Abstract
Centrobin resides in daughter centriole and play a critical role in centriole duplication. Nucleation and stabilization of microtubules are known biological activities of centrobin. Here, we report a specific localization of centrobin outside the centrosome. Centrobin was associated with the stable microtubules. In hippocampal cells, centrobin formed cytoplasmic dots in addition to the localization at both centrosomes with the mother and daughter centrioles. Such specific localization pattern suggests that cytoplasmic centrobin is not just a reserved pool for centrosomal localization but also has a specific role in the cytoplasm. In fact, centrobin enhanced microtubule formation outside as well as inside the centrosome. These results propose specific roles of the cytoplasmic centrobin for noncentrosomal microtubule formation in specific cell types and during the cell cycle.
Introduction
Microtubules may be assembled and disassembled without an enzyme activity, which is best described with the dynamic instability model.Citation1 In reality, however, the nucleation and stability of microtubules in live cells are largely controlled by protein factors. A number of microtubule-associated proteins have been identified to have a nucleating and stabilizing activity of microtubules in vitro. For example, doublecortin enhances microtubule nucleation and stabilization with a specific interaction with protofilaments.Citation2,3
Microtubules of animal cells are strongly influenced by the centrosome where the minus ends of microtubules are concentrated. Meanwhile, a fraction of microtubules may be nucleated outside the centrosomes, especially in neuronal, epithelial and muscle cells. The noncentrosomal microtubules may be generated de novo or transported from the centrosome to the site.Citation4 A list of microtubule-associated factors for the noncentrosomal, cytoplasmic microtubules has been identified.Citation5
Centrobin was initially known as a daughter centriole-specific protein which is required for centriole duplication and elongation.Citation6 Centrobin binds to microtubules and stabilizes them.Citation7-9 Two different kinases are known to regulate the microtubule stabilizing activity of centrobin in opposite ways. PLK1 enhances the microtubule stabilizing activity of centrobin during mitosis whereas NEK2 antagonizes it in interphase cells.Citation8,10 The microtubule organizing activity of centrobin is critical for asymmetric division of Drosophila neuroblasts.Citation11 Centrobin is necessary and sufficient for daughter centriole anchoring at the apical region of the neuroblasts.Citation12
Studies on the biological activity of centrobin have been limited to the centrosome. In this study, we expand biological function of centrobin to the cytoplasm. We determined subcellular localization of the cytoplasmic centrobin. Furthermore, we revealed that the cytoplasmic centrobin is essential for noncentrosomal microtubule formation.
Results
Centrobin is essential for microtubule nucleation at both the centrosome and cytoplasm
We performed immunocytochemical analysis to determine cytoplasmic distribution of centrobin in U2OS cells. The cytoplasmic signals of centrobin became visible when most of microtubules except the stable ones were disassembled at a low temperature (). The cytoplasmic centrobin was detected along with the stable microtubules (). This result is consistent with the previous report in which ectopic centrobin was colocalized with the bundles of stable microtubules.Citation7
Figure 1. Microtubule regrowth assays in the centrobin-depleted cells. (A) U2OS cells were placed on ice for 45 min and coimmunostained with the α-tubulin (green) and centrobin (red) antibodies. Nuclei were stained with DAPI (blue). The magnified view shows the cytoplasmic centrobin along with stable microtubules. Scale bar, 10 μm. (B) Immunoblot and immunostaining analyses were carried out to confirm centrobin depletion with siRNA transfection. Scale bar, 10 μm. Insets are magnified views of the centrosomes. (C) Microtubule regrowth assays were performed with the centrobin-depleted U2OS cells. The cells were immunostained with the α-tubulin antibody. The cytoplasmic microtubules were enlarged from the 60-second time point. The centrosomal microtubule intensities, the number and length of microtubules from a centrosome, and the number of cells with cytoplasmic microtubules were quantified at the 10-second time point. (D) Microtubule regrowth assays were performed for extended time periods up to 10 min. Microtubule intensities in the centrosome and cytoplasm were determined. Scale bar, 10 μm. At least 30 cells per an experimental group were measured in each of 3 independent experiments. Data show the mean±s.d.. *P < 0.05, in comparison to the control.
