Abstract
Functional loss of expression of breast cancer susceptibility gene 1(BRCA1) has been implicated in genomic instability and cancer progression. There is emerging evidence that BRCA1 gene product (BRCA1) also plays a role in cancer cell migration. We performed a quantitative proteomics study of EOC patient tumor tissues and identified changes in expression of several key regulators of actin cytoskeleton/cell adhesion and cell migration (CAPN1, 14-3-3, CAPG, PFN1, SPTBN1, CFN1) associated with loss of BRCA1 function. Gene expression analyses demonstrate that several of these proteomic hits are differentially expressed between early and advanced stage EOC thus suggesting clinical relevance of these proteins to disease progression. By immunohistochemistry of ovarian tumors with BRCA1+/+ and BRCA1null status, we further verified our proteomic-based finding of elevated PFN1 expression associated with BRCA1 deficiency. Finally, we established a causal link between PFN1 and BRCA1-induced changes in cell migration thus uncovering a novel mechanistic basis for BRCA1-dependent regulation of ovarian cancer cell migration. Overall, findings of this study open up multiple avenues by which BRCA1 can potentially regulate migration and metastatic phenotype of EOC cells.
Abbreviations
| EOC | = | Epithelial Ovarian Cancer |
| BRCA1 | = | Breast cancer susceptibility gene 1 |
| BRCA2 | = | Breast cancer susceptibility gene 2 |
| PFN1 | = | Profilin-1 |
| ID1 | = | Inhibitor of differentiation-1 |
| ERM | = | Ezrin-Radixin-Moesin |
| CFN1 | = | Cofilin-1 |
| CAPG | = | Macrophage capping protein |
| SPTBN1 | = | Non-erythrocytic spectrin β Chain-1 |
| PP2A | = | Protein phosphatase 2A |
| HYOU1 | = | Hypoxia upregulated protein 1 |
| CAPN1 | = | Calpain-1 |
| FFPE | = | Formalin-fixed paraffin-embedded |
| WT | = | Wild-type |
| Luc | = | luciferase |
| IHC | = | Immunohistochemistry |
| LC MS-MS | = | Liquid chromatography tandem mass spectrometry |
Introduction
There are an estimated 21,290 new cases of epithelial ovarian cancer (EOC) and 14,180 deaths attributable to this disease in 2015 (Cancer Facts and Figures 2015, American Cancer Society). Of these new cases, approximately 10–15% are estimated to harbor underlying germline mutations in either BRCA1 (breast cancer type 1 susceptibility protein) or BRCA2 (breast cancer type 2 susceptibility protein).Citation1,2 There is increasing evidence that many EOC tumors harbor somatic mutations in BRCA1 or 2, and these tumors are phenotypically and clinically similar to tumors with germline BRCA mutations.Citation3,4 It is also increasingly appreciated that the abundance level of the BRCA1 protein (BRCA1) can be variable in EOC, resulting from either genetic or epigenetic mechanisms.Citation5 As such, it is imperative to further our understanding of BRCA from a molecular standpoint to better understand etiology, disease progression, and response to chemotherapy and/or molecularly-targeted therapy in this group of patients.
BRCA1 encodes an 1863 amino acid protein that contains several functional regions including RING (Really interesting new gene) and BRCT (BRCA1 C-terminal) domains at the N- and C-termini, respectively. It functions as an E3-class ubiquitin ligase when it is in heterodimer with BARD1 (BRCA1-associated RING domain protein-1). BRCA1 is responsible for multiple functions related to DNA damage response and repair, transcription, cell-cycle check-point regulation, DNA decatenation, protein ubiquitination and apoptosis.Citation6-10 There is emerging evidence that BRCA1 also plays a role in cell migration and invasion. Full-length BRCA1 restoration in immortalized human mammary epithelial cell line that harbors BRCA1 mutation leads to alteration in the expression of several proteins that are important for initiation of invasion and metastasis (E-cadherin, P-cadherin, caveolin and ID1 (inhibitor of differentiation-1)) with concomitant inhibition of cell migration and invasion.Citation11 Whether there is a direct causal relationship between BRCA1-dependent changes in the expression of any of those proteins and cell motility is yet to be demonstrated. Another study revealed that BRCA1 interacts with ezrin-radixin-moesin (ERM), a family of proteins that connect plasma membrane to the underlying actin cytoskeleton, and co-localizes with F-actin at the plasma membrane at the leading edges and focal adhesions. Disruption of this endogenous interaction through forced expression of a truncated form of BRCA1 that retains its C-terminus but lacks the N-terminal ubiquitin ligase domain induces delay in spreading but increases the spontaneous motility of human breast cancer cells. These findings led to a model that BRCA1 suppresses motility of breast cancer cells through its ubiquitin ligase activity at least partly via regulating ERM protein content at the membrane.Citation12 How BRCA1 affects migration of other types of carcinoma cells has not been studied.
BRCA1 is involved in multiple levels of gene expression control. To date, attempts to define a molecular profile of BRCA deficiency have focused primarily on DNA microarray-based gene expression analyses.Citation13-15 However, these types of analyses fail to reveal post-transcriptional and post-translational changes in gene expression. In this study, we assessed for the first time global proteomic changes associated with BRCA1 deficiency in highly annotated ovarian cancer patient tumor specimens to identify novel targets of BRCA1 that are involved in actin cytoskeletal remodeling and cell migration, and have clinical association to advanced stage ovarian cancer.
