeast chronological aging we investigated in this study, are significantly more abundant and functionally active in yeast cells limited in calorie supply than they are in yeast cells on a high-calorie diet;Citation37,38 (2) the longevity-extending efficacy of LCA accumulated in mitochondria of yeast cultured under CR conditions significantly exceeds that of yeast cultured under non-CR conditions;Citation38 (3) LCA extends yeast chronological lifespan under CR conditions, under which such key pro-aging routes of longevity regulation as the target of rapamycin (TOR) and cAMP/protein kinase A (cAMP/PKA) signaling pathways are fully suppressed;Citation38 and (4) LCA delays chronological aging in yeast by targeting ″constitutive″ or ″housekeeping″ longevity pathways; these pathways modulate longevity irrespective of the number of available calories and do not overlap with the ″adaptable″ TOR and cAMP/PKA pathways that are under the stringent control of calorie availability.Citation38
Results
LCA elicits age-related changes in mitochondrial proteome
We hypothesized that LCA accumulated in mitochondria of chronologically aging yeast may alter not only the membrane lipidome of these organellesCitation36 but also their proteome. To test our hypothesis, we used quantitative mass spectrometry to compare proteins that were recovered in mitochondria purified from wild-type (WT) cells cultured in the presence of LCA or in its absence. We found that LCA alters the age-related dynamics of changes in levels of numerous mitochondrial proteins implicated in many essential mitochondrial functions ( and Table S1), including the following: (1) various enzymes of the TCA cycle;Citation39 (2) Icl1p and Mls1p, enzymes of the glyoxylate cycle in mitochondria;Citation40,41 (3) various protein components of the ETC;Citation39 (4) Aat1p, Gdh3p, Ilv5p, Ilv6p and Lys12p, enzymes involved in the biosynthesis of several amino acids, including aspartate, asparagine, threonine, glycine, isoleucine, glutamate, leucine, valine and lysine;Citation42,43 (5) the Cox10p, Cyc3p and Hem14p proteins implicated in heme synthesis and attachment;Citation44,45 (6) the Grx5p protein involved in the synthesis and assembly of ISC, indispensable inorganic cofactors of various mitochondrial, nuclear and cytosolic proteins;Citation9,46 (7) Pos5p, an enzyme catalyzing the synthesis of NADPH from NADH;Citation47,48 (8) key protein components of reactive oxygen species (ROS) detoxification in mitochondria;Citation39 (9) stress response proteins that have been implicated in mitochondrial protein import, folding and stress protection;Citation39 (10) the Mia40p and Tim23p components of a machinery involved in protein import into the inner membrane, intermembrane space and matrix of mitochondria;Citation44,49,50 (11) the Caf4p and Mdv1p protein components of mitochondrial fission machinery;Citation1 (12) proteins that are essential for the synthesis, processing and translation of various mitochondrial RNA species;Citation39 and (13) various mtDNA-binding proteins with essential roles in mitochondrial nucleoid replication, maintenance, protection from damage and inheritance.Citation39
Figure 1. LCA alters the age-related dynamics of changes in levels of numerous mitochondrial proteins implicated in many essential mitochondrial functions. Wild-type cells were cultured in the nutrient-rich YP medium initially containing 0.2% glucose, with 50 50 μM mu;M LCA or without it. Mitochondria were purified from cells recovered on days 2, 5 and 9 of culturing (D, PD and ST growth phases, respectively) as described in “Materials and Methods”. Mass spectrometry-based identification and quantitation of proteins recovered in purified mitochondria were performed as described in “Materials and Methods”. Relative levels of proteins in mitochondria of cells cultured in the presence of LCA (fold difference relative to those in the absence of LCA) are shown. Abbreviations: D, diauxic growth phase; ETC, the mitochondrial electron transport chain; mtDNA, mitochondrial DNA; PD, post-diauxic growth phase; ROS, reactive oxygen species; ST, stationary growth phase; TCA, the tricarboxylic acid cycle in mitochondria.
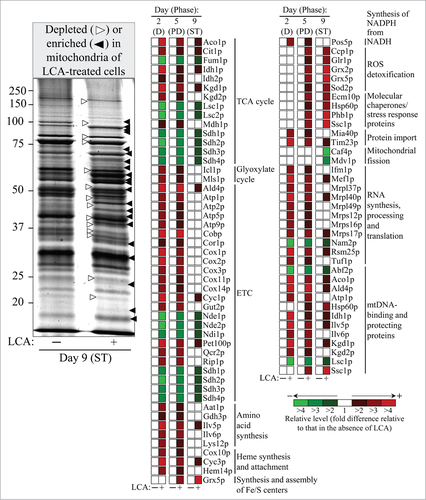
With the help of the SPELL online search engine,Citation51 we used each of these mitochondrial proteins as a query for analyzing extensive datasets of gene expression profiles in yeast mutants that lack (1) various transcription factors; (2) protein components of several signaling pathways known to be modulated by mitochondria; or (3) mitochondrial proteins shown to be essential for modulating these signaling pathways. Such bioinformatic analysis revealed that the mitochondrial proteins whose levels were altered in an age-related fashion in yeast cells cultured with LCA can be divided into 2 regulons, each modulated by a different kind of mitochondrial dysfunction. We call these 2 regulons a partial mitochondrial dysfunction (PMD) regulon and an oxidative stress (OS) regulon (). Based on expression profiles of the genes encoding mitochondrial proteins composing the PMD regulon and taking into consideration published data on regulation of such proteins in response to certain mitochondrial dysfunctions, it can be divided into 6 “clusters” (). Each of these clusters denotes a distinct type of partial mitochondrial dysfunction that elicits a different signaling pathway governed by a distinct set of transcription factors. The PMD regulon includes the following clusters: (1) rho0 cluster (which is governed by Rtg2p, a sensor of an age-related reduction of mitochondrial membrane potential);Citation4,41,52-56 (2) S1 cluster;Citation4,52,54,55 (3) general TCA cycle dysfunction cluster;Citation4,52,53,55 (4) kgd1Δ, kgd2Δ or lpd1Δ cluster;Citation4,53-55 (5) yme1Δ mdl1Δ cluster;Citation4,55 and (6) afo1Δ cluster (which is governed by Sfp1p, a transcription activator of genes encoding cytoplasmic ribosomal proteins).Citation4,57 The OS regulon includes the following clusters: (1) a cluster governed by the transcription factor Yap1p, a primary determinant in the antioxidant response of yeast cells;Citation58-64 (2) a cluster governed by the transcription factors Msn2p/Msn4p, which are required for expression of numerous genes in response to thermal, oxidative and other types of stress;Citation56,62-65 (3) a cluster governed by the transcription factor Skn7p, which is involved in the osmotic and oxidative stress responses;Citation58-64 and (4) a cluster governed by Hog1p, a mitogen-activated protein kinase orchestrating an osmosensing signal transduction pathway in yeast.Citation64,66,67
Figure 2. The mitochondrial proteins whose levels are altered in an age-related fashion in yeast cells cultured with LCA can be divided into 2 regulons called a partial mitochondrial dysfunction (PMD) regulon and an oxidative stress (OS) regulon. Each regulon is modulated by a different kind of mitochondrial dysfunction. Based on expression profiles of the genes encoding mitochondrial proteins composing the PMD and OS regulons and considering published data on regulation of such proteins in response to certain mitochondrial dysfunctions, each regulon can be divided into several “clusters”. Each cluster denotes a distinct type of partial mitochondrial dysfunction that elicits a different signaling pathway mediated by a distinct set of transcription factors. Because mitochondrial proteins constituting the PMD and OS regulons exhibit 3 different patterns of the age-related dynamics of changes in their cellular levels, each of these regulons is separated into regulons “type 1”, “type 2” and “type 3”. The names of proteins that belong to more than one PMD or OS regulon are italicized; the names of proteins that are members of both a PMD regulon and an OS regulon are underlined. Abbreviations: D, diauxic growth phase; PD, post-diauxic growth phase; ST, stationary growth phase.
