Peroxisomes and mitochondria are highly dynamic organelles with diverse roles in cellular signaling and metabolism. Oxidative metabolism and in particular the production and detoxification of reactive oxygen species (ROS) link peroxisomes and mitochondria to cell death and aging. Interestingly, peroxisomes and mitochondria exist in close functional and spatial relationship, the “peroxisome-mitochondrion connection,” mediated by components of the ERMES complex, which establishes contact between the endoplasmic reticulum (ER) and mitochondria.Citation1 These interorganellar contacts seem crucial for proper organelle function, fission and distribution as well as for selective degradation via autophagy in organisms ranging from yeast to mammals. The shape, size and number of peroxisomes and mitochondria rapidly adapts to specific cellular requirements. Thus, a sophisticated system governed by the dynamin-related proteins Dnm1 and/or Vps1 regulates their fission and proliferation. While the fission of peroxisomes can be executed either via Dnm1 or Vps1 (mainly depending on the available carbon source), mitochondrial fission solely relies on Dnm1. Cytosolic Dnm1 is recruited to either peroxisomes or mitochondria by the adaptor proteins Fis1 and Mdv1/Caf4 at sites where membrane fission will occur.Citation2 This molecular machinery is highly conserved across phylae and any imbalance affects life span. Lack of either Dnm1, Fis1 or Mdv1 causes longevity in yeast, which was supposed to be due to a reduction of mitochondrial fission.Citation3 However, in their current paper, Lefevre, Kumar and Van der Klei provided evidence for an important role of peroxisomal fission in the determination of yeast life span.Citation4 They suggest that the absence of peroxisomal but not mitochondrial fission is the main reason for longevity caused by genetic impairment of the Dnm1/Fis1 machinery ().
Figure 1. Schematic illustration of peroxisomal fission. Two independent dynamin-related GTPases, Vps1 and Dnm1, govern peroxisomal division. Lefevre, Kumar and Van der Klei could show that in Δvps1 cells, the additional deletion FIS1 and thus the complete absence of peroxisomal fission leads to an extended maximum chronological life span.
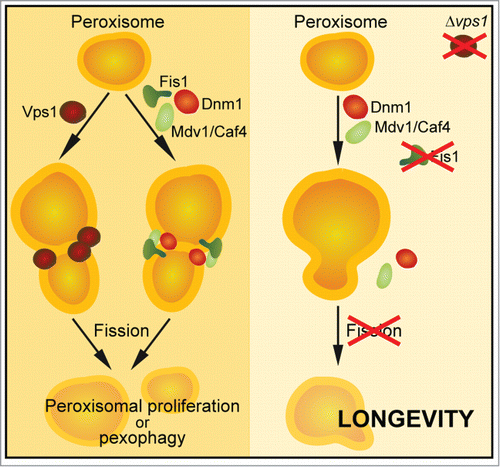
In order to selectively examine the contribution of peroxisomal and mitochondrial fission to survival during chronological aging, the authors used a Δvps1 mutant background, which completely relies on the Dnm1/Fis1 machinery for peroxisomal fission. Deletion of FIS1 in Δvps1 cells increased the maximum chronological life span (CLS), and re-introduction of a modified Fis1 protein exclusively localized to the peroxisome prevented this pro-survival effect. Thus, the maximum CLS was significantly enhanced in strains with blocked peroxisomal and mitochondrial fission, but not in strains only inhibited in mitochondrial fission. The authors further demonstrate that cells lacking Pex3, an essential protein for peroxisome biogenesis,Citation5 did not respond to the life span-prolonging effect of FIS1 deletion. Δpex3 cells were not able to produce normal peroxisomes but apparently displayed intact mitochondria, indicating that blocked peroxisomal and mitochondrial fission via deletion of FIS1 only influenced CLS when intact peroxisomes were present.
Together, these findings suggest that a block of peroxisomal but not mitochondrial fission leads to an enhancement of maximum (but not mean) CLS in yeast. Since peroxisomes have the enzymatic capacity to prevent ROS-driven necrotic death during chronological aging,Citation6 the inhibition of peroxisomal fission might somehow improve this cytoprotective function, potentially via a reduction of peroxisomal degradation. On the other hand, peroxisomes can not only detoxify but also generate ROS, indicating that the mechanisms underlying life span control by peroxisomal fission are most probably more complex.
Peroxisomal and mitochondrial fission have been shown to be prerequisites for the breakdown of these organelles by selective autophagy (pexophagy and mitophagy, respectively). Interestingly, regular peroxisomal fission as well as pexophagy-specific fission, mediated either by Dnm1 or Vps1, occurs in the immediate vicinity of mitochondria, indicating that both proliferation and selective degradation of peroxisomes requires functional mitochondria.Citation7 Peroxisomes and mitochondria are metabolically linked to each other and a functional coordination of fission and selective degradation of these organelles seems to be important during aging. Thus, exploration of mechanistic connections between peroxisomes and mitochondria, in particular regarding the regulation of peroxisomal versus mitochondrial fission as well as their selective degradation is needed. While the decisive role of mitochondrial fusion and fission in the physiology and pathophysiology of aging is beyond dispute, the importance of peroxisomal fission might still be underestimated.
References
- Klecker T, et al. Trends Cell Biol 2014; 24:537-45; PMID:24786308; http://dx.doi.org/10.1016/j.tcb.2014.04.004
- Westermann B. Nat Rev Mol Cell Biol 2010; 11:872-84; PMID:21102612; http://dx.doi.org/10.1038/nrm3013
- Scheckhuber C, et al. Nat Cell Biol 2007; 9:99-105; PMID:17173038; http://dx.doi.org/10.1038/ncb1524
- Lefevre S, et al. Cell Cycle 2015; 14(11):1698-703; PMID:25840089; http://dx.doi.org/10.1080/15384101.2015.1029685
- Hettema EH, et al. EMBO J 2000; 19(2):223-33; PMID:305556; http://dx.doi.org/10.1093/emboj/19.2.223
- Jungwirth H, et al. FEBS Lett 2008; 582:2882-6; PMID:18656474; http://dx.doi.org/10.1016/j.febslet.2008.07.023
- Mao K, et al. Autophagy 2014; 10:652-61; PMID:24451165; http://dx.doi.org/10.4161/auto.27852