Results and Discussion
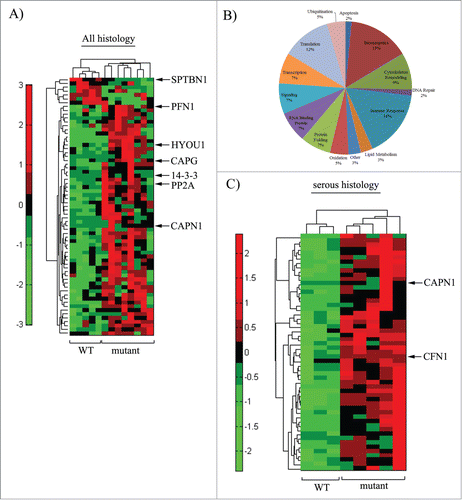
To assess proteomic changes associated by BRCA1 deficiency in EOC, we performed global proteomic analyses of formalin-fixed, paraffin-embedded (FFPE) EOC patient tissue specimens (8 patients with a known deleterious mutation in BRCA1 and 5 patients with wild-type (WT) BRCA1 status, Table S1). Tumor stages were defined by 2014 International Federation of Gynecology and Obstetrics (FIGO) guidelines (Stage I: tumor confined to ovaries; Stage II: tumor involves one or both ovaries with pelvic extension (i.e below the pelvic brim) or primary peritoneal cancer; Stage III: tumor involves one or both ovaries, confirmed spread to extra-pelvic peritoneum and/or metastasis to the retroperitoneal lymph nodes; Stage IV: distant metastasis excluding peritoneal metastasis). We found 67 differentially abundant proteins between the 2 cohorts with P < 0.05 (see for cluster diagram and Table S2 for a complete list of those proteins and associated fold-changes, respectively). Differentially expressed proteins could be categorized into several functional groups including bioenergetics, cytoskeletal support and regulation, lipid metabolism, gene transcription, protein translation, immune regulation, RNA-binding, protein ubiquitination, protein folding and secretion, and signal transduction (). Functional diversity of differentially expressed proteins suggests that loss of BRCA1 function has pleotropic effects in ovarian cancer cells. Clearly our observation that expressions of several proteins involved in ubiquitination pathway (cullin-associated NEDD8-dissociated protein, 2 proteasome subunits) are altered in BRCA1-deficient tumors is interesting in light of previous findings that implicated BRCA1 controls cell motility at least partly via its ubiquitin ligase activity.Citation12 We identified 7 candidate proteins that showed at least 1.5-fold change in abundance and have established connections to regulation of cell migration and invasion. Six out of these 7 proteins are known to either directly or indirectly influence actin cytoskeleton and/or cell adhesion. These include:
Figure 1. Quantitative global proteomic analyses of BRCA1-WT and BRCA1-null ovarian tumors. (A) Hierarchical clusters show differentially abundant proteins between the cohorts of BRCA1-mutant and –WT tumors. (B) Pie chart representing the differentially expressed proteins based on functional groups. (C) Hierarchical clusters for differentially abundant proteins between cohorts of tumors with papillary serous histology only. See Tables S2 and S3 for the list of proteins differentially expressed in BRCA1-mutant vs –WT tumors for these 2 sets of analyses. Hits that either directly or indirectly regulate actin cytoskeleton/cell adhesion and impact cell migration are indicated by arrows in cluster diagrams.
Calpain-1 catalytic subunit (CAPN1 - increased by 16.9-fold): CAPN1 is the ubiquitously expressed member of CAPN family of calcium-dependent cysteine proteases that is implicated in regulation of actin cytoskeleton and cell migration.Citation16 As adhesion-associated proteins are proteolytic targets of CAPN, inhibition of CAPN leads to increased stability of cell-substrate adhesion and reduced cell migration.Citation17,18 CAPN1 has been also shown to proteolyze RhoA which can inhibit cell spreading,Citation19 an important aspect for cell migration. Depending on the tumor type, CAPN family of proteins is differentially expressed in tumors relative to their normal counterparts. Overexpression of one or more members of CAPN family of proteins (CAPN1, CAPN2) has been noted in breast, colorectal, meningiomas and renal cell carcinomas.Citation20-23 However, in the case of gynecological (ovarian, endometrial) cancers, CAPN expression/activity appears to be inversely correlated with the tumor grade and/or overall survival as suggested by a limited number of clinical correlation studies.Citation24,25 Therefore, the role of CAPN family proteins in tumor pathogenesis and progression appears to be context-specific.
14-3-3 (increased by 2.8-fold): Because of its ability to interact with many functionally diverse signaling proteins (kinases, phosphatases, and transmembrane receptors), 14-3-3 is traditionally recognized as a signal transduction protein. However, it also associates with actin cytoskeleton Citation26 and regulates actin remodeling pathways. For example, it inhibits Rho-GDI (a negative regulator of Rho GTPases) and in turn activates Rho-ROCK (Rho-associated coiled-coiled kinase) pathway, an important signaling arm for control of actin polymerization.Citation27 It also prevents inactivation of cofilin (CFN - an F-actin-depolymerizing factor which is required for accelerating actin dynamics at the leading edge of migrating cells) through increasing the cellular pool of slingshot (a phosphatase that dephosphorylates and maintains cofilin in an active state).Citation28 14-3-3 family proteins promote cell migration Citation29 and have been reported to be overexpressed and associated with malignancy in human cancers including ovarian cancer.Citation30
Protein phosphatase 2A (PP2A - increased by 5.3-fold): PP2A, a broad spectrum serine/threonine phosphatase is an important regulator of many signal transduction pathways that involve serine/threonine phosphorylation. It regulates the activities of CAPN1Citation31 and focal adhesion proteins (such as paxillin)Citation32 thereby influencing the stability of focal adhesion complexes. PP2A is also involved in dephosphorylation of CFN1 and therefore regulation of actin cytoskeleton.Citation33 Presumably through some of these actions, PP2A regulates cell migration. PP2A has been considered to be a suppressor of tumor growth and metastasis, and it is frequently mutated in gynecological cancers (more prevalent in endometrial than ovarian cancer) with poor prognosis.Citation34
Macrophage capping protein (CAPG - increased by 2.0 fold): CAPG modulates actin filament dynamics through capping the barbed ends of actin filaments. It acts as an oncogene in various carcinomas (pancreatic, melanoma),Citation35,36 promotes cell migration and invasion of both breast and ovarian cancer cells, and has been implicated as a biomarker for stage III ovarian cancer.Citation37,38
Non-erythrocytic spectrin beta-chain (SPTBN1 - decreased by 2.5-fold): Spectrin acts as scaffolding protein for the actin cytoskeleton by linking the plasma membrane to actin filaments. Spectrin plays a key role in determining the cell shape and organization of organelles in the cell, and reduced expression of beta1-spectrin (SPTBN1) correlates with poorer prognosis for pancreatic cancer patients.Citation39 Although the role of spectrin has not been directly investigated in the context of ovarian cancer cell migration and metastasis, spectrin family proteins have been implicated in drug resistance to cisplatin therapy in serous ovarian cancer.Citation40
Profilin1 (PFN1 - increased by 1.75-fold): PFN1 is a ubiquitously expressed G-actin-binding protein that is directly involved in regulating actin polymerization in cells. In most physiological contexts, functional loss of PFN1 causes defects in membrane protrusion and/or cell-matrix adhesion leading to impaired cell motility and ECM invasion.Citation41-44 Consistent with this pro-migratory function of PFN1, high cytoplasmic expression of PFN1 has been reported to be associated with advanced stage and shorter disease-free survival in clear cell renal cell carcinoma.Citation45 On contrary, breast cancer cells become hypermotile upon downregulation of either PFN1 or its close isoform PFN2, and this correlates with the clinical findings of prominent PFN1 downregulation associated with metastatic propensity in human breast cancer.Citation46-48 Thus, PFN1 appears to have a tissue context-specific role in cell migration and tumor malignancy. PFN1's role is yet to be studied in the context of gynecological cancer.