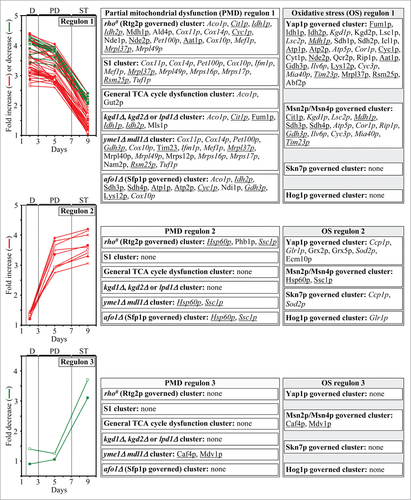
It needs to be emphasized that mitochondrial proteins constituting the PMD and OS regulons exhibit 3 different patterns of the age-related dynamics of changes in their cellular levels; to underscore the existence of such differences in expression, we separated each of the PMD and OS regulons into regulons “type 1”, “type 2” and “type 3” ( and Table S1). The cellular levels of mitochondrial proteins comprising the PMD and OS regulons type 1 remained nearly unchanged during diauxic (D) and post-diauxic (PD) growth phases but underwent a 2-3 fold increase or decrease during the subsequent stationary (ST) growth phase (; PMD regulon 1 and OS regulon 1). Furthermore, the cellular levels of mitochondrial proteins comprising the PMD and OS regulons type 2 elevated by 2-3 folds during D and PD growth phases but remained almost unaltered during the following ST growth phase (; PMD regulon 2 and OS regulon 2). Moreover, the cellular levels of mitochondrial proteins comprising the PMD and OS regulons type 3 remained nearly the same during the most of D and PD growth phases but underwent a 3-4 fold reduction during the subsequent ST growth phase (; PMD regulon 3 and OS regulon 3). Noteworthy, the expression of genes encoding mitochondrial proteins that belong to the PMD regulons type 1, 2 and 3 is known to be regulated by the transcription factors Rtg1p/Rtg2p/Rtg3p, Sfp1p and Aft1p.Citation4,56,57 The expression of genes coding for mitochondrial proteins that belong to the OS regulons type 1, 2 and 3 has been shown to be under the control of the transcription factors Yap1p, Msn2p/Msn4p, Skn7p and Hog1p.Citation58-63,65-67
LCA causes age-related changes in cellular proteome
Age-related alterations in the rates and efficiencies of several processes within mitochondria are known to modulate the capacity of these organelles to make and release certain molecular signals; outside mitochondria, such signals have been shown to cause changes in the rates and efficiencies of longevity-defining processes in other cellular locations.Citation3-6,16,20,25,31,33,56 Of note, we found that a treatment of yeast cells with LCA alters the age-related chronology of these mitochondrial processes.Citation5,36,38,68-70 We therefore hypothesized that LCA may impact not only the levels of numerous mitochondrial proteins but also the levels of proteins in cellular locations outside mitochondria. To validate our hypothesis, we used quantitative mass spectrometry to compare proteins that were recovered in total lysates of WT cells cultured in the presence of LCA or in its absence. This quantitative analysis of cellular proteins confirmed the data of such analysis for proteins recovered in purified mitochondria; indeed, we revealed that LCA alters the age-related dynamics of changes in levels of many mitochondrial proteins known for their essential roles in vital mitochondrial functions and aging in yeast (compare , as well as Tables S1 and S2). Moreover, we found that LCA causes age-related changes in levels of numerous proteins known to be located outside of mitochondria and shown to be involved in various cellular processes ( and Table S2). These cellular processes include the following: (1) glycogen degradation; (2) the glycolytic pathway; (3) the pentose phosphate pathway; (4) pyruvate conversion to acetyl-CoA; (5) the maintenance of redox balance between NAD and NADH with the help of carnitine and glycerol-3-phosphate shuttles; (6) ROS detoxification; (7) stress response; (8) glutathione synthesis; (9) gluconeogenesis; (10) ethanol formation; (11) the synthesis and hydrolytic degradation of triacylglycerols (TAG) and ergosteryl esters (EE), the 2 major neutral lipids; (12) the synthesis of various amino acids; (13) nucleotide synthesis; (14) the assembly of the 40S and 60S ribosomal subunits from numerous protein components whose levels were altered by LCA; and (15) proteasomal and vacuolar protein degradation.Citation4,5,39,41,38,71,72
Figure 3. LCA alters the age-related dynamics of changes in levels of many mitochondrial proteins and numerous proteins located outside of mitochondria; these mitochondrial and non-mitochondrial proteins have been implicated in various cellular processes. Wild-type cells were cultured in the nutrient-rich YP medium initially containing 0.2% glucose, with 50 50 μM mu;M LCA or without it. Cells were recovered on days 2, 5 and 9 of culturing (D, PD and ST growth phases, respectively). Mass spectrometry-based identification and quantitation of proteins recovered in total lysates of yeast cells were performed as described in “Materials and Methods”. Relative levels of proteins in cells cultured in the presence of LCA (fold difference relative to those in the absence of LCA) are shown. Abbreviations: C, cytosol; D, diauxic growth phase; EE, ergosteryl esters; ETC, the mitochondrial electron transport chain; M, mitochondria; mtDNA, mitochondrial DNA; PD, post-diauxic growth phase; ROS, reactive oxygen species; ST, stationary growth phase; TAG, triacylglycerols; TCA, the tricarboxylic acid cycle in mitochondria.
