Abstract
Anti-integrin-linked kinase (ILK) therapies result in aberrant mitosis including altered mitotic spindle organization, centrosome declustering and mitotic arrest. In contrast to cells that expressed the retinoblastoma tumor suppressor protein Rb, we have shown that in retinoblastoma cell lines that do not express Rb, anti-ILK therapies induced aberrant mitosis that led to the accumulation of temporarily viable multinucleated cells. The present work was undertaken to: 1) determine the ultimate fate of cells that had survived anti-ILK therapies and 2) determine whether or not Rb expression altered the outcome of these cells. Our data indicate that ILK, a chemotherapy drug target is expressed in both well-differentiated, Rb-negative and relatively undifferentiated, Rb-positive retinoblastoma tissue. We show that small molecule targeting of ILK in Rb-positive and Rb-deficient cancer cells results in increased centrosomal declustering, aberrant mitotic spindle formation and multinucleation. However, anti-ILK therapies in vitro have different outcomes in retinoblastoma and glioblastoma cell lines that depend on Rb expression. TUNEL labeling and propidium iodide FACS analysis indicate that Rb-positive cells exposed to anti-ILK therapies are more susceptible to apoptosis and senescence than their Rb-deficient counterparts wherein aberrant mitosis induced by anti-ILK therapies exhibit mitotic arrest instead. These studies are the first to show a role for ILK in chemotherapy-induced senescence in Rb-positive cancer lines. Taken together these results indicate that the oncosuppressive outcomes for anti-ILK therapies in vitro, depend on the expression of the tumor suppressor Rb, a known G1 checkpoint and senescence regulator.
Abbreviations
| ILK | = | Integrin-linked kinase |
| Rb1 | = | Retinoblastoma gene |
| Rb | = | Retinoblastoma protein |
| DMEM | = | Dulbecco's Modified Eagle Medium |
| NGS | = | Normal Goat Serum |
| BSA | = | Bovine Serum Albumin |
| PI | = | Propidium iodide |
| siRNA | = | Small Interfering RNA |
| DMSO | = | Dimethyl Sulfoxide |
| TBS | = | Tris Buffered Saline |
| TUNEL | = | Terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end. |
Introduction
During cell division, integrin-linked kinase (ILK) regulates microtubule dynamics and centrosome clustering,Citation1-6 processes involved in cell cycle progression and malignant transformation.Citation7 This regulation occurs in a wide variety of human cancers cells including retinoblastoma.Citation8 ILK expression is upregulated in retinoblastomas,Citation9 and is a key regulator of G1/S cyclin-cdk activities,Citation10,11 a critical step in the retinoblastoma signaling pathway. Furthermore, in cells containing a functional retinoblastoma gene RB1 and in which ILK is overexpressed, ILK directly regulates retinoblastoma protein (Rb) activity.Citation11 Retinoblastoma is a retinal tumor that traditionally carries RB1 mutations, and often lack expression of the tumor suppressor protein Rb.Citation12-15 However, some recently discovered retinoblastomas have been shown to express an apparently functional Rb tumor suppressor that is normally phosphorylated preventing its interaction with the transcription factor E2F.Citation12,13 Although the transformation of retinal cells and the development of tumors are not fully understood, the progression of this cancer in the majority of retinoblastomas is considered intimately related to deficient Rb signaling, increased and inappropriate proliferation and the ability to survive mitotic infidelity. To date, the Rb-dependent nature of ILK's mitotic function has not been studied.
Molecular events underlying the role of ILK in mitotic regulation are emerging. An ILK-targeted small molecule inhibitor was shown to selectively effect cancer cells with supernumerary centrosomes imposing centrosome declustering, multipolar division, and cell death.Citation2 This has led to the proposal that ILK is a valid anti-mitotic chemotherapy drug target.Citation7 When ILK is downregulated, multipolar cells undergoing mitosis may be exhibiting mitotic catastrophe or anaphase catastrophe, 2 different mechanisms that underlie decreased mitotic fidelity. Although poorly understood, mitotic catastrophe originates from aberrations in the mitotic apparatus that is accompanied by some degree of mitotic arrest giving rise to multinucleated cells.Citation16,17 Anaphase catastrophe is a variant of mitotic catastrophe that satisfies the spindle assembly checkpoint and some investigators have proposed that ILK inhibition triggers anaphase catastrophe.Citation17 An earlier study from our laboratory has provided evidence that ILK inhibition increases mitotic catastrophe in retinoblastoma cells.Citation8 This was evident by: aberrant mitotic division, increased multinucleation, mitotic arrest and aberrant chromosomal segregation.Citation8 Both mitotic catastrophe and anaphase catastrophe underlie compromised mitotic fidelity and are thought to be oncosuppressive in that they ultimately result in cell death or cell senescence. Hallmarks of cellular senescence include a large flat morphology, senescence-associated ß-galactosidase (SA-ß-gal) expression, and mitotic infidelity.Citation18,19 Here we tested whether anti-ILK therapies regulated the induction of cellular senescence, and whether this was impacted by the Rb-status of the targeted cells.