The only other protein which showed prominent change in expression associated with BRCA1 deficiency and is known to influence cell migration is Hypoxia upregulated protein 1 (HYOU1 - increased by 4.8-fold), a molecular regulator of cell signaling. HYOU1 is an oxygen-regulated molecular chaperone that belongs to the family of heat shock and ER-stress proteins. HYOU1 is upregulated in invasive human breast and head and neck cancers, and, at least, in the case of breast cancer, overexpression of HYOU1 is associated with increased lymphovascular infiltration.Citation49,50 HYOU1 has been shown to influence cell migration through regulating chemokine secretion.Citation51
Given the heterogeneity of the patient population, a supervised cluster analysis was also performed taking into account tumor histology. When the cohorts of BRCA1 mutant (N = 5) and WT (N = 3) tumors were evaluated using only serous histology, a more striking clustering of differentially abundant proteins was observed (; see Table S2 for the list of 55 proteins that were found to be elevated in BRCA1-mutated cohort with P < 0.05). Note that ∼38% of hits found in mixed histology proteomic analyses (equivalent to 26 proteins) were also represented in the list of differentially expressed proteins for serous histology tumors. In addition to CAPN1 (increased by 8.8 fold), cofilin-1 (CFN1), another prominent regulator of actin cytoskeleton and cell migration, was found to be elevated (by 1.6 fold) in BRCA1-mutated cohort of serous histology. Upregulation of signaling pathways that inactivate CFN1 has been shown to promote ovarian cancer cell migration, and accordingly, expression of CFN1 positively correlates with differentiation status and progression-free survival of patients in ovarian cancer.Citation52 We noticed that limiting global proteomic analyses on serous tumor subset led to exclusion of PFN1, 14-3-3, HYOU 1, PP2A1, CAPG and SPTBN1 from the differentially expressed protein hits in the cluster diagram. We asked whether at least some of these proteins failed to meet the criterion for statistical significance because of non-parametric Wilcoxon Rank test adopted in the global proteomic analyses and even smaller sample size in serous cohort analyses. Indeed, when we used parametric Student's T-test to individually compare the mean spectral counts of these proteins, PFN1 (2-fold change; p = 0.005) and PP2A (8-fold change; p = 0.01) but not others were found to be differentially expressed between WT and BRCA1–mutant serous cohorts.
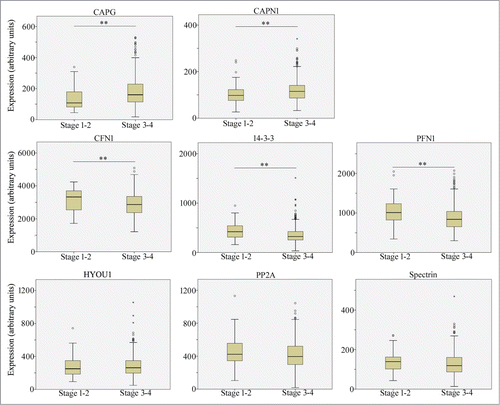
We next queried whether any of the actin cytoskeleton- and/or cell adhesion-modulatory proteins that are differentially expressed in BRCA1-deficient tumors has clinical association to advanced stage ovarian cancer. We compared the mean expression level of our genes of interest (CAPN1, 14-3-3, PP2A, CAPG, SPTBN1, PFN1, HYOU1, CFN1) between advanced (stages III and IV combined) and early (stages I and II combined) stage EOC from curated microarray dataset (source: TCGA (The Cancer Genome Atlas) database) derived from a total of 580 patient tumor samples. Five out of the 8 candidate genes (PFN1, 14-3-3, CFN1, CAPG, CAPN1) showed statistically significant difference in expression in primary tumors between advanced and early stage EOC. CAPG and CAPN1 exhibited an increase in expression in advanced stage EOC while PFN1, CFN1 and 14-3-3 showed a reverse trend (). Together, our proteomic and gene expression analyses studies demonstrate that BRCA1 deficiency in EOC is associated with alteration in expression of several key actin cytoskeleton- and/or cell-adhesion regulatory proteins that also have clinical relevance to disease progression.
Figure 2. A subset of cell motility-regulatory proteins impacted by loss of BRCA1 function exhibit differential expression association to disease progression in ovarian cancer. Box plots show the comparison of mRNA levels of indicated genes between early stage (Stages I and II) and late stage (stages III and IV) serous ovarian cancer tumor samples, based on the analyses of TCGA microarray data set (** indicates P < 0.05).