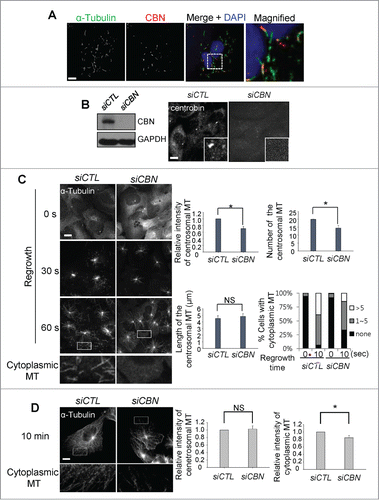
To study involvement of centrobin in microtubule assembly, we performed microtubule regrowth assays with centrobin-depleted U2OS cells. We depleted endogenous centrobin with siRNA transfection into U2OS cells (). The results showed that the intensity and the number of centrosomal microtubules were reduced in centrobin-depleted cells (). The number of cytoplasmic microtubules was also reduced in the centrobin-depleted cells (). However, an average length of the centrosomal microtubules was not affected with the centrobin depletion (). These results suggest that centrobin is essential for microtubule nucleation in both the centrosome and cytoplasm, but not for microtubule elongation. We also observed microtubule regrowth for extended time periods up to 10 minutes to determine involvement of centrobin in microtubule anchoring.Citation13 The results showed that microtubule networks were reduced and disorganized in the centrobin-depleted cells (). However, no significant difference in the centrosomal microtubules was observed between the control and centrobin-depleted cells, suggesting that centrobin may not affect the microtubule anchoring ().Citation13
C-terminus is required for microtubule nucleating activity of centrobin
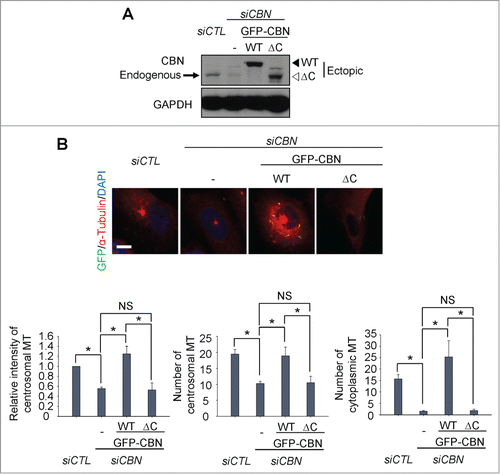
The C-terminal half of centrobin includes a domain for the microtubule stabilizing activity.Citation7 In fact, a domain at the 765–903 residues is responsible for interaction with tubulin and for localization to the centrosome.Citation9 We generated inducible U2OS cell lines in which the wild type (GFP-CBN) and a C-terminal end-truncated mutant (GFP-CBNΔC) of centrobin were expressed. We carefully adjusted the ectopic centrobin levels comparable to the endogenous levels using the leaky expression of ectopic GFP-CBN without doxycycline treatment (). An siRNA specific to centrobin 5'UTR (siCBN) was transfected to deplete endogenous centrobin (). We then performed microtubule regrowth assays with the GFP-CBN- and GFP-CBNΔC-rescued cells. The results showed that intensity and the number of centrosomal microtubules and the number of cytoplasmic microtubules was rescued with GFP-CBN but not with GFP-CBNΔC (). These results indicate that the C- terminal domain of centrobin is crucial for microtubule nucleation both at the centrosome and cytoplasm.
Figure 2. Microtubule regrowth assays with a C-terminus deletion mutant of centrobin. (A) Immunoblot was performed to determine the GFP-CBN and GFP-CBNΔC levels in centrobin-depleted U2OS cells. (B) Microtubule regrowth assays were performed with the centrobin-depleted U2OS cells rescued with GFP-CBN or GFP-CBNΔC. The cells were immunostained with the GFP (green) and α-tubulin (red) antibodies. Scale bar, 10 μm. The centrosomal microtubule intensities, the number of microtubules from a centrosome, and the number of cytoplasmic microtubules were quantified at the 10-second time point. At least 30 cells per an experimental group were measured in each of 3 independent experiments. Data show the mean±s.d.. *P < 0.05, in comparison to the centrobin-depleted cells.