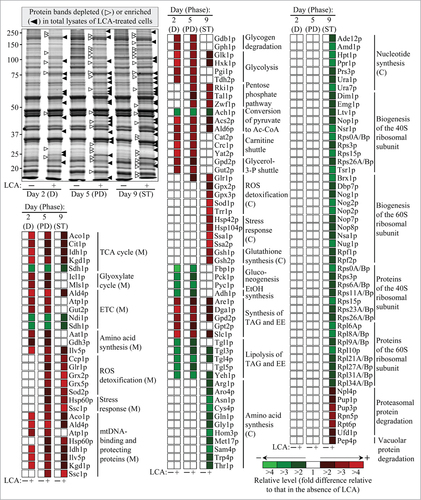
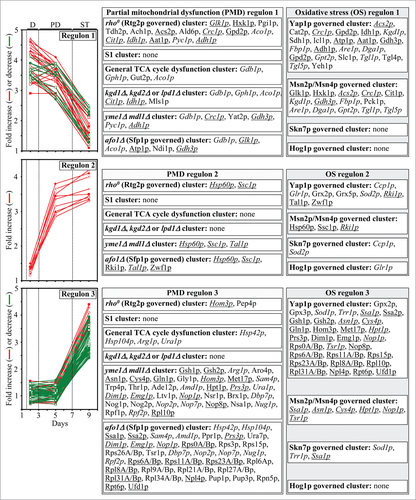
We then subjected cellular proteins whose levels were changed in yeast grown in a medium supplemented with LCA to bioinformatic analysis with the help of the SPELL online search engine,Citation51 as described above for mitochondrial proteins. Just as our bioinformatic analysis of mitochondrial proteins revealed (see above), we found that each of the cellular proteins whose level was altered in yeast cultured with LCA belongs to the following 2 multi-clustered regulons: (1) the PMD regulon, which consisted of the rho0 (Rtg2p governed) cluster, S1 cluster, general TCA cycle dysfunction cluster, kgd1Δ, kgd2Δ or lpd1Δ cluster, yme1Δmdl1Δ cluster, and afo1Δ (Sfp1p governed) cluster;Citation4,5,41,52-57 and (2) the OS regulon, which included the Yap1p governed cluster, Msn2p/Msn4p governed cluster, Skn7p governed cluster and Hog1p governed cluster ().Citation4,5,56,58-63,65-67 We also found that, akin to mitochondrial proteins constituting the PMD and OS regulons, cellular proteins that belong to each of them displays either of the following 3 patterns of age-related changes in their levels: (1) the levels of cellular proteins that belong to the PMD and OS regulons type 1 remained nearly unchanged during D and PD growth phases but underwent a 3–4 fold rise or reduction during the subsequent ST growth phase (; PMD regulon 1 and OS regulon 1); (2) the levels of cellular proteins that belong to the PMD and OS regulons type 2 increased by 3–4 folds during D and PD growth phases but remained almost unaltered during the following ST growth phase (; PMD regulon 2 and OS regulon 2); and (3) the levels of cellular proteins that belong to the PMD and OS regulons type 3 remained practically the same during the most of D and PD growth phases but underwent a 2-4 fold-reduction or rise during the subsequent ST growth phase (; PMD regulon 3 and OS regulon 3). Moreover, we noted that: (1) similar to mitochondrial proteins constituting the 3 different PMD regulons, the expression of genes for cellular proteins that belong to the PMD regulons type 1, 2 and 3 has been shown to be governed by the transcription factors Rtg1p/Rtg2p/Rtg3p, Sfp1p and Aft1p;Citation4,56,57 and (2) akin to mitochondrial proteins constituting the 3 different OS regulons, the expression of genes for cellular proteins that belong to the OS regulons type 1, 2 and 3 is known to be controlled by the transcription factors Yap1p, Msn2p/Msn4p, Skn7p and Hog1p.Citation58-63,65-67
Figure 4. Each of the cellular proteins whose level is changed in yeast cultured with LCA belongs to the following 2 multi-clustered regulons, each modulated by a different kind of mitochondrial dysfunction: 1) the partial mitochondrial dysfunction (PMD) regulon, which consisted of the rho0 (Rtg2p governed) cluster, S1 cluster, general TCA cycle dysfunction cluster, kgd1Δ, kgd2Δ or lpd1Δ cluster, yme1Δmdl1Δ cluster, and afo1Δ (Sfp1p governed) cluster; and 2) the oxidative stress (OS) regulon, which included the Yap1p governed cluster, Msn2p/Msn4p governed cluster, Skn7p governed cluster and Hog1p governed cluster. Each cluster denotes a distinct type of partial mitochondrial dysfunction that elicits a different signaling pathway governed by a distinct set of transcription factors. Because cellular proteins that belong to the PMD and OS regulons display 3 different patterns of age-related changes in their levels, each of these regulons is separated into regulons “type 1”, “type 2” and “type 3”. The names of cellular proteins that belong to more than one PMD or OS regulon are italicized; the names of cellular proteins that are members of both a PMD regulon and an OS regulon are underlined. Abbreviations: D, diauxic growth phase; PD, post-diauxic growth phase; ST, stationary growth phase.
Longevity extension by LCA requires a distinct set of transcription factors
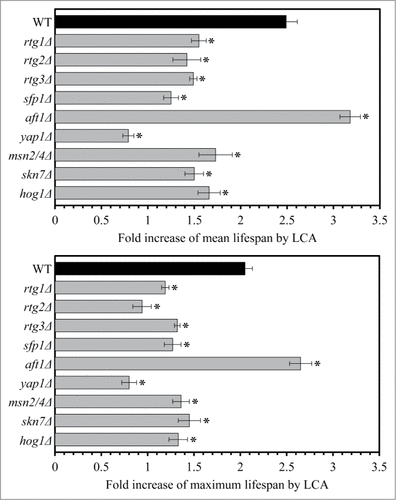
We found that gene-deletion mutations eliminating either the Rtg1p, Rtg2p, Rtg3p, Sfp1p, Yap1p, Msn2p/Msn4p, Skn7p or Hog1p transcription factor(s) significantly reduce the extent to which LCA extends yeast longevity ( and S1); all these factors are known to cause a development of an anti-aging cellular pattern by activating specific transcriptional programs in the nucleus.Citation4,5,55-63,65-67 In contrast, a single-gene-deletion mutation eliminating the transcription factor Aft1p increased the longevity-extending efficiency of LCA ( and S1); this factor has been shown to respond to reduced levels of cellular ISC by eliciting a pro-aging transcriptional program in the nucleus.Citation4,5,73,74 In sum, these findings suggest that, by establishing a specific pattern of an age-related expression of numerous nuclear genes encoding mitochondrial and non-mitochondrial proteins that belong to the PMD or OS regulons of type 1, 2 or 3, each of these transcription factors plays an essential role in longevity extension by LCA.
Figure 5. Gene-deletion mutations eliminating the Rtg1p, Rtg2p, Rtg3p, Sfp1p, Aft1p, Yap1p, Msn2p/Msn4p, Skn7p or Hog1p transcription factor significantly alter the extent to which LCA extends yeast longevity. Wild-type and mutant cells lacking one (or 2, as in case of msn2/4Δ mutant cells) of the above transcription factors were cultured in the nutrient-rich YP medium initially containing 0.2% glucose, with 50 50 μM mu;M LCA or without it. The chronological lifespans were measured as described in “Materials and Methods”. Data are presented as means ± SEM (n = 4–6; #p < 0.01).