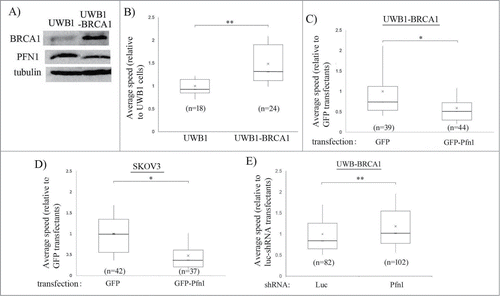
Of those 5 candidate actin cytoskeleton- and/or cell-adhesion regulatory proteins which revealed differential expression association to both BRCA1 status and advanced stage in ovarian cancer (CAPG, CAPN1, CFN1, PFN1, 14-3-3), we chose to further our studies on PFN1 as it is the only gene on this list that has not been studied at all in the context of ovarian cancer. We first corroborated the proteomic results by performing PFN1 immunostaining of tumor histosections from patient samples which showed the general trend of increased PFN1 expression in BRCA1-deficient tumors (). The average PFN1 immunoreactivity of BRCA1-mutant tumors was approximately ∼50% higher than that of WT tumor and the difference was statistically significant (P < 0.01) (). In alignment with these patient sample data, SKOV3, a BRCA1-proficient EOC cell line, exhibited markedly lower PFN1 expression when compared to BRCA1-deficient SNU-251 cell line (). Given our data showing differential expression of PFN1 associated with BRCA1 status in EOC, we asked whether BRCA1 can impact EOC cell motility through modulating PFN1 expression. To query BRCA1's role in EOC motility, we used UWB1.289 (UWB1 – a cell line derived from a serous EOC patient with a germline mutation in BRCA1 (2594delC)) and its isogenic counterpart UWB1.289-BRCA1 (UWB1-BRCA - in this cell line, BRCA1 is restored via stable transfection of full length BRCA1)Citation53 cell lines. As expected, UWB1 cells presented higher PFN1 expression than UWB1-BRCA1 cells () further validating our proteomics- and immunohistochemistry-based finding of PFN1 elevation associated with BRCA1 mutation. BRCA1 restoration increased the average speed of UWB1 cells by ∼50% thus demonstrating that BRCA1 can promote EOC cell motility (). The average speed of UWB1-BRCA1 cells was dramatically reduced when PFN1 expression was elevated by transient overexpression of GFP-PFN1 (). PFN1-induced inhibition of cell motility was also observed in the case of BRCA1-proficient SKOV3 cells (). Conversely, PFN1 knockdown by transient transfection with a small hairpin RNA (shRNA) further enhanced the motility of UWB1-BRCA1 cells [; the average reduction in Pfn1 expression by shRNA transfection was ∼75%, as judged by immunostaining method (Fig. S1)]. Together, these results demonstrate that PFN1 has an inhibitory action on EOC cell motility, and BRCA1 impacts EOC cell motility at least partly through altering PFN1 expression.
Figure 3. BRCA1 mutation is associated with increased PFN1 expression in EOC. (A) Fluorescence micrographs of WT- vs BRCA1-mutated ovarian cancer FFPE histosections stained with anti-PFN1 and anti-pan-cytokeratin antibodies. (B) Bar-graph comparing the average fluorescence intensity of PFN1 immunoreactivity between WT and BRCA1-mutated tumors (only cytokeratin-positive areas were scored) (** indicates P < 0.01). Counter-imaging of hematoxylin and eosin-stained slides further ensured that only tumor tissues were scored. (C) Total extracts of SNU-251 and SKOV3 cells were immunoblotted with anti-PFN1, and anti-tubulin (loading control) antibodies.
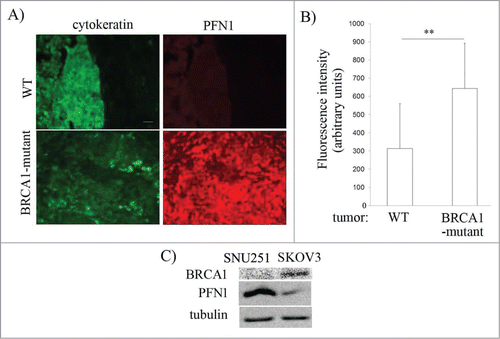
Figure 4. BRCA1 promotes EOC cell motility through downregulating PFN1 expression. (A) Total extracts of UWB1 vs UWB1-BRCA1 cells were immunoblotted with anti-BRCA1, anti-PFN1, and anti-tubulin. (B) A box and whisker plot depict the average speed of randomly migrating UWB1 and UWB1-BRCA1 cells (n indicates number of cells from 2 independent experiments, ** P < 0.01). (C, D) Box and whisker plots represent the average speeds of UWB-BRCA1 (C) and SKOV3 cells (D) expressing either GFP (control) or GFP-PFN1 (n indicates number of cells from 3 independent experiments). (E) A box and whisker plot represents the average speed of UWB-BRCA1 cell line expressing shRNAs targeting either Luciferase (luc – control) or PFN1. Transfected cells were identified by expression of an RFP reporter encoded by the plasmid. (‘n’ indicates number of cells from 5 independent experiments, *P < 0.05, **P < 0.01).
How might PFN1 exert an anti-migratory action in ovarian cancer cells? Besides actin, PFN1 also interacts with membrane phosphoinositides and a wide range of proteins containing poly-L-proline (PLP) motifs including many prominent regulators of actin cytoskeleton (e.g., N-WASP (Neural Wiskott Aldrich Syndrome protein), Arp (actin-related protein)-2/3, formins and Enabled/VASP (vasodilator activated phosphoprotein)).Citation54 We previously demonstrated that PFN1's inhibitory action on breast cancer cell migration/invasion is mediated by its phosphoinositide-binding rather than its interaction with actin, and this is through suppression of phosphoinositide-dependent recruitment of pro-migratory protein complexes to the leading edge.Citation46 It is possible that a similar mechanism might be applicable in the case of EOC cells. Recently it has been demonstrated that depletion of PFN1 increases F-actin and Arp2/3 complex (a PLP-ligand of Pfn1 that initiates branched F-actin network formation through actin nucleation thereby leading to membrane protrusion) content at the lamellipodia in cells.Citation55 Therefore, an alternative possibility is that Pfn1 inhibits EOC cell motility via putting a brake on Arp2/3-mediated actin assembly at the leading edge. In addition to addressing these mechanistic questions, future studies should also explore whether there is any causal relationship between PFN1 downregulation and increased disseminative propensity of EOC cells in vivo.
Finally, how functional status of BRCA1 impacts PFN1 expression is not clear. It is possible that BRCA1 directly impinges on one of the regulatory pathways of PFN1 expression Gene expression analyses of TCGA data sets showed no significant change in PFN1 mRNA expression between BRCA1-WT vs –mutant tumors (data not shown). This could be due to very low number of BRCA-mutated samples (11 out of a total 304 patient tumors in TCGA data set that were characterized for somatic mutation) in the cohort failing to give us adequate power for statistical analyses. Based on the findings of our proteomic analyses that revealed alteration in expression of several proteins in protein ubiquitination pathway (cullin-associated NEDD8-dissociated protein, ubiquitin-thioesterase OTUB1 and 2 proteasome subunits – see Tables S2 and S3) in BRCA1-deficient tumors, it is highly possible that loss of BRCA1 function perturbs protein turnover pathways in cells thereby post-translationally influencing the expression of a range of target proteins including PFN1. Future studies are needed to resolve these outstanding questions.