Microtubule regrowth is limited to the centrosome with the centrobin-PACT fusion protein
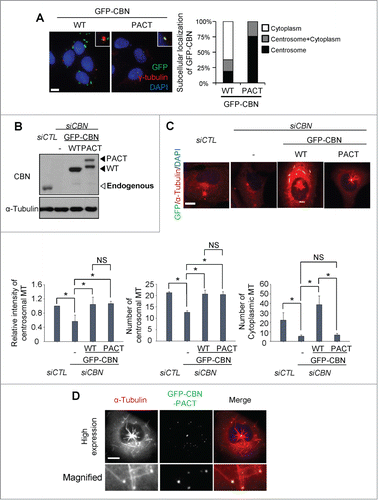
In order to limit ectopic centrobin to the centrosome, we linked the PACT (Pericentrin-AKAP450 centrosomal targeting) domain to GFP-CBN.Citation14 As expected, most of GFP-CBN-PACT localized to the centrosome whereas GFP-CBN was detected in both the centrosome and cytoplasm (). We adjusted the cellular GFP-CBN and GFP-CBN- PACT levels to comparable to endogenous centrobin. Then, we performed the microtubule regrowth assays with the GFP-CBN- and GFP-CBN-PACT-rescued cells after the depletion of endogenous centrobin (). The results showed that the centrosomal microtubule levels and the number of the centrosomal microtubules were efficiently rescued in both the GFP-CBN- and GFP-CBN-PACT-rescued cells (). However, the number of cytoplasmic microtubules was not effectively rescued with GFP-CBN-PACT (). We also performed microtubule regrowth assays with U2OS cells with excess amounts of GFP- CBN-PACT (). Excess amounts of GFP-CBN-PACT saturated the centrosomes and even formed small dots in the cytoplasm. Furthermore, microtubules were grown from the cytoplasmic dots as well as from the centrosomes (). These results confirm that centrobin is essential for microtubule nucleation irrespective of the subcellular localization.
Figure 3. Microtubule regrowth assays with centrobin-PACT. (A) Immunostaining analysis was performed to determine subcellular distribution of GFP-CBN and GFP-CBN- PACT in U2OS cells. The cells were coimmunostained with the GFP (green) and γ-tubulin (red) antibodies. Nuclei were stained with DAPI (blue). Insets are magnified views of the centrosomes. Scale bar, 10 μm. The number of cells with cytoplasmic and/or centrosomal signals of ectopic GFP-CBN was counted. (B) Immunoblot analysis of centrobin was performed with the centrobin-depleted U2OS cells rescued with GFP-CBN and GFP-CBN- PACT. (C) Microtubule regrowth assays were performed with the centrobin-depleted cells rescued with GFP-CBN or GFP-CBN-PACT. The cells were coimmunostained with the GFP (green) and α-tubulin (red) antibodies. Scale bar, 10 μm. The intensity of centrosomal microtubules, the number of microtubules from centrosome, and the number of microtubules at the cytoplasm were quantified at the 10-second time point. At least 30 cells per an experimental group were measured in each of 3 independent experiments. Data show the mean±s.d.. *P < 0.05, in comparison to the centrobin depleted cells. (D) Immunostaining analysis was performed with centrobin-depleted U2OS cells rescued with an excess amount of GFP-CBN-PACT. The magnified view shows the regrown microtubules from the cytoplasmic centrobin. Scale bar, 10 μm.
