Although over 95% of pancreatic ductal adenocarcinoma (PDA) harbor Kras mutations, the presence of an oncogenic allele of Kras alone is not sufficient to develop pancreatic cancer. Moreover, mutant Kras is not constitutively-active per se,Citation1 and requires additional stimulation to initiate a feed forward loop for enhanced pathological level of activity.Citation2 In addition, a cooperation of signaling pathways activated by mutant Kras with signaling pathways initiated by epidermal growth factor receptor (EGFR)-activated wildtype Kras is needed for the development of PDA.Citation3 Given that Kras is difficult to target, an ideal strategy would be to target an enzyme that serves as a “bottleneck,” converging both pathways. In our recent work we have shown that the serine/threonine kinase Protein Kinase D1 (PKD1) functions downstream of oncogenic Kras and activated wildtype Kras (Fig. 1) and may represent such an ideal target.Citation4
The protein kinase D family of protein kinases actually consists of 3 isoforms PKD1, PKD2 and PKD3. In normal pancreas, only PKD3 is expressed in acinar cells, whereas PKD1 is only expressed in islets of Langerhans and pancreatic ducts.Citation4 This expression pattern changes when pancreatic acinar cells acquire an oncogenic Kras mutation or aberrant EGFR activation. In response to such signaling acinar cells undergo acinar-to-ductal metaplasia (ADM). During ADM cells down-regulate expression of PKD3 and upregulate expression of PKD1, whereas PKD2 expression remains unchanged.Citation4 In addition to increased expression, PKD1 activity is also elevated in presence of a mutant Kras, or after EGFR-mediated activation of endogenous wildtype Kras.Citation4 As a result of such signaling, PKD1 expression and activity can be detected in regions of ADM, PanIN1 and PanIN2 pre-neoplastic lesions, while the 2 other PKD isoforms are not involved in these processes.Citation4 Questions remaining are i) how PKD1 expression is upregulated by both, mutant Kras and EGFR signaling?; and ii) how both pathways can mediate activation of PKD1? Since PKD1 activity downstream of Kras was determined by measuring nPKC-mediated activating phosphorylations, an involvement of the novel PKCs PKCε and/or PKCδ is most likely, and this is currently under investigation.
In vitro explant culture studies, as well as in vivo animal models comparing mice that express oncogenic Kras in acinar cells (p48cre;LSL-KrasG12D mice) to mice with an additional acinar cell-specific knockout of PKD1, demonstrated that PKD1 not only drives the ADM process, but also contributes to Kras-mediated formation of precancerous lesions and to their progression.Citation4 We have shown that PKD1 regulates ADM by activating the Notch pathway, which previously had been established as a driver of acinar cell re-programming.Citation5 On one hand active PKD1 down-regulated the expression of known suppressors of Notch (e.g. Cbl, Sel1l). On the other hand, active PKD1 also upregulated the expression of Notch target genes (e.g., Hes-1, Hey-1), molecules that are involved in Notch signaling (e.g. MAP2K7), stem cell markers (e.g., CD44), as well as proteinases, including Adam10, Adam17 and MMP7, that mediate Notch activation by S2 cleavage.Citation4,6 Moreover, expression of active PKD1 led to formation of the Notch intracellular domain (NICD), which is caused by a γ-secretase-mediated cleavage step (S3 cleavage) subsequent to S2 cleavage. However, since PKD1 also regulates golgi transport processes, it is possible that it controls Notch1 activation at additional levels. Another interesting question is if PKD1-activated Notch can cooperate or crosstalk with other transcription factors which previously have been implicated in the development of pancreatic disease. For example, PKD1 has been shown to activate nuclear factor κ-B (NF-κB); and NF-κB and Notch both cooperate in some signaling pathways. Therefore, PKD1 is downstream of 2 critical signaling cascades driving PDA, and upstream of 2 critical transcription factors. It is tempting to speculate if PKD1 also contributes to activation of other key signaling events that regulate gene expression driving the formation of PDA, including activation of ERK1/2 and STAT3.
In animal models mimicking human disease, initiation of pancreatic cancer is driven by PKD1, but not the other isoforms of this kinase family.Citation4 Similarly, in previous studies using cell lines, PKD1 also has been implicated to drive proliferation, angiogenesis and chemoresistance, However, recent studies have implicated that in order to metastasize tumor cells need to undergo an isoform switch to expression of PKD2.Citation7 This is an interesting aspect of PKD biology, and similar isoform switches (PKD1 to PKD2/3 switch) were observed for other cancers including invasive breast, colorectal or prostate cancers. However, the functional dichotomy that is caused by such isoform switches is not well understood, yet. It is also important to keep in mind that so far most of the data regarding the roles of PKD isoforms in pancreatic cancer was obtained with cancer cell lines, which may not fully reflect their roles in tumor development or progression in organisms.
In summary, since PKD1 is upregulated during acinar cell transformation, PanIN progression and formation of pancreatic cancer, and since it converges important signaling pathways that drive these processes, it has potential for both, development as a biomarker and a drug target.
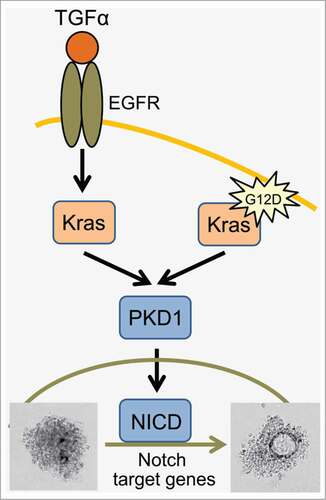
Figure 1. Schematic overview of how PKD1 is involved in regulation of ADM downstream of EGFR and mutant Kras. Activation of wildtype Kras via EGFR or acquisition of an oncogenic Kras mutation (here KrasG12D) both lead to upregulation and activation of PKD1. PKD1 once activated mediates ADM through Notch.
References
- Huang H, et al. Oncogene 2014; 33:532-5; PMID:23334325; https://doi.org/10.1038/onc.2012.619
- Daniluk J, et al. J Clin Invest 2012; 122:1519-28; PMID:22406536; https://doi.org/10.1172/JCI59743
- Ardito CM, et al. Cancer Cell 2012; 22:304-17; PMID:22975374; https://doi.org/10.1016/j.ccr.2012.07.024
- Liou GY, et al. Nat Commun 2015; 6:6200; PMID:25698580; https://doi.org/10.1038/ncomms7200
- Apelqvist A, et al. Nature 1999; 400:877-81; PMID:10476967; https://doi.org/10.1038/23716
- Sawey ET, et al. Cell Cycle 2008; 7:566-9; PMID:18239463; https://doi.org/10.4161/cc.7.5.5531
- Wille C, et al. Mol Biol Cell 2014; 25:324-36; PMID: 24336522; https://doi.org/10.1091/mbc.E13-06-0334