In conclusion, we revealed association of functional status of BRCA1 with expression of several major regulators of actin cytoskeletal/cell adhesion remodeling and cell migration in EOC and further demonstrated differential expression of a subset of these proteins between early and advanced stage patient tumors. Therefore, this study opens up multiple possible avenues by which BRCA1 can potentially regulate migration and metastatic phenotype of EOC cells. Through further establishing a causal link between PFN1 and BRCA1-induced changes in EOC cell migration, we uncovered a novel mechanistic basis for BRCA1-mediated regulation of cell motility.
Methods and Materials
Liquid chromatography-tandem mass spectrometry (LC-MS/MS)
For these experiments, thin sections were prepared from FFPE samples on standard microscope slides, deparaffinized in xylene and rehydrated through graded ethanol washes. Cancerous epithelial cells were identified through microscopic evaluation and harvested by manual scraping. The harvested tissue samples were processed and digested with porcine sequencing grade modified trypsin (Promega) as previously described.Citation56 Samples were and desalted with PepClean columns according to the manufacturer's procedure (Pierce). Samples were dried by vacuum centrifugation and stored at −80°C until proteomic analysis. Peptide digests were resolved by nanoflow reversed-phase liquid chromatography (Ultimate 3000, Dionex Inc.) coupled online via electrospray ionization to a hybrid linear ion trap-Orbitrap Velos mass spectrometer (MS, ThermoFisher Scientific Inc.). Tandem mass spectra were searched against the UniProt human protein database (02/13 release) from the UniProt Knowledgebase (UniProtKB) website (http://www.uniprot.org/), using SEQUEST (Thermo Proteome Discoverer, v1.4.1.14, ThermoScientific). Search criteria were set as follows: peptides were searched fully tryptic with up to 2 missed cleavage sites, dynamic modification of methionine oxidation (15.9949 Da), precursor mass tolerance of 20 ppm and fragment ion tolerance of 0.5 Da. Peptide and protein identifications were further screened using Peptide Prophet algorithm and Protein Prophet Algorithm which was included in Scaffold proteome platform (v4.3.0, Proteome Software Inc.). Peptide identifications were accepted if they could be established at greater than 95.0% probability as specified by the Peptide Prophet. Protein identifications were accepted if they could be established at greater than 99.0% probability and contained at least 2 identified peptides. A false protein discovery rate of 0.9% and a false peptide discovery rate of 0.16% were determined by this criterion. A total of 55054 spectra and 775 protein groups were accepted. The qualitative differences in protein abundance between samples were derived by summing the total CID events that resulted in a positively identified peptide for a given protein accession across all samples (spectral counting). The proteins with differential abundance were identified using Wilcoxon rank-sum test. Significance level was set to P < 0.05. Hierarchical clustering was carried out using MATLAB (vR2011a, MathWorks, Inc.). The values for spectral counts were standardized for each protein so that each had a mean of 0 and a standard deviation of 1. Both sample distance and protein feature distance were calculated using Pearson's correlation and average linkage.
Fluorescence immunohistochemistry
Histosections were cleared using xylenes and rehydrated prior to antigen retrieval (DAKO, S1700, 10 mM Citrate, pH 6.0, 95° C, 25 min) Following retrieval, histosections were blocked with a H2O2 solution (0.75% H2O2 in methanol) for 30 minutes and then again in .3% BSA w/v in TBS-T. The primary target (anti-PFN1 (1:200; Novus Biologicals, http://www.uniprot.org/)) was secondarily stained with DAKO EnVision + System HRP with Cy5-Tyramide Reagent Pack (1:50; PerkinElmer, K4002). Sections were also stained with anti-pan-cytokeratin (1:100; DAKO, http://www.dako.com/us/ar38/p103580/prod_products.htm) and cytokeratin was counterstained with Alexa-546 (1:100; Life Technologies, http://www.lifetechnologies.com/order/catalog/product/A11030).
Cell culture
UWB1.289 and UWB-BRCA1 cell lines (ATCC (CRL-2945 and CRL-2946)) were cultured in 50% RPMI and 50% MEGM, supplemented with 3% fetal bovine serum at 37°C and 5% CO2. SKOV3 (ATCC) cell line was cultured in RPMI, supplemented with 10% fetal bovine serum at 37°C and 5% CO2.
Transfections
For all experiments, plasmid transfection was performed by Lipofectamine LTX (Life Technologies, http://www.lifetechnologies.com/order/catalog/product/15338100) transfection agent. Motility experiments were performed 48 h after transfection.
Cell motility assay
For cell motility assessment, cells were sparsely plated on a 48-well tissue-culture plate and after an overnight incubation, time-lapse imaging of randomly migrating cells was performed simultaneously at 4 locations and at an interval of 1 min for a total duration of 120 min. The acquired images were analyzed using the NIH ImageJ software to compute the total distance traveled by cells during the observation time. For box and whisker plots, x represents the mean, middle line of box indicates median, top of the box indicates 75th percentile, bottom of the box measures 25th percentile and the 2 whiskers indicate the 10th and 90th percentiles, respectively.
Protein extraction and immunoblotting
Whole cell protein extracts were prepared from ∼70% confluent cultures using 0.1% SDS-containing lysis buffer (50 mM Tris-HCl, 1 mM EDTA, 1 mM phenyl-methane-sulphonylfloride). Antibody concentrations used for immunobloting were as follows: antibodies. The concentrations of primary antibodies for immunoblotting were: anti-profilin-1 (1:5000; Novus Biologicals), anti-α tubulin (1:3000; Sigma-Aldrich, http://www.sigmaaldrich.com/catalog/product/sigma/t9026?lang=en®ion=US), and anti-BRCA1 (1:1000; EMD Millipore, http://www.emdmillipore.com/US/en/product/Anti-BRCA1-%28Ab-1%29-Mouse-mAb-%28MS110%29,EMD_BIO-OP92).
Gene differential expression analysis
Gene expression (Affymetrix platform) and clinical data were downloaded from UCSC Cancer browser (version 2013-12-18), and a Welch Two Sample t-test was performed in R software to query the differential expression.