Importance of NEK2 phosphorylation of centroin in the cytoplasmic microtubule formation
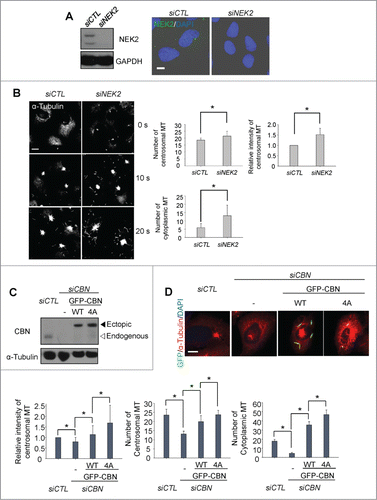
NEK2 phosphorylates centrobin at specific residues including T35, S36, S41 and S45.Citation10 The phospho-resistant mutant of centrobin at these residues (CBN4A) enhanced microtubule stabilizing activity, suggesting that NEK2 antagonizes the centrobin activity by phosphorylation.Citation10 First, we depleted endogenous NEK2 of U2OS cells with siRNA transfection and performed microtubule regrowth assays (). The results showed that the microtubule nucleation activities in both the centrosome and cytoplasm were enhanced in the NEK2-depleted cells (). Next, we examined whether centrobin mediates the inhibitory effects of NEK2 on microtubule network formation or not. To determine the microtubule nucleating activity of the phospho-resistant centrobin, we generated inducible U2OS cell lines in which the wild type (GFP-CBN) and phospho-resistant mutant (GFP- CBN4A) of centrobin are stably expressed. We, then, depleted the endogenous centrobin and performed microtubule regrowth assays (). The results showed that the centrosomal microtubule levels and the number of centrosomal microtubules were enhanced in the GFP- CBN4A-rescued cells in comparison to the GFP-CBN-rescued cells (). The number of cytoplasmic microtubules was also enhanced significantly in the GFP-CBN4A-rescued cells (). These results indicate that NEK2 phosphorylation antagonizes the microtubule nucleating activity of centrobin in both the centrosome and cytoplasm.
Figure 4. Importance of NEK2 phosphorylation of centrobin in microtubule formation. (A) Immunoblot and immunostaining analyses were carried out to confirm NEK2 depletion with siRNA transfection. Scale bar, 10 μm. (B) Microtubule regrowth assays were performed with the NEK2-depleted U2OS cells. The cells were immunostained with the α-tubulin antibody. The centrosomal microtubule intensities, the number of microtubules from a centrosome, and the number of cells with cytoplasmic microtubules were quantified. Scale bar, 10 μm. (C) Centrobin-depleted U2OS cells were stably rescued with the wild type (WT) or a phospho-resistant mutant of centrobin at T35, S36, S41, S45 (4A). Immunoblot analysis was performed with the centrobin and α-tubulin antibodies. (D) Microtubule regrowth assays were performed for 20 seconds with the centrobin-rescued U2OS cells. The cells were coimmunostained with the GFP (green) and α-tubulin (red) antibodies. Scale bar, 10 μm. The centrosomal microtubule intensities, number of microtubules from a centrosome, and number of cytoplasmic microtubules were quantified at the 10-second time point. At least 30 cells per an experimental group were measured in each of 3 independent experiments. Data show the mean±s.d.. *P < 0.05, in comparison to the centrobin depleted cells.
Centrobin in the mouse hippocampal cells
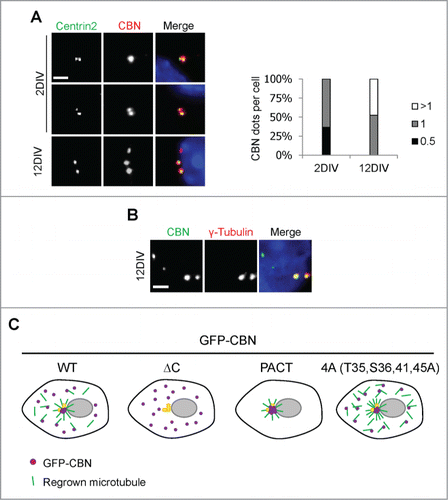
We observed subcellular localization of centrobin in the mouse hippocampal cells. The centrobin signals were detected at both the mother and daughter centrioles in the young hippocampal cells (2 d in vitro; 2DIV) (). This result was unexpected because centrobin is known to localize exclusively at the daughter centrioles in most cells examined.Citation6,7 In the mature 12DIV hippocampal cells, additional centrobin signals were also detected out of the centrosomes (). We confirmed these results with 2 different centrobin antibodies (data not shown). However, the additional centrobin dots were not co-immunostained with the γ-tubulin antibody, suggesting that they may not function as microtubule organizing centers ().