Discussion
This study and our recent published dataCitation36 suggest that LCA-driven changes in mitochondrial lipidome alter mitochondrial proteome and functionality, thereby enabling mitochondria to function as signaling organelles that orchestrate an establishment of an anti-aging transcriptional program for many longevity-defining nuclear genes (). It is conceivable that the reduced mitochondrial membrane potential seen in chronologically “young” yeast cells cultured with LCACitation36 triggers the mitochondrial retrograde (RTG) signaling pathway of cellular aging regulation (); the Rtg2p protein component of this pathway is known to respond to a decline in mitochondrial membrane potential by stimulating nuclear import of the Rtg1p-Rtg3p heterodimeric transcription factor, which in the nucleus triggers an anti-aging transcriptional program.Citation4,5,56 Furthermore, the observed in chronologically “old” yeast cells cultured with LCA reduction in the levels of several protein components of the large and small subunits of mitochondrial ribosome () is likely to activate the so-called back-signaling pathway (); in response to reduced levels of mitochondrial ribosomal proteins this pathway is known to trigger an anti-aging transcriptional program which in the nucleus is activated by the transcription factor Sfp1p.Citation4,5,57 Moreover, it is plausible that the observed in chronologically “old” yeast cells cultured with LCA rise in the levels of proteins involved in the synthesis and assembly of ISC in mitochondria () may diminish activity and/or nuclear import of Aft1p (), a transcription factor known to respond to reduced levels of cellular ISC by driving a pro-aging transcriptional program in the nucleus.Citation4,5,73,74 It is also conceivable that the observed in chronologically “young” and “old” yeast cells cultured with LCA significant changes in the levels of numerous mitochondrial and non-mitochondrial proteins comprising the yme1Δmdl1Δ cluster of the PMD regulon () activate a distinct retrograde response signaling pathway that triggers an anti-aging transcriptional program in the nucleus (); this pathway is known to be elicited in response to a simultaneous lack of the mitochondrial i-AAA protease Yme1p and the mitochondrial ABC-transporter Mdl1p involved in peptide export from mitochondria.Citation4,5,55 Finally, the observed ability of LCA to amplify the “hormetic”, anti-aging effect of mitochondrially generated ROS by enabling to maintain their levels in chronologically “old” yeast cells relatively high but below a cytotoxic thresholdCitation5,36 is likely to delay aging by activating Yap1p, Msn2p/Msn4p, Skn7p or Hog1p (); these transcription factors are known to respond to “hormetic” levels of ROS by triggering an anti-aging transcriptional program in the nucleus.Citation4,5,58-63,65-67
Figure 6. A model for how LCA-driven changes in mitochondrial lipidome alter mitochondrial proteome and functionality, thereby enabling mitochondria to function as signaling organelles modulating transcription of many longevity-defining nuclear genes. See text for details. Abbreviations: Ac-CoA, acetyl-CoA; ETC, the mitochondrial electron transport chain; mtDNA, mitochondrial DNA; ROS, reactive oxygen species; ST, stationary growth phase; TCA, the tricarboxylic acid cycle in mitochondria.
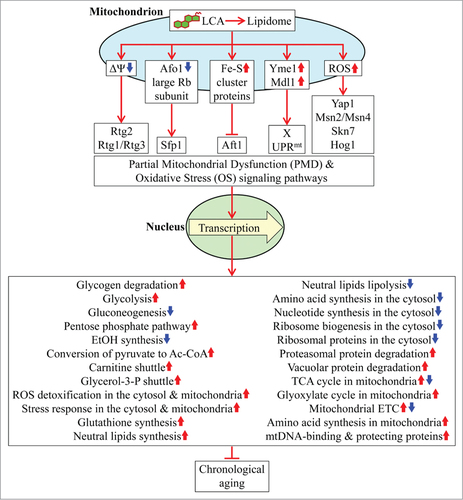
Of note, the expression of many proteins that belong to the PMD and OS regulons types 1 and 3 is reduced in the presence of LCA (); yet, most of the above transcription factors are known to activate transcription of nuclear genes encoding many of these proteins.Citation4,5,55-63,65-67,73,74 It is conceivable therefore that LCA-driven changes in mitochondrial lipidome, proteome and functionality may orchestrate an establishment of an anti-aging transcriptional program for many longevity-defining nuclear genes by: (1) inhibiting activities of some of these transcriptional activators with respect to some of their target genes, thereby repressing transcription of these genes; (2) remodeling multimeric protein complexes formed by some of these transcriptional activators in the nucleus, thus repressing transcription of some of the target genes; and/or (3) eliciting certain other, presently unknown, changes in the activities and/or target specificities of some of these transcriptional activators.
Furthermore, a substantial increase in the levels of proteins constituting the PMD and OS regulons type 2 occurs during D and PD growth phases; this expression pattern precedes the significant alterations in the levels of proteins comprising the PMD and OS regulons types 1 and 3 during the subsequent ST phase (). One could assume therefore that proteins constituting the PMD and OS regulons type 2 may somehow (directly or indirectly) modulate the expression of proteins comprising the PMD and OS regulons types 1 and 3. Because none of the proteins within the type 2 regulons is known to be a transcription factor (),Citation39 a mechanism underlying such hypothetical relationship between these regulons is unlikely to involve transcriptional regulation of the genes that encode proteins within the PMD and OS regulons types 1 and 3.
Moreover, this study and our published dataCitation5,36,38,68-70,72 suggest the following model for how LCA-driven changes in mitochondrial proteome and functionality early and late in life of chronologically aging yeast cause a stepwise development of an anti-aging cellular pattern and its maintenance throughout lifespan ().
Figure 7. For figure legend, see page 1654. Figure 7 (See previous page). A model for how LCA-driven changes in mitochondrial proteome and functionality early and late in life of chronologically aging yeast orchestrate a stepwise development of an anti-aging cellular pattern and its maintenance throughout lifespan. From the data of proteomic analysis () and based on the data of biochemical and cell biological analyses,Citation36,38,68–70,72,84,85 we inferred an outline of metabolic pathways and processes that were activated (red arrows) or inhibited (green arrows) in cells cultured with exogenous LCA. Arrows next to the names of metabolites, proteins or processes denote those of them whose concentrations or efficiencies were elevated (red arrows) or reduced (green arrows) in cells cultured with exogenous LCA. Abbreviations: Ac-CoA, acetyl-CoA; D, diauxic growth phase; EE, ethyl esters; ER, endoplasmic reticulum; EtOH, ethanol; ETC, the mitochondrial electron transport chain; FFA, free fatty acids; IMM, inner mitochondrial membrane; LD, lipid droplets; mtDNA, mitochondrial DNA; OMM, outer mitochondrial membrane; PD, post-diauxic growth phase; ST, stationary growth phase; TAG, triacylglycerols; TCA, the tricarboxylic acid cycle in mitochondria. See text for details.