Immunostaining
Cells were fixed with 3.7% formaldehyde for 10 min, permeabilized with 0.5% Triton X-100 in DPBS for 10 min before blocking in 10% goat serum and incubating with anti-PFN1 (1:200; Novus Biologicals) in 10% goat serum. Cells were washed 5 times (3 times with DPBS containing 0.02% tween then incubated with secondary FITC antibody (1:100; Jackson ImmunoResearch Laboratories Inc., 111-546-144).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
1036203_supplemental_files.zip
Download Zip (707.9 KB)Funding
This research was supported by a grant from the National Institute of Health (2R01-CA108607 to PR), a generous gift from Ray and Stefanie Lane to Magee-Women's Research Foundation, National Science Foundation Graduate Research Fellowship (Grant No. 2012139050 to DMG), and by the University of Pittsburgh Cancer Institute Cancer Biomarkers Facility that is supported in part by award P30-CA047904.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website
References
- Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Kwan E, Jack E, Vesprini DJ, Kuperstein G, Abrahamson JL, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet 2001; 68:700-10; PMID:11179017; http://dx.doi.org/10.1086/318787
- Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, LaPolla J, Hoffman M, Martino MA, Wakeley K, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer 2005; 104:2807-16; PMID:16284991; http://dx.doi.org/10.1002/cncr.21536
- Hennessy BT, Timms KM, Carey MS, Gutin A, Meyer LA, Flake DD, 2nd, Abkevich V, Potter J, Pruss D, Glenn P, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol 2010; 28:3570-6; PMID:20606085; http://dx.doi.org/10.1200/JCO.2009.27.2997
- Tan DS, Rothermundt C, Thomas K, Bancroft E, Eeles R, Shanley S, Ardern-Jones A, Norman A, Kaye SB, Gore ME. “BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol 2008; 26:5530-6; PMID:18955455; http://dx.doi.org/10.1200/JCO.2008.16.1703
- Chan KY, Ozcelik H, Cheung AN, Ngan HY, Khoo US. Epigenetic factors controlling the BRCA1 and BRCA2 genes in sporadic ovarian cancer. Cancer Res 2002; 62:4151-6; PMID:12124354
- Wu J, Lu LY, Yu X. The role of BRCA1 in DNA damage response. Protein Cell 2010; 1:117-23; PMID:21203981; http://dx.doi.org/10.1007/s13238-010-0010-5
- Scully R, Chen J, Ochs RL, Keegan K, Hoekstra M, Feunteun J, Livingston DM. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell 1997; 90:425-35; PMID:9267023; http://dx.doi.org/10.1016/S0092-8674(00)80503-6
- Fabbro M, Savage K, Hobson K, Deans AJ, Powell SN, McArthur GA, Khanna KK. BRCA1-BARD1 complexes are required for p53Ser-15 phosphorylation and a G1/S arrest following ionizing radiation-induced DNA damage. J Biol Chem 2004; 279:31251-8; PMID:15159397; http://dx.doi.org/10.1074/jbc.M405372200
- Xu B, Kim S, Kastan MB. Involvement of Brca1 in S-phase and G(2)-phase checkpoints after ionizing irradiation. Mol Cell Biol 2001; 21:3445-50; PMID:11313470; http://dx.doi.org/10.1128/MCB.21.10.3445-3450.2001
- Zhang F, Ma J, Wu J, Ye L, Cai H, Xia B, Yu X. PALB2 links BRCA1 and BRCA2 in the DNA-damage response. Curr Biology 2009; 19:524-9; PMID:19268590; http://dx.doi.org/10.1016/j.cub.2009.02.018
- Yasmeen A, Liu W, Dekhil H, Kassab A, Aloyz R, Foulkes WD, Al Moustafa AE. BRCA1 mutations contribute to cell motility and invasion by affecting its main regulators. Cell Cycle 2008; 7:3781-3; PMID:19098453; http://dx.doi.org/10.4161/cc.7.23.6993
- Coene ED, Gadelha C, White N, Malhas A, Thomas B, Shaw M, Vaux DJ. A novel role for BRCA1 in regulating breast cancer cell spreading and motility. J Cell Biol 2011; 192:497-512; PMID:21282464; http://dx.doi.org/10.1083/jcb.201004136
- Pradhan M, Risberg BA, Trope CG, van de Rijn M, Gilks CB, Lee CH. Gross genomic alterations and gene expression profiles of high- grade serous carcinoma of the ovary with and without BRCA1 inactivation. BMC Cancer 2010; 10:493; PMID:20843305; http://dx.doi.org/10.1186/1471-2407-10-493
- Konstantinopoulos PA, Spentzos D, Karlan BY, Taniguchi T, Fountzilas E, Francoeur N, Levine DA, Cannistra SA. Gene expression profile of BRCAness that correlates with responsiveness to chemotherapy and with outcome in patients with epithelial ovarian cancer. J Clin Oncol 2010; 28:3555-61; PMID:20547991; http://dx.doi.org/10.1200/JCO.2009.27.5719
- Jazaeri AA, Yee CJ, Sotiriou C, Brantley KR, Boyd J, Liu ET. Gene expression profiles of BRCA1-linked, BRCA2-linked, and sporadic ovarian cancers. J Natl Cancer Inst 2002; 94:990-1000; PMID:12096084; http://dx.doi.org/10.1093/jnci/94.13.990
- Wells A, Huttenlocher A, Lauffenburger DA. Calpain proteases in cell adhesion and motility. Int Rev Cytol 2005; 245:1-16; PMID:16125543; http://dx.doi.org/10.1016/S0074-7696(05)45001-9
- Dourdin N, Bhatt AK, Dutt P, Greer PA, Arthur JS, Elce JS, Huttenlocher A. Reduced cell migration and disruption of the actin cytoskeleton in calpain-deficient embryonic fibroblasts. J Biol Chem 2001; 276:48382-8; PMID:11602605