Figure 5. Centrobin expression in mouse hippocampal cells. (A) Mouse hippocampal cells at the 2DIV and 12DIV developmental stages were coimmunostained with the centrobin (red) and centrin2 (green) antibodies. Nuclei were stained with DAPI (blue). Scale bar, 2 μm. The number of centrobin dots in a hippocampal cell was counted. At least 30 cells per an experimental group were measured in each of 3 independent experiments. Data show the mean±s.d. (B) The 12DIV mouse hippocampal cells were coimmunostained with the centrobin (green) and γ-tubulin (red) antibodies. Nuclei were stained with DAPI (blue). Scale bar, 2 μm. (C) Model. Centrobin in both the centrosome and cytoplasm contributes to microtubule nucleation/stabilization. NEK2 phosphorylation reduces the microtubule nucleation/stabilization activity of centrobin.
Discussion
In this study, we detected a specific localization of cytoplasmic centrobin at the stable microtubules. In hippocampal cells, centrobin forms cytoplasmic dots in addition to the localization at both centrosomes with the mother and daughter centrioles. Such specific localization pattern suggests that cytoplasmic centrobin is not just a reserved pool for centrosomal localization but also has a specific role in the cytoplasm (). In fact, centrobin enhanced microtubule formation outside as well as inside the centrosome. These results suggest that cytoplasmic centrobin participates in microtubule network formation in a cell ().
Centrobin was initially known to be a daughter centriole-specific protein which is critical for centriole duplication.Citation6,7 A known biological activity of centrobin, however, is microtubule nucleation and stabilization.Citation8 In fact, we observed that the number of centrosomal microtubules was reduced in centrobin-depleted cells and rescued with the wild type and PACT-linked centrobin. The result indicates that centrosomal centrobin is involved in microtubule nucleation at the centrosome. In consistent with our view, centrobin is essential for the microtubule nucleation in the daughter centrioles of Drosophila neuroblasts.Citation12 It is not clear how the microtubule stabilization activity of centrobin is essential for procentriole assembly. It is possible that centrobin might play a role in nucleation and stabilization of the triplet microtubules for procentriole formation. Recently, it was reported that centrobin induces centriole elongation through regulating the CPAP level which is also a crucial factor for centriole growth.Citation15,16
It remains to be investigated what is the biological significance of cytoplasmic centrobin. It is known that both NEK2 and PLK1 phosphorylate centrobin at specific sites but result in opposite outcomes.Citation8,10 NEK2 phosphorylation reduces the microtubule nucleation activity of centrobin while PLK1 phosphorylation enhances it.Citation8,10 In fact, we observed that the phospho-resistant mutant centrobin against NEK2 enhanced the microtubule formation in both centrosome and cytoplasm, implying that centrobin mediates NEK2 regulation on microtubule formation (). Since the NEK2 activity is highest at G2 phase, NEK2 may control centrobin functions prior to mitosis, possibly for reorganization of microtubule networks. Once cells enter mitosis, PLK1 becomes active and phosphorylates centrobin. An enhanced centrobin activity may be critical for formation of stable mitotic spindle.Citation8 In fact, PLK1 phosphorylation is critical for enhanced microtubule organizing activity of the spindle pole with a daughter centriole during the mitosis of Drosophila neuroblasts.Citation12 We currently investigate involvement of centrobin in cellular morphology of non-dividing cells, such as neuronal cells.
Materials and Methods
Antibodies
The centrobin antibody was previously described.Citation7 We also purchased the centrobin antibody from Abcam. The polyclonal and monoclonal α-tubulin antibodies were purchased from Abcam and Sigma, respectively. The antibodies against centrin2, γ-tubulin, GAPDH and GFP were purchased from Millipore, Santa Cruz, Ambion and Santa Cruz, respectively. The Alexa-fluorescence secondary antibodies were purchased from Invitrogen.