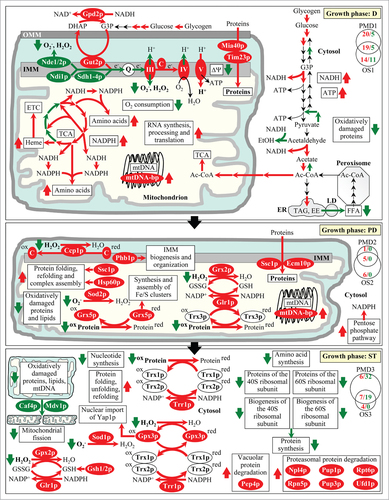
Early in life of a chronologically aging yeast cell, during the D phase of growth, LCA within the inner and outer membranes of mitochondria reduces the capacities of coupled mitochondrial respiration, mitochondrial membrane potential and mitochondrial ROS production ()-likely by decreasing the levels of the “upstream” protein components (i.e. Ndi1p, Nde1p, Nde2p and Sdh1p-Sdh4p) of the mitochondrial ETC and increasing the levels of its “downstream” protein components (i.e., cytochrome c as well as respiratory complexes III, IV and V) (). It is conceivable that such changes in expression pattern are responsible in part for the observed ability of mitochondria in yeast treated with LCA to sustain the concentration of ROS at a sub-lethal (hormetic) level ();Citation36,38,68-70 if sustained at such level, ROS are known to promote the development and maintenance of an anti-aging cellular pattern.Citation3-6 By differentially altering the levels of proteins involved in the mitochondrial TCA cycle (), LCA increases the efficiencies with which some of the intermediates of this cycle are converted into amino acids, NADPH and heme within mitochondria (); the rates of such conversion during D growth phase are further elevated by the LCA-elicited rise in the levels of mitochondrial enzymes catalyzing the key reactions of the conversion (). The rise in the levels of proteins known to be essential for the synthesis, processing and translation of various mitochondrial RNA species, which is observed in mitochondria of cultured with LCA cells during D growth phase (), improves mitochondrial functionality by stimulating these processes within mitochondria (). Furthermore, the elevated levels of mitochondrial components involved in protein import into the inner membrane, intermembrane space and matrix of mitochondria () accelerate these processes during D growth phase, thus further improving mitochondrial functionality () (these components include Mia40p, Tim23p and Ssc1p;Citation44,49,50 it remains to be determined whether or not the observed elevated levels of only these 3 key members of the multi-component machinery driving mitochondrial protein import are sufficient to accelerate such import in yeast treated with LCA). Additionally, by increasing the levels of mtDNA-binding proteins during D growth phase (), LCA stabilizes the mitochondrial nucleoid and protects it from oxidative and other forms of age-related damage (). During the D phase of growth, LCA within the inner and outer mitochondrial membranes also elevates the levels of mitochondrial membrane proteins required for acetyl-CoA uptake by mitochondria (); moreover, the altered functionality of these mitochondria triggers several mitochondria-to-nucleus signaling pathways that differentially affect cellular levels of non-mitochondrial proteins involved in various metabolic pathways for carbohydrates and lipids (). The resulting remodeling of these pathways in yeast cells cultured in the presence of LCA (1) stimulates glycogen degradation, glycolysis, acetyl-CoA formation in the cytosol and neutral lipids synthesis in the ER - thus increasing the levels of NADH, ATP and neutral lipids; and (2) inhibits ethanol formation from acetaldehyde, acyl-CoA conversion to acetate, gluconeogenetic metabolism of pyruvate and the lipolytic conversion of neutral lipids into fatty acids - thereby further stimulating acetyl-CoA formation in the cytosol, from which it is then transported with the increased efficiency into mitochondria to improve their functionality ().Citation4,5
During the subsequent PD phase of growth, LCA within the inner and outer mitochondrial membranes not only maintains its ability to decrease the levels of the “upstream” protein components of the mitochondrial ETC and to increase the levels of its “downstream” protein components but also elevates the level of the mitochondrial cytochrome-c peroxidase Ccp1p () – thus enabling to sustain reduced capacities of coupled mitochondrial respiration, mitochondrial membrane potential and mitochondrial ROS production (). By increasing the levels of stress response proteins implicated in mitochondrial protein import, folding, stress protection and membrane proteins biogenesis (), LCA stimulates all these longevity-defining processes in mitochondria of yeast cells that have entered the PD phase of growth (). Furthermore, a sustained ability of LCA to increase the levels of mtDNA-binding proteins during PD growth phase () enables it to support mitochondrial nucleoid stability and protection from age-related damage (). Moreover, by increasing the levels of numerous mitochondrial proteins integrated into a network of antioxidant scavenger reactions for ROS decomposition (), LCA attenuates oxidative damage to mitochondrial proteins, lipids and nucleic acids (). In addition, the altered functionality of mitochondria in cells cultured with LCA triggers mitochondria-to-nucleus signaling that during PD growth phase increases the levels of non-mitochondrial proteins involved in the pentose phosphate pathway (); this pathway is known to generate NADPH, the primary source of cellular reducing equivalents required for the reductive synthesis of fatty acids, sterols and some amino acids as well as for the protection of numerous thiol-containing cytosolic, nuclear and mitochondrial proteins from oxidative damage ().Citation5,75
During ST phase, LCA within mitochondria maintains its ability to elevate the levels of many mitochondrial proteins involved in antioxidant scavenger reactions for ROS decomposition (), thus reducing the extent of oxidative damage to mitochondrial proteins, lipids and nucleic acids (). In addition, LCA attenuates mitochondrial fragmentation during ST phase by reducing the levels of the Caf4p and Mdv1p protein components of mitochondrial fission machinery ()-thus delaying an age-related form of programmed apoptotic cell death (). During ST phase, the altered functionality of mitochondria in yeast cultured in the presence of LCA also stimulates several mitochondria-to-nucleus signaling pathways that differentially affect cellular levels of many non-mitochondrial proteins involved in various longevity-defining processes (). The resulting remodeling of these processes outside mitochondria (1) reduces the extent of oxidative damage to cytosolic proteins; (2) stimulates protein folding, unfolding and refolding in the cytosol; (3) promotes vacuolar and proteasomal protein degradation; (4) slows down amino acid and nucleotide synthesis in the cytosol; and (5) decelerates protein synthesis in the cytosol by attenuating the assembly of the 40S and 60S ribosomal subunits ().
In the future, it would be important to further explore the following key aspects of the mechanism proposed here through which yeast mitochondria function as signaling organelles orchestrating a stepwise development of a longevity-defining cellular pattern. First, it is intriguing to investigate how several genetic interventions known to cause various changes in the mitochondrial membrane lipidomeCitation76-79 influence the ability of LCA (1) to extend longevity of chronologically aging yeast; (2) to elicit a characteristic set of age-related changes in mitochondrial proteome and functionality; and (3) to cause a distinct kind of age-related alterations in the levels of proteins outside mitochondria. Second, it is interesting to elucidate how genetic interventions known to alter mitochondrial membrane potential, mitochondrial ribosomal proteins, mitochondrial synthesis and assembly of ISC, mitochondrial protein degradation and peptide efflux, or mitochondrial ROSCitation4,5 impact the capability of LCA to trigger a characteristic pattern of age-related changes in the concentrations of proteins located outside of mitochondria. Third, it will be important to use quantitative mass spectrometric metabolomics to confirm the anti-aging “metabolic signature” whose stepwise development is driven by LCA that accumulates in mitochondria of chronologically aging yeast.
Materials and Methods
Yeast strains and growth conditions
The WT strain BY4742 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0), single-gene-deletion mutant strains in the BY4742 genetic background (all from Thermo Scientific/Open Biosystems) and the msn2/4Δ mutant strain (constructed as previously described, see ref. Citation80) were grown in YP medium (1% yeast extract, 2% peptone) initially containing 0.2% glucose as carbon source. Cells were cultured at 30°C with rotational shaking at 200 rpm in Erlenmeyer flasks at a “flask volume/medium volume” ratio of 5:1.
Pharmacological manipulation of chronological lifespan
Chronological lifespan assay and pharmacological manipulation of chronological lifespan by addition of LCA (Sigma) were performed as previously described.Citation38 The stock solution of LCA in dimethyl sulfoxide (DMSO) was made on the day of adding this compound to cell cultures. LCA was added to growth medium in DMSO at the final concentration of 50 50 μM mu;M immediately following cell inoculation into the medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v).
Miscellaneous procedures
Preparation of total cell lysates,Citation37 purification of mitochondria,Citation81 SDS-PAGE,Citation82 quantitative mass spectrometric analysis of proteinsCitation37 and statistical analysisCitation83 were performed as previously described.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
1026493_Supplemental_Materials.pdf
Download PDF (240.6 KB)Acknowledgments
We acknowledge the Centre for Biological Applications of Mass Spectrometry and the Centre for Structural and Functional Genomics, both at Concordia University, for outstanding services.