- Huttenlocher A, Palecek SP, Lu Q, Zhang W, Mellgren RL, Lauffenburger DA, Ginsberg MH, Horwitz AF. Regulation of cell migration by the calcium-dependent protease calpain. J Biol Chem 1997; 272:32719-22; PMID:9407041; http://dx.doi.org/10.1074/jbc.272.52.32719
- Kulkarni S, Goll DE, Fox JE. Calpain cleaves RhoA generating a dominant-negative form that inhibits integrin-induced actin filament assembly and cell spreading. J Biol Chem 2002; 277:24435-41; PMID:11964413; http://dx.doi.org/10.1074/jbc.M203457200
- Lakshmikuttyamma A, Selvakumar P, Kanthan R, Kanthan SC, Sharma RK. Overexpression of m-calpain in human colorectal adenocarcinomas. Cancer Epidemiol Biomarkers Prev 2004; 13:1604-9; PMID:15466976
- Storr SJ, Lee KW, Woolston CM, Safuan S, Green AR, Macmillan RD, Benhasouna A, Parr T, Ellis IO, Martin SG. Calpain system protein expression in basal-like and triple-negative invasive breast cancer. Ann Oncol 2012; 23:2289-96; PMID:22745213; http://dx.doi.org/10.1093/annonc/mds176
- Kimura Y, Koga H, Araki N, Mugita N, Fujita N, Takeshima H, Nishi T, Yamashima T, Saido TC, Yamasaki T, et al. The involvement of calpain-dependent proteolysis of the tumor suppressor NF2 (merlin) in schwannomas and meningiomas. Nat Med 1998; 4:915-22; PMID:9701243; http://dx.doi.org/10.1038/nm0898-915
- Braun C, Engel M, Seifert M, Theisinger B, Seitz G, Zang KD, Welter C. Expression of calpain I messenger RNA in human renal cell carcinoma: correlation with lymph node metastasis and histological type. Int J Cancer 1999; 84:6-9; PMID:9988224; http://dx.doi.org/10.1002/(SICI)1097-0215(19990219)84:1%3c6::AID-IJC2%3e3.0.CO;2-T
- Salehin D, Fromberg I, Haugk C, Dohmen B, Georg T, Bohle RM, Bauerschlag D, Maass N, Friedrich M. Immunhistochemical analysis for expression of calpain 1, calpain 2 and calpastatin in endometrial cancer. Anticancer Res 2010; 30:2837-43; PMID:20683020
- Salehin D, Fromberg I, Haugk C, Dohmen B, Georg T, Bohle RM, Bauerschlag D, Thill M, Friedrich M. Immunhistochemical analysis for expression of calpain 1, calpain 2 and calpastatin in ovarian cancer. Eur J Gynaecol Oncol 2011; 32:628-35; PMID:22335024
- Chen XQ, Yu AC. The association of 14-3-3gamma and actin plays a role in cell division and apoptosis in astrocytes. Biochem Biophys Res Commun 2002; 296:657-63; PMID:12176032; http://dx.doi.org/10.1016/S0006-291X(02)00895-1
- Xiao Y, Lin VY, Ke S, Lin GE, Lin FT, Lin WC. 14-3-3tau promotes breast cancer invasion and metastasis by inhibiting RhoGDIalpha. Mol Cell Biol 2014; 34:2635-49; PMID:24820414; http://dx.doi.org/10.1128/MCB.00076-14
- Kim JS, Huang TY, Bokoch GM. Reactive oxygen species regulate a slingshot-cofilin activation pathway. Mol Biol cell 2009; 20:2650-60; PMID:19339277; http://dx.doi.org/10.1091/mbc.E09-02-0131
- Chen M, Liu T, Xu L, Gao X, Liu X, Wang C, He Q, Zhang G, Liu L. Direct interaction of 14-3-3zeta with ezrin promotes cell migration by regulating the formation of membrane ruffle. J Mol Biol 2014; 426:3118-33; PMID:25020230; http://dx.doi.org/10.1016/j.jmb.2014.06.021
- Neal CL, Yao J, Yang W, Zhou X, Nguyen NT, Lu J, Danes CG, Guo H, Lan KH, Ensor J, et al. 14-3-3zeta overexpression defines high risk for breast cancer recurrence and promotes cancer cell survival. Cancer Res 2009; 69:3425-32; PMID:19318578; http://dx.doi.org/10.1158/0008-5472.CAN-08-2765
- Xu L, Deng X. Suppression of cancer cell migration and invasion by protein phosphatase 2A through dephosphorylation of mu- and m-calpains. J Biol Chem 2006; 281:35567-75; PMID:16982626; http://dx.doi.org/10.1074/jbc.M607702200
- Ito A, Kataoka TR, Watanabe M, Nishiyama K, Mazaki Y, Sabe H, Kitamura Y, Nojima H. A truncated isoform of the PP2A B56 subunit promotes cell motility through paxillin phosphorylation. EMBO J 2000; 19:562-71; PMID:10675325; http://dx.doi.org/10.1093/emboj/19.4.562
- Ambach A, Saunus J, Konstandin M, Wesselborg S, Meuer SC, Samstag Y. The serine phosphatases PP1 and PP2A associate with and activate the actin-binding protein cofilin in human T lymphocytes. Eur J Immunol 2000; 30:3422-31; PMID:11093160; http://dx.doi.org/10.1002/1521-4141(2000012)30:12%3c3422::AID-IMMU3422%3e3.0.CO;2-J
- Perrotti D, Neviani P. Protein phosphatase 2A: a target for anticancer therapy. Lancet Oncol 2013; 14:e229-38; PMID:23639323; http://dx.doi.org/10.1016/S1470-2045(12)70558-2
- Thompson CC, Ashcroft FJ, Patel S, Saraga G, Vimalachandran D, Prime W, Campbell F, Dodson A, Jenkins RE, Lemoine NR, et al. Pancreatic cancer cells overexpress gelsolin family-capping proteins, which contribute to their cell motility. Gut 2007; 56:95-106; PMID:16847067; http://dx.doi.org/10.1136/gut.2005.083691
- Berthier-Vergnes O, Kharbili ME, de la Fouchardiere A, Pointecouteau T, Verrando P, Wierinckx A, Lachuer J, Le Naour F, Lamartine J. Gene expression profiles of human melanoma cells with different invasive potential reveal TSPAN8 as a novel mediator of invasion. Br J Cancer 2011; 104:155-65; PMID:21081927; http://dx.doi.org/10.1038/sj.bjc.6605994
- Renz M, Betz B, Niederacher D, Bender HG, Langowski J. Invasive breast cancer cells exhibit increased mobility of the actin-binding protein CapG. Int J Cancer 2008; 122:1476-82; PMID:18059028; http://dx.doi.org/10.1002/ijc.23215