Cell culture
U2OS cells were cultured in McCoy's 5A media supplemented with 10% FBS. The plasmid DNA was transfected in U2OS cells using Fugene HD (Promega) according to the manufacturer's instruction. The Tet-on stable U2OS cell lines were generated using 400 μg/ml of G418 (Calbiochem) selection. The TRE double stable U2OS cell lines were generated with hygromycin B (Clontech) according to the manufacturer's instruction.
The mouse hippocampus cells were cultured following a general protocol with modifications.Citation17 Hippocampi were extracted from E17 mouse embryos and dissociated mechanically after trypsin treatment. The cells were washed with HBSS, and then 250,000 cells/cm2 were plated on the poly-D-lysine coated coverslips in plating media (10% FBS, 0.45% glucose, 0.1 mg/mL sodium pyruvate in 2 mM glutamine-containing MEM/EBSS). Cells were cultured in the neurobasal media supplemented with B27, glutamax and penicillin/streptomycin after 3 h of recovery.
Plasmids and RNA interference
The wild type and mutant centrobin cDNA were subcloned into pTRE2hyg-GFP. siRNAs specific to centrobin (5′-GGATGGTTCTAAGCATATC-3′), 5′-UTR of centrobin (5′-GGACTTTGCTAAAGCAGAA-3′), and NEK2 (5′-GGCAAATTCAGGCGAATTC-3′) were purchased from ST Pharm, and was transfected using RNAiMAX (Invitrogen) according to the manufacturer's instruction. Non-specific control siRNA (5′- GCAATCGAAGCTCGGCTAC-3′) was used.
Immunoblot analysis
The protein samples for immunoblot were lysed using sodium dodecyl sulfate sample buffer, and then subjected to SDS-polyacrylamide gel electrophoresis. The gel was transferred to a nitrocellulose membrane. The membrane was blocked with 5% skim milk in 0.1% TBST (Tris-buffered saline TBS with 0.1% Tween 20) for 1 h. The membrane was incubated with primary antibody overnight at 4˚C, and then washed with 0.1% TBST 3 times. The membrane was incubated with horseradish peroxidase-conjugated secondary antibody for 30 min at room temperature, and then washed with 0.1% TBST 3 times. The ECL solution was treated onto the membrane, and then exposed to an X-ray film.
Immunocytochemistry, image processing and statistical analysis
The cells on the coverslip were fixed with methanol for 10 min and washed with phosphate-buffered saline (PBS) 3 times. The fixed cells were blocked with 3% bovine serum albumin in 0.5% PBST (PBS with 0.5% Triton X-100) for 20 min, incubated with the primary antibody for 1 h, washed with 0.1% PBST 3 times, incubated with the secondary antibody for 30 min, and then washed with 0.1% PBST 3 times. The coverslips were mounted on a slide glass using Prolong gold mounting solution (Invitrogen) after DAPI incubation. The immunostained cells were observed using fluorescence microscope with a CCD (Qi-cam Fast 1394; Qimaging) camera and processed with ImagePro 5.0 (Media Cybernetics, Inc.) and Adobe Photoshop software. The length of microtubule was measured using ImagePro 5.0 2(Media Cybernetics, Inc.). The intensity of centrosomal microtubule was measured by drawing the circle including the nucleated microtubules, and background was excluded by drawing same size circle in the nearest cytoplasm. All distinguished centrosomal and cytoplasmic microtubules were counted per cell for quantifying the centrosomal and cytoplasmic microtubules, and statistically analyzed with Student's t-test.
Microtubule regrowth assay
The microtubule regrowth assay was conducted following an optimized method for observing cytoplasmic microtubules.Citation18 To disrupt microtubule network, U2OS cells were placed on the ice for up to 90 min. To induce microtubule regrowth, the cells were incubated with warmed McCoy's 5A media for indicated time periods, and immediately fixed with 4% paraformaldehyde in PEM buffer (80mM PEPES pH6.9, 1 mM MgCl2, 5 mM EGTA, 0.5% Triton X-100) for 10 min. The fixed cells were incubated with 0.3% PBST for 5 minutes to increase permeability.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by a grant from the Basic Research Laboratory (NRF-2014R1A4A10052590) through the National Research Foundation of Korea. BKK was supported by the Korean Honor Scientist Program.