Funding
This study was supported by grants from the Natural Sciences and Engineering Research Council (NSERC) of Canada and Concordia University Chair Fund. A.B., V.R.R. and T.B.V. were supported by Frederick Banting and Charles Best Doctoral Scholarship Awards from the Canadian Institutes of Health Research (CIHR). P.K. was supported by Doctoral Research Fellowship Awards from the Fonds de recherché en santé du Quebec and from the Fonds québécois de la recherche sur la nature et les technologies. A.P. was supported by a Frederick Banting and Charles Best Canada Master's Scholarship Award from the CIHR. V.S. was supported by an NSERC Undergraduate Summer Research Award. V.I.T. is a Concordia University Research Chair in Genomics, Cell Biology and Aging.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 2010; 11:872-84; PMID:21102612
- Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science 2011; 333:1109-12; PMID:21868666; http://dx.doi.org/10.1126/science.1201940
- Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell 2012; 148:1145-59; PMID:22424226; http://dx.doi.org/10.1016/j.cell.2012.02.035
- Leonov A, Titorenko VI. A network of interorganellar communications underlies cellular aging. IUBMB Life; 2013; 65:665-74; PMID:23818261; http://dx.doi.org/10.1002/iub.1183
- Arlia-Ciommo A, Leonov A, Piano A, Svistkova V, Titorenko VI. Cell-autonomous mechanisms of chronological aging in the yeast Saccharomyces cerevisiae. Microbial Cell 2014; 1:164-78
- Chandel NS. Mitochondria as signaling organelles. BMC Biol 2014; 12:34; PMID:24884669
- Green DR, Galluzzi L, Kroemer G. Cell biology. Metabolic control of cell death. Science 2014; 345:1250256; PMID:25237106; http://dx.doi.org/10.1126/science.1250256
- Kaelin WG Jr, McKnight SL. Influence of metabolism on epigenetics and disease. Cell 2013; 153:56-69; PMID:23540690; http://dx.doi.org/10.1016/j.cell.2013.03.004
- Lill R, Hoffmann B, Molik S, Pierik AJ, Rietzschel N, Stehling O, Uzarska MA, Webert H, Wilbrecht C, Mühlenhoff U. The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism. Biochim Biophys Acta 2012; 1823:1491-508; PMID:22609301; http://dx.doi.org/10.1016/j.bbamcr.2012.05.009
- Hekimi S, Lapointe J, Wen Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol 2011; 21:569-76; PMID:21824781
- Ristow M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat Med 2014; 20:709-11; PMID:24999941; http://dx.doi.org/10.1038/nm.3624
- Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 2014; 24:R453-62; PMID:24845678; http://dx.doi.org/10.1016/j.cub.2014.03.034
- Stuart JA, Maddalena LA, Merilovich M, Robb EL. A midlife crisis for the mitochondrial free radical theory of aging. Longev Healthspan 2014; 3:4; PMID:24690218; http://dx.doi.org/10.1186/2046-2395-3-4
- Yun J, Finkel T. Mitohormesis. Cell Metab 2014; 19:757-66; PMID:24561260; http://dx.doi.org/10.1016/j.cmet.2014.01.011
- Haynes CM, Fiorese CJ, Lin YF. Evaluating and responding to mitochondrial dysfunction: the mitochondrial unfolded-protein response and beyond. Trends Cell Biol 2013; 23:311-8; PMID:23489877; http://dx.doi.org/10.1016/j.tcb.2013.02.002
- Pellegrino MW, Nargund AM, Haynes CM. Signaling the mitochondrial unfolded protein response. Biochim Biophys Acta 2013; 1833:410-6; PMID:22445420; http://dx.doi.org/10.1016/j.bbamcr.2012.02.019
- Mottis A, Jovaisaite V, Auwerx J. The mitochondrial unfolded protein response in mammalian physiology. Mamm Genome 2014; 25:424-33; PMID:24898297; http://dx.doi.org/10.1007/s00335-014-9525-z
- Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol 2012; 13:780-8; PMID:23175281; http://dx.doi.org/10.1038/nrm3479
- Galluzzi L, Kepp O, Trojel-Hansen C, Kroemer G. Non-apoptotic functions of apoptosis-regulatory proteins. EMBO Rep 2012; 13:322-30; PMID:22402666; http://dx.doi.org/10.1038/embor.2012.19
- Tait SW, Green DR. Mitochondria and cell signalling. J Cell Sci 2012; 125:807-15; PMID:22448037; http://dx.doi.org/10.1242/jcs.099234
- Esseltine JL, Scott JD. AKAP signaling complexes: pointing towards the next generation of therapeutic targets? Trends Pharmacol Sci 2013; 34:648-55; PMID:24239028; http://dx.doi.org/10.1016/j.tips.2013.10.005
- Koshiba T. Mitochondrial-mediated antiviral immunity. Biochim Biophys Acta 2013; 1833:225-32; PMID:22440325
- Taylor SS, Zhang P, Steichen JM, Keshwani MM, Kornev AP. PKA: lessons learned after twenty years. Biochim Biophys Acta 2013; 1834:1271-8; PMID:23535202; http://dx.doi.org/10.1016/j.bbapap.2013.03.007
- Giménez-Cassina A, Garcia-Haro L, Choi CS, Osundiji MA, Lane EA, Huang H, Yildirim MA, Szlyk B, Fisher JK, Polak K, et al. Regulation of hepatic energy metabolism and gluconeogenesis by BAD. Cell Metab 2014; 19:272-84; PMID:24506868; http://dx.doi.org/10.1016/j.cmet.2013.12.001
- Kasahara A, Scorrano L. Mitochondria: from cell death executioners to regulators of cell differentiation. Trends Cell Biol 2014; 24:761-70; PMID:25189346; http://dx.doi.org/10.1016/j.tcb.2014.08.005
- Liu L, Sakakibara K, Chen Q, Okamoto K. Receptor-mediated mitophagy in yeast and mammalian systems. Cell Res 2014; 24:787-95; PMID:24903109; http://dx.doi.org/10.1038/cr.2014.75
- Westermann B. Mitochondrial inheritance in yeast. Biochim Biophys Acta 2014; 1837:1039-46; PMID:24183694
- van der Laan M, Bohnert M, Wiedemann N, Pfanner N. Role of MINOS in mitochondrial membrane architecture and biogenesis. Trends Cell Biol 2012; 22:185-92; PMID:22386790; http://dx.doi.org/10.1016/j.tcb.2012.01.004
- Kaufman RJ, Malhotra JD. Calcium trafficking integrates endoplasmic reticulum function with mitochondrial bioenergetics. Biochim Biophys Acta 2014; 1843:2233-9; PMID:24690484; http://dx.doi.org/10.1016/j.bbamcr.2014.03.022
- Klecker T, Böckler S, Westermann B. Making connections: interorganelle contacts orchestrate mitochondrial behavior. Trends Cell Biol 2014; 24:537-45; PMID:24786308; http://dx.doi.org/10.1016/j.tcb.2014.04.004
- Naon D, Scorrano L. At the right distance: ER-mitochondria juxtaposition in cell life and death. Biochim Biophys Acta 2014; 1843:2184-94; PMID:24875902; http://dx.doi.org/10.1016/j.bbamcr.2014.05.011