- Glaser J, Neumann MH, Mei Q, Betz B, Seier N, Beyer I, Fehm T, Neubauer H, Niederacher D, Fleisch MC. Macrophage capping protein CapG is a putative oncogene involved in migration and invasiveness in ovarian carcinoma. BioMed Res Int 2014; 2014:379847; PMID:24804218; http://dx.doi.org/10.1155/2014/379847
- Jiang X, Gillen S, Esposito I, Giese NA, Michalski CW, Friess H, Kleeff J. Reduced expression of the membrane skeleton protein beta1-spectrin (SPTBN1) is associated with worsened prognosis in pancreatic cancer. Histol Histopathol 2010; 25:1497-506; PMID:20886430
- Maeda O, Shibata K, Hosono S, Fujiwara S, Kajiyama H, Ino K, Nawa A, Tamakoshi K, Kikkawa F. Spectrin alphaII and betaII tetramers contribute to platinum anticancer drug resistance in ovarian serous adenocarcinoma. Int J Cancer 2012; 130:113-21; PMID:21328338; http://dx.doi.org/10.1002/ijc.25983
- Ding Z, Lambrechts A, Parepally M, Roy P. Silencing profilin-1 inhibits endothelial cell proliferation, migration and cord morphogenesis. J Cell Sci 2006; 119:4127-37; PMID:16968742; http://dx.doi.org/10.1242/jcs.03178
- Ding Z, Gau D, Deasy B, Wells A, Roy P. Both actin and polyproline interactions of profilin-1 are required for migration, invasion and capillary morphogenesis of vascular endothelial cells. Exp Cell Res 2009; 315:2963-73; PMID:19607826; http://dx.doi.org/10.1016/j.yexcr.2009.07.004
- Bottcher RT, Wiesner S, Braun A, Wimmer R, Berna A, Elad N, Medalia O, Pfeifer A, Aszodi A, Costell M, et al. Profilin 1 is required for abscission during late cytokinesis of chondrocytes. EMBO J 2009; 28:1157-69; PMID:19262563; http://dx.doi.org/10.1038/emboj.2009.58
- Kullmann JA, Neumeyer A, Gurniak CB, Friauf E, Witke W, Rust MB. Profilin1 is required for glial cell adhesion and radial migration of cerebellar granule neurons. EMBO Rep 2012; 13:75-82; http://dx.doi.org/10.1038/embor.2011.211
- Karamchandani JR, Gabril MY, Ibrahim R, Scorilas A, Filter E, Finelli A, Lee JY, Ordon M, Pasic M, Romaschin AD, et al. Profilin-1 expression is associated with high grade and stage and decreased disease-free survival in renal cell carcinoma. Hum Pathol 2015; 46(5):673-80 PMID:25704627; http://dx.doi.org/10.1016/j.humpath.2014.11.007
- Bae YH, Ding Z, Das T, Wells A, Gertler F, Roy P. Profilin1 regulates PI(3,4)P2 and lamellipodin accumulation at the leading edge thus influencing motility of MDA-MB-231 cells. Proc Natl Acad Sci U S A 2010; 107:21547-52; PMID:21115820; http://dx.doi.org/10.1073/pnas.1002309107
- Ding Z, Joy M, Bhargava R, Gunsaulus M, Lakshman N, Miron-Mendoza M, Petroll M, Condeelis J, Wells A, Roy P. Profilin-1 downregulation has contrasting effects on early vs late steps of breast cancer metastasis. Oncogene 2014; 33:2065-74; PMID:23686314; http://dx.doi.org/10.1038/onc.2013.166
- Mouneimne G, Hansen SD, Selfors LM, Petrak L, Hickey MM, Gallegos LL, Simpson KJ, Lim J, Gertler FB, Hartwig JH, et al. Differential remodeling of actin cytoskeleton architecture by profilin isoforms leads to distinct effects on cell migration and invasion. Cancer Cell 2012; 22:615-30; PMID:23153535; http://dx.doi.org/10.1016/j.ccr.2012.09.027
- Stojadinovic A, Hooke JA, Shriver CD, Nissan A, Kovatich AJ, Kao TC, Ponniah S, Peoples GE, Moroni M. HYOU1/Orp150 expression in breast cancer. Med Sci Monit 2007; 13:BR231-9; PMID:17968289
- Chiu CC, Lin CY, Lee LY, Chen YJ, Lu YC, Wang HM, Liao CT, Chang JT, Cheng AJ. Molecular chaperones as a common set of proteins that regulate the invasion phenotype of head and neck cancer. Clin Cancer Res 2011; 17:4629-41; PMID:21642380; http://dx.doi.org/10.1158/1078-0432.CCR-10-2107
- Giffin L, Yan F, Ben Major M, Damania B. Modulation of Kaposi's sarcoma-associated herpesvirus interleukin-6 function by hypoxia-upregulated protein 1. J Virol 2014; 88:9429-41; PMID:24920810; http://dx.doi.org/10.1128/JVI.00511-14
- Zhou J, Wang Y, Fei J, Zhang W. Expression of cofilin 1 is positively correlated with the differentiation of human epithelial ovarian cancer. Oncol Lett 2012; 4:1187-90; PMID:23205117
- DelloRusso C, Welcsh PL, Wang W, Garcia RL, King MC, Swisher EM. Functional characterization of a novel BRCA1-null ovarian cancer cell line in response to ionizing radiation. Mol Cancer Res 2007; 5:35-45; PMID:17259345; http://dx.doi.org/10.1158/1541-7786.MCR-06-0234
- Ding Z, Bae YH, Roy P. Molecular insights on context-specific role of profilin-1 in cell migration. Cell Adh Migr 2012; 6:442-9; PMID:23076048; http://dx.doi.org/10.4161/cam.21832
- Rotty JD, Wu C, Haynes EM, Suarez C, Winkelman JD, Johnson HE, Haugh JM, Kovar DR, Bear JE. Profilin-1 serves as a gatekeeper for actin assembly by Arp2/3-dependent and -independent pathways. Dev Cell 2015; 32:54-67; PMID:25543281; http://dx.doi.org/10.1016/j.devcel.2014.10.026
- Alkhas A, Hood BL, Oliver K, Teng PN, Oliver J, Mitchell D, Hamilton CA, Maxwell GL, Conrads TP. Standardization of a sample preparation and analytical workflow for proteomics of archival endometrial cancer tissue. J Proteome Res 2011; 10:5264-71; PMID:21932769; http://dx.doi.org/10.1021/pr2007736