References
- Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature 1984; 312:237-42; PMID:6504138
- Moores CA, Perderiset M, Francis F, Chelly J, Houdusse A, Milligan RA. Mechanism of microtubule stabilization by doublecortin. Mol Cell 2004; 14:833-9; PMID:15200960
- Bechstedt S, Brouhard GJ. Doublecortin recognizes the 13-protofilament microtubule cooperatively and tracks microtubule ends. Dev Cell 2012; 23:181-92; PMID:22727374; http://dx.doi.org/10.1016/j.devcel.2012.05.006
- Bartolini F, Gundersen GG. Generation of noncentrosomal microtubule arrays. J Cell Sci 2006; 119:4155-63; PMID:17038542
- Cassimeris L, Spittle C. Regulation of microtubule-associated proteins. Int Rev Cytol 2001; 210:163-226; PMID:11580206
- Zou C, Li J, Bai Y, Gunning WT, Wazer DE, Band V, Gao Q. Centrobin: a novel daughter centriole-associated protein that is required for centriole duplication. J Cell Biol 2005; 171:437-45; PMID:16275750
- Jeong Y, Lee J, Kim K, Yoo JC, Rhee K. Characterization of NIP2/centrobin, a novel substrate of Nek2, and its potential role in microtubule stabilization. J Cell Sci 2007; 120:2106-16; PMID:17535851
- Lee J, Jeong Y, Jeong S, Rhee K. Centrobin/NIP2 is a microtubule stabilizer whose activity is enhanced by PLK1 phosphorylation during mitosis. J BIol Chem 2010; 285: 25476-84; PMID:20511645; http://dx.doi.org/10.1074/jbc.M109.099127
- Gudi R, Zou C, Li J, Gao Q. Centrobin-tubulin interaction is required for centriole elongation and stability. J Cell Biol 2011; 193:711-25; PMID:21576394; http://dx.doi.org/10.1083/jcb.201006135
- Park J, Rhee K. NEK2 phosphorylation antagonizes the microtubule stabilizing activity of centrobin. Biochem Biophys Res Commu 2013; 431:302-8; PMID:23291182; http://dx.doi.org/10.1016/j.bbrc.2012.12.106
- Januschke J, Llamazares S, Reina J, Gonzalez C. Drosophila neuroblasts retain the daughter centrosome. Nat Comm 2011; 2:1-6; PMID:21407209; http://dx.doi.org/10.1038/ncomms1245
- Januschke J, Reina J, Llamazares S, Bertran T, Rossi F, Roig J, Gonzalez C. Centrobin controls mother-daughter centriole asymmetry in Drosophila neurobalsts. Nat Cell Biol 2013; 15:241-8; PMID:23354166; http://dx.doi.org/10.1038/ncb2671
- Delgehyr N, Sillibourne J, Bornens M. Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J Cell Biol 2005; 118:1565-75; PMID:15784680
- Gillingham AK, Munro S. The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Rep 2000; 1:524-9; PMID:11263498
- Gudi R, Zou C, Dhar J, Gao Q, Vasu C. Centrobin-CPAP interaction promotes CPAP localization to the centrioles during centriole duplication. J Biol Chem 2014; 289:15166-78; PMID:24700465; http://dx.doi.org/10.1074/jbc.M113.531152
- Gudi R, Haycraft CJ, Bell PD, Li Z, Vasu C. Centroin-mediated regulation of the Centrosomal Protein 4.1-associated Protein (CPAP) level limits centriole length during elongation stage. J Biol Chem 2015; 290:6890-902; PMID:25616662; http://dx.doi.org/10.1074/jbc.M114.603423
- Kaech S, Banker G. Culturing hippocampal neurons. Nature Protocols 2006; 1:2406-15; PMID:17406484
- Choi YK, Liu P, Sze SK, Dai C, Qi RZ. CDK5RAP2 stimulates microtubule nucleation by γ-tublin ring complex. J Cell Biol 2010; 191:1089-95; PMID:21135143; http://dx.doi.org/10.1083/jcb.201007030