- Prinz WA. Bridging the gap: membrane contact sites in signaling, metabolism, and organelle dynamics. J Cell Biol 2014; 205:759-69; PMID:24958771; http://dx.doi.org/10.1083/jcb.201401126
- van Vliet AR, Verfaillie T, Agostinis P. New functions of mitochondria associated membranes in cellular signaling. Biochim Biophys Acta 2014; 1843:2253-62; PMID:24642268; http://dx.doi.org/10.1016/j.bbamcr.2014.03.009
- Mohanty A, McBride HM. Emerging roles of mitochondria in the evolution, biogenesis, and function of peroxisomes. Front Physiol 2013; 4:268; PMID:24133452; http://dx.doi.org/10.3389/fphys.2013.00268
- Sugiura A, McLelland GL, Fon EA, McBride HM. A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J 2014; 33:2142-56; PMID:25107473; http://dx.doi.org/10.15252/embj.201488104
- Beach A, Richard VR, Leonov A, Burstein MT, Bourque SD, Koupaki O, Juneau M, Feldman R, Iouk T, Titorenko VI. Mitochondrial membrane lipidome defines yeast longevity. Aging (Albany NY) 2013; 5:551-74; PMID:23924582
- Goldberg AA, Bourque SD, Kyryakov P, Gregg C, Boukh-Viner T, Beach A, Burstein MT, Machkalyan G, Richard V, Rampersad S, et al. Effect of calorie restriction on the metabolic history of chronologically aging yeast. Exp Gerontol 2009; 44:555-71; PMID:19539741; http://dx.doi.org/10.1016/j.exger.2009.06.001
- Goldberg AA, Richard VR, Kyryakov P, Bourque SD, Beach A, Burstein MT, Glebov A, Koupaki O, Boukh-Viner T, et al. Chemical genetic screen identifies lithocholic acid as an anti-aging compound that extends yeast chronological life span in a TOR-independent manner, by modulating housekeeping longevity assurance processes. Aging (Albany NY) 2010; 2:393-414; PMID:20622262
- The Saccharomyces Genome Database [Internet]. Stanford (CA): Department of Genetics at the School of Medicine, Stanford University; 1996 - [cited 2015 Apr 19]. Available from: http://www.yeastgenome.org/
- Titorenko VI, Terlecky SR. Peroxisome metabolism and cellular aging. Traffic 2011; 12:252-9; PMID:21083858; http://dx.doi.org/10.1111/j.1600-0854.2010.01144.x
- Beach A, Burstein MT, Richard VR, Leonov A, Levy S, Titorenko VI. Integration of peroxisomes into an endomembrane system that governs cellular aging. Front Physiol 2012; 3:283; PMID:22936916; http://dx.doi.org/10.3389/fphys.2012.00283
- Aris JP, Fishwick LK, Marraffini ML, Seo AY, Leeuwenburgh C, Dunn WA Jr. Amino acid homeostasis and chronological longevity in Saccharomyces cerevisiae. Subcell Biochem 2012; 7:161-86
- Ljungdahl PO, Daignan-Fornier B. Regulation of amino acid, nucleotide, and phosphate metabolism in Saccharomyces cerevisiae. Genetics 2012; 190:885-929
- Fox TD. Mitochondrial protein synthesis, import, and assembly. Genetics 2012; 192:1203-34; PMID:23212899
- Soto IC, Fontanesi F, Liu J, Barrientos A. Biogenesis and assembly of eukaryotic cytochrome c oxidase catalytic core. Biochim Biophys Acta. 2012; 1817:883-97; PMID:21958598
- Rodríguez-Manzaneque MT, Tamarit J, Bellí G, Ros J, Herrero E. Grx5 is a mitochondrial glutaredoxin required for the activity of iron/sulfur enzymes. Mol Biol Cell 2002; 13:1109-21; PMID:11950925; http://dx.doi.org/10.1091/mbc.01-10-0517
- Strand MK, Stuart GR, Longley MJ, Graziewicz MA, Dominick OC, Copeland WC. POS5 gene of Saccharomyces cerevisiae encodes a mitochondrial NADH kinase required for stability of mitochondrial DNA. Eukaryot Cell 2003; 2:809-20
- Murray DB, Haynes K, Tomita M. Redox regulation in respiring Saccharomyces cerevisiae. Biochim Biophys Acta 2011; 1810:945-58; PMID:21549177
- Dudek J, Rehling P, van der Laan M. Mitochondrial protein import: common principles and physiological networks. Biochim Biophys Acta. 2013; 1833:274-85; PMID:22683763; http://dx.doi.org/10.1016/j.bbamcr.2012.05.028
- Pareek G, Krishnamoorthy V, D'Silva P. Molecular insights revealing interaction of Tim23 and channel subunits of presequence translocase. Mol Cell Biol 2013; 33:4641-59; PMID:24061477; http://dx.doi.org/10.1128/MCB.00876-13
- SPELL: Serial Pattern of Expression Levels Locator - S. cerevisiae [Internet]. Stanford (CA): Department of Genetics at the School of Medicine, Stanford University; 2007 - [cited 2015 Apr 19]. Available from: http://spell.yeastgenome.org/search/about
- Hughes TR, Marton MJ, Jones AR, Roberts CJ, Stoughton R, Armour CD, Bennett HA, Coffey E, Dai H, He YD, et al. Functional discovery via a compendium of expression profiles. Cell 2000; 102:109-26; PMID:10929718; http://dx.doi.org/10.1016/S0092-8674(00)00015-5
- Traven A, Wong JM, Xu D, Sopta M, Ingles CJ. Interorganellar communication. Altered nuclear gene expression profiles in a yeast mitochondrial DNA mutant. J Biol Chem 2001; 276:4020-7; PMID:11054416; http://dx.doi.org/10.1074/jbc.M006807200
- McCammon MT, Epstein CB, Przybyla-Zawislak B, McAlister-Henn L, Butow RA. Global transcription analysis of Krebs tricarboxylic acid cycle mutants reveals an alternating pattern of gene expression and effects on hypoxic and oxidative genes. Mol Biol Cell 2003; 14:958-72; PMID:12631716; http://dx.doi.org/10.1091/mbc.E02-07-0422
- Arnold I, Wagner-Ecker M, Ansorge W, Langer T. Evidence for a novel mitochondria-to-nucleus signalling pathway in respiring cells lacking i-AAA protease and the ABC-transporter Mdl1. Gene 2006; 367:74-88; PMID:16403607; http://dx.doi.org/10.1016/j.gene.2005.09.044
- Jazwinski SM. The retrograde response and other pathways of interorganelle communication in yeast replicative aging. Subcell Biochem 2012; 57:79-100; PMID:22094418; http://dx.doi.org/10.1007/978-94-007-2561-4_4
- Heeren G, Rinnerthaler M, Laun P, von Seyerl P, Kössler S, Klinger H, Hager M, Bogengruber E, Jarolim S, Simon-Nobbe B, et al. The mitochondrial ribosomal protein of the large subunit, Afo1p, determines cellular longevity through mitochondrial back-signaling via TOR1. Aging (Albany NY) 2009; 1:622-36; PMID:20157544
