The mitotic checkpoint, conserved from yeast to humans, delays the onset of anaphase until all chromosomes are aligned at the metaphase plate ensuring equal chromosome segregation. The RZZ complex, an essential mitotic checkpoint component present only in higher eukaryotes regulates checkpoint activation and silencing. Checkpoint activation occurs by RZZ mediated Mad1-Mad2 kinetochore recruitment whereas checkpoint silencing is by dynein/dynactin kinetochore recruitment through Spindly. Here we discuss recent developments in the mitotic checkpoint function of Spindly.
Human Spindly recruits dynein to kinetochores and facilitates its binding to dynactin, a regulatory factor of dynein.Citation1 Spindly knockdown causes prometaphase delay, alignment defects, loss of dynein/dynactin kinetochore localization and delayed removal of checkpoint proteins from bi-polar attached kinetochores independent of dynein/dynactin.Citation1,2 Gassmann et al. elegantly showed that kinetochore retention of Spindly box point mutants (S256A and F258A), incapable of recruiting dynein, lead to metaphase arrest rather than prometaphase delay as observed in Spindly depleted cells suggesting a dynein independent checkpoint silencing mechanism is not activated while Spindly is present at kinetochores.Citation3 Furthermore, these point mutants rescue chromosome misalignment demonstrating a dynein independent role of Spindly in kinetochore-microtubule attachment.Citation3 Exactly how Spindly is recruited to kinetochores has remained unknown.
We mapped Spindly's kinetochore localization domain to its C-terminal 294–605 amino acids.Citation4 Deletions in residues 596–605, or substitution of the C-terminal cysteine were not tolerated indicating their importance in Spindly kinetochore localization, with the C-terminal cysteine being essential.Citation2,4 Spindly C-terminal residues contain a CAAX farnesylation motif that is conserved in vertebrates but not in worms and insects.Citation4,5 Prenylation is a post translational lipid modification adding either a farnesyl or geranylgeranyl lipid group onto a C-terminal cysteine.Citation6 Farnesyl transferase inhibitor (FTI) treatment prevents Spindly kinetochore localization but not RZZ complex, and 2 previously known farnesylated mitotic proteins CENP-E and CENP-F.Citation4 Holland et al. observed the same results in HeLa and DLD-1 cells (with loss of kinetochore dynein as expected) in a parallel study, although they reported CENP-E and CENP-F kinetochore levels were affected albeit to a lesser extent.Citation5 The observed differences in the 2 studies pertaining to CENP-E and CENP-F kinetochore levels can be attributed to 48 hour FTI treatment as compared to 24 hour in our study and these effects were more prominent in DLD-1 cells compared to HeLa. In addition, we showed that a CENP-F cysteine to alanine mutant (of the farnesylation motif) localized to kinetochores suggesting that CENP-F farnesylation is not essential for its kinetochore localization.
We have demonstrated that Spindly is farnesylated in vivo and that this lipid modification is required for its interaction with the RZZ complex (). To date, farnesylation has only been reported to modulate protein-protein interaction strength but we present for the first time that farnesylation is essential for the interaction of Spindly with the RZZ complex and its subsequent kinetochore localization. Holland et al. also found that Spindly is farnesylated using a different approach. We also showed that farnesylation inhibition moderately reduced Spindly protein levels (∼25%) in consensus with a previous study that showed the RZZ complex/Spindly interaction stabilizes the Spindly protein.Citation1 Swapping Spindly's farnesylation motif with the CENP-E or CENP-F farnesylation motif did not affect its ability to undergo farnesylation and kinetochore localization. Since substitution with a geranylgeranylation motif did not target Spindly to kinetochores, we concluded that Spindly specifically requires farnesylation for kinetochore localization.
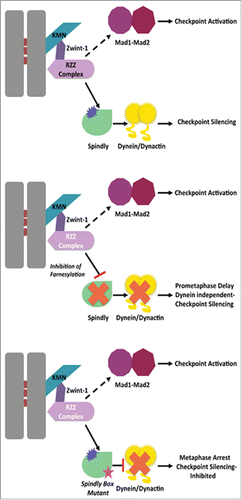
Figure 1. Farneyslation regulates kinetochore recruitment of Spindly. The RZZ complex is required for the kinetochore recruitment of dynein/dynactin (through Spindly) as well as Mad1-Mad2 (unknown mechanism dotted line). Farnesylation inhibition prevents RZZ/Spindly interaction and Spindly dependent dynein/dynactin localization leading to prometaphase delay and dynein independent checkpoint silencing. No silencing occurs when Spindly box point mutants localize but do not recruit dynein/dynactin.
Live cell imaging studies comparing the mitotic phenotype of Spindly knockdown and FTI treated cells concluded that the mitotic effects of FTIs are essentially caused by the loss of Spindly function although CENP-E and CENP-F might contribute to some extent.Citation4,5 FTIs have shown a good therapeutic potential in Progeria patients, and are being explored for malaria, African sleeping sickness, hepatitis and multiple sclerosis.Citation6 However the effect of FTIs on Spindly function in mitosis, and hence in the maintenance of the genomic integrity should not be ignored.
We showed that residues upstream of the Spindly farnesylation motif are important for kinetochore binding affinity, which is analogous to membrane binding in RAS proteins.Citation7 KRAS4B undergoes serine phosphorylation in the polybasic region upstream of the farnesylation site that facilitates its translocation from the plasma membrane to the endomembranes referred to as farnesyl-electrostatic switch.Citation7 In the case of Spindly the hydrophobicity, charge and perhaps phosphorlyation of upstream residues may contribute to kinetochore binding affinity. We observed that Spindly phospho mutants showed premature transport to spindle poles indicating phosphorylation regulation facilitates Spindly transport to poles (unpublished results). Whether hSpindly undergoes farnesyl-electrostatic switch to promote its release from kinetochores upon bi-orientation of chromosomes remains to be investigated.
In conclusion, a novel role of farnesylation in mitosis has been uncovered raising several questions. Why did cells adopt Spindly farnesylation as a regulatory mechanism during evolution and how do insect and worm cells lacking the Spindly farnesylation motif regulate this interaction. How does lipidation regulate the interaction at the molecular level and with which RZZ subunit? Does Spindly regulate the RZZ complex KT dynamics and how does it contribute to kinetochore-microtubule attachments? Farnesylation regulating kinetochore protein interactions as presented here will hopefully lead to a better understanding of the intriguing complexity of kinetochore protein assembly.
References
- Chan YW, et al. J Cell Biol 2009; 185:859–74; PMID:19468067; http://dx.doi.org/10.1083/jcb.200812167
- Barisic M, et al. Mol Biol Cell 2010; 21:1968–81; PMID:20427577; http://dx.doi.org/10.1091/mbc.E09-04-0356
- Gassmann R, et al. Genes Dev 2010; 24:957–71; PMID:20439434; http://dx.doi.org/10.1101/gad.1886810
- Moudgil DK, et al. J Cell Biol 2015; 208:881–96; PMID:25825516; http://dx.doi.org/10.1083/jcb.201412085
- Holland AJ, et al. Mol Biol Cell 2015; PMID:25808490; http://dx.doi.org/10.1091/mbc.E14-11-1560
- Ochocki J, et al. Medchemcomm 2013; 4:476–92; PMID:25530833; http://dx.doi.org/10.1039/C2MD20299A
- Bivona TG, et al. Mol Cell 2006; 21:481–93, PMID:16483930; http://dx.doi.org/10.1016/j.molcel.2006.01.012