- Lee J, Godon C, Lagniel G, Spector D, Garin J, Labarre J, Toledano MB. Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. J Biol Chem 1999; 274:16040-6; PMID:10347154; http://dx.doi.org/10.1074/jbc.274.23.16040
- Temple MD, Perrone GG, Dawes IW. Complex cellular responses to reactive oxygen species. Trends Cell Biol 2005; 15:319-26; PMID:15953550; http://dx.doi.org/10.1016/j.tcb.2005.04.003
- Brombacher K, Fischer BB, Rüfenacht K, Eggen RI. The role of Yap1p and Skn7p-mediated oxidative stress response in the defence of Saccharomyces cerevisiae against singlet oxygen. Yeast 2006; 23:741-50; PMID:16862604
- Rodrigues-Pousada C, Menezes RA, Pimentel C. The Yap family and its role in stress response. Yeast 2010; 27:245-58; PMID:20148391; http://dx.doi.org/10.1002/yea.1752
- Aung-Htut MT, Ayer A, Breitenbach M, Dawes IW. Oxidative stresses and ageing. Subcell Biochem 2012; 57:13-54; PMID:22094416; http://dx.doi.org/10.1007/978-94-007-2561-4_2
- Morano KA, Grant CM, Moye-Rowley WS. The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genetics 2012; 190:1157-95; PMID:22209905
- Engelberg D, Perlman R, Levitzki A. Transmembrane signaling in Saccharomyces cerevisiae as a model for signaling in metazoans: State of the art after 25years. Cell Signal 2014; 26:2865-78; PMID:25218923
- Galdieri L, Mehrotra S, Yu S, Vancura A. Transcriptional regulation in yeast during diauxic shift and stationary phase. OMICS 2010; 14:629-38; PMID:20863251; http://dx.doi.org/10.1089/omi.2010.0069
- Barbosa AD, Graça J, Mendes V, Chaves SR, Amorim MA, Mendes MV, Moradas-Ferreira P, Côrte-Real M, Costa V. Activation of the Hog1p kinase in Isc1p-deficient yeast cells is associated with mitochondrial dysfunction, oxidative stress sensitivity and premature aging. Mech Ageing Dev 2012; 133:317-30; PMID:22445853; http://dx.doi.org/10.1016/j.mad.2012.03.007
- Brewster JL, Gustin MC. Hog1: 20 years of discovery and impact. Sci Signal 2014; 7:re7; PMID:25227612
- Burstein MT, Kyryakov P, Beach A, Richard VR, Koupaki O, Gomez-Perez A, Leonov A, Levy S, Noohi F, Titorenko VI. Lithocholic acid extends longevity of chronologically aging yeast only if added at certain critical periods of their lifespan. Cell Cycle 2012; 11:3443-62; PMID:22894934; http://dx.doi.org/10.4161/cc.21754
- Arlia-Ciommo A, Piano A, Svistkova V, Mohtashami S, Titorenko VI. Mechanisms underlying the anti-aging and anti-tumor effects of lithocholic bile acid. Int J Mol Sci 2014; 15:16522-43; PMID:25238416; http://dx.doi.org/10.3390/ijms150916522
- Burstein MT, Titorenko VI. A mitochondrially targeted compound delays aging in yeast through a mechanism linking mitochondrial membrane lipid metabolism to mitochondrial redox biology. Redox Biol 2014; 2:305-7; PMID:24563847; http://dx.doi.org/10.1016/j.redox.2014.01.011
- Goldberg AA, Bourque SD, Kyryakov P, Boukh-Viner T, Gregg C, Beach A, Burstein MT, Machkalyan G, Richard V, Rampersad S, et al. A novel function of lipid droplets in regulating longevity. Biochem Soc Trans. 2009; 37:1050-5; PMID:19754450; http://dx.doi.org/10.1042/BST0371050
- Beach A, Titorenko VI. In search of housekeeping pathways that regulate longevity. Cell Cycle 2011; 10:3042-4; PMID:21862878; http://dx.doi.org/10.4161/cc.10.18.16947
- Veatch JR, McMurray MA, Nelson ZW, Gottschling DE. Mitochondrial dysfunction leads to nuclear genome instability via an iron-sulfur cluster defect. Cell 2009; 137:1247-58; PMID:19563757; http://dx.doi.org/10.1016/j.cell.2009.04.014
- Xu XM, Møller SG. Iron-sulfur clusters: biogenesis, molecular mechanisms, and their functional significance. Antioxid Redox Signal 2011; 15:271-307; PMID:20812788; http://dx.doi.org/10.1089/ars.2010.3259
- Brandes N, Tienson H, Lindemann A, Vitvitsky V, Reichmann D, Banerjee R, Jakob U. Time line of redox events in aging postmitotic cells. eLife 2013; 2:e00306; PMID:23390587
- Horvath SE, Daum G. Lipids of mitochondria. Prog Lipid Res 2013; 52:590-614; PMID:24007978; http://dx.doi.org/10.1016/j.plipres.2013.07.002
- Schlattner U, Tokarska-Schlattner M, Rousseau D, Boissan M, Mannella C, Epand R, Lacombe ML. Mitochondrial cardiolipin/phospholipid trafficking: the role of membrane contact site complexes and lipid transfer proteins. Chem Phys Lipids 2014; 179:32-41; PMID:24373850; http://dx.doi.org/10.1016/j.chemphyslip.2013.12.008
- Tamura Y, Sesaki H, Endo T. Phospholipid transport via mitochondria. Traffic 2014; 15:933-45; PMID:24954234; http://dx.doi.org/10.1111/tra.12188
- Tatsuta T, Scharwey M, Langer T. Mitochondrial lipid trafficking. Trends Cell Biol 2014; 24:44-52; PMID:24001776; http://dx.doi.org/10.1016/j.tcb.2013.07.011
- Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science 2001; 292:288-90; PMID:11292860; http://dx.doi.org/10.1126/science.1059497
- Gregg C, Kyryakov P, Titorenko VI. Purification of mitochondria from yeast cells. J Vis Exp 2009; 30:1417; PMID:19704406
- Titorenko VI, Smith JJ, Szilard RK, Rachubinski RA. Pex20p of the yeast Yarrowia lipolytica is required for the oligomerization of thiolase in the cytosol and for its targeting to the peroxisome. J Cell Biol 1998; 142:403-20; PMID:9679140
- Kyryakov P, Beach A, Richard VR, Burstein MT, Leonov A, Levy S, Titorenko VI. Caloric restriction extends yeast chronological lifespan by altering a pattern of age-related changes in trehalose concentration. Front Physiol 2012; 3:256; PMID:22783207; http://dx.doi.org/10.3389/fphys.2012.00256
- Sheibani S, Richard VR, Beach A, Leonov A, Feldman R, Mattie S, Khelghatybana L, Piano A, Greenwood M, Vali H, et al. Macromitophagy, neutral lipids synthesis, and peroxisomal fatty acid oxidation protect yeast from “liponecrosis”, a previously unknown form of programmed cell death. Cell Cycle 2014; 13:138-47; PMID:24196447
- Richard VR, Leonov A, Beach A, Burstein MT, Koupaki O, Gomez-Perez A, Levy S, Pluska L, Mattie S, Rafesh R, et al. Macromitophagy is a longevity assurance process that in chronologically aging yeast limited in calorie supply sustains functional mitochondria and maintains cellular lipid homeostasis. Aging (Albany NY) 2013; 5:234-69; PMID:23553280