The expression of the Rb tumor suppressor is significant, as several genes with known functions in mitosis are expressed in an E2F-dependent manner following phospho-Rb–mediated derepression.Citation20 Moreover, Rb has been shown to be important in regulating a checkpoint that acts subsequent to mitotic errors to block proliferation of cells that have entered G1 with a multinucleated status.Citation21,22 The Rb tumor suppressor is a master regulator of senescence, and inactivation of this signaling pathway has been shown to prevent the induction of senescence.Citation23-26 Although most tumor cells are able to induce senescence-related pathways in response to chemotherapies, cells without functional Rb, have been shown to initially overcome senescence.Citation26 We have compared the mitotic effects of anti-ILK therapies in retinoblastoma and glioblastoma cell lines that express normal levels of Rb and those that do not and find that outcomes in vitro are dependent on the Rb status of the cell. Specifically, we find that in cancer cell lines expressing Rb, ILK inhibition or downregulation increases aberrant mitosis that results in apoptosis and senescence. In contrast, although anti-ILK therapies increase aberrant mitosis in Rb deficient cells, these cells exhibit mitotic arrest instead.
Results
ILK and Rb expression in retinoblastoma tissue and cancer cell lines
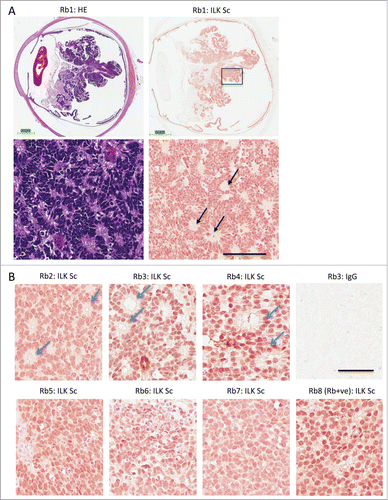
Positive staining for ILK has previously been reported for the majority of retinoblastoma tissue (15 of the 17 specimens).Citation27 However, histological features of ILK immunoreactive retinoblastoma tissue were not described in detail. ILK expression in rare Rb-positive tumors was also not studied. We observed nuclear and cytoplasmic ILK immunoreactivity in all 8 retinoblastoma patient samples studied. These samples included well-differentiated and undifferentiated tissue samples as well as Rb-positive and Rb-negative samples. Prominent ILK staining was observed in areas of tissue exhibiting Homer Wright rosettes (see rosettes highlighted by black arrow). Homer Wright rosettes consist of a halo of tumor cells surrounding a central eosinophilic region containing neuropil. These rosettes are seen in well-differentiated Rb-deficient retinoblastoma and in other neuroblastic tumors.Citation28,29 The presence of this type of rosette can be verified by comparing similar regions of tissue in serial sections that have been stained either for ILK or for haematoxylin and eosin (; the area enclosed in a black box at low magnification is shown below at high magnification). Similarly, areas of other patient tumors that exhibit ILK immunoreactivity have classic Flexner-Wintersteiner rosettes, a histological feature of well-differentiated retinoblastoma tissue (). Flexner-Wintersteiner rosettes contain tumor cells surrounding a clear central lumen. Undifferentiated cells with large, round nuclei, seen in the Rb-expressing retinoblastoma tissue sample also stains positively for ILK (; see panel labeled Rb8). In contrast to well-differentiated Rb-deficient retinoblastoma tumor samples, this largely undifferentiated tissue lacked classic Flexner-Wintersteiner rosettes, a finding that has been previously reported.Citation13
Figure 1. ILK expression in Rb positive and Rb deficient retinoblastoma. (A) Sections of retinoblastoma tissue taken from 8 different patients that were Rb mutant (Rb1–7) or Rb positive (Rb8). Serial sections of Rb mutant retinoblastoma tissue were stained with haemoxylin and eosin (HE) or with a monoclonal antibody to ILK (ILK Sc). The area enclosed by the black box in the low magnification view (upper) is shown at a higher magnification below (lower). Black arrows depict Homer Wright rosettes seen in well-differentiated retinoblastoma tissue and other tumors of neuronal origin. Calibration bars represent 3 mm (upper) and 100 μm (lower) (B) Immunohistochemical staining of well differentiated retinoblastoma tissue (P2–4) or more undifferentiated tissue (P5–8) for ILK. Well-differentiated tissue exhibit classic Flexner-Wintersteiner rosettes, characteristic of retinoblastoma (blue arrows) while Rb positive tissue (P8) have a preponderance of undifferentiated cells. Calibration bar represent 50 μm.
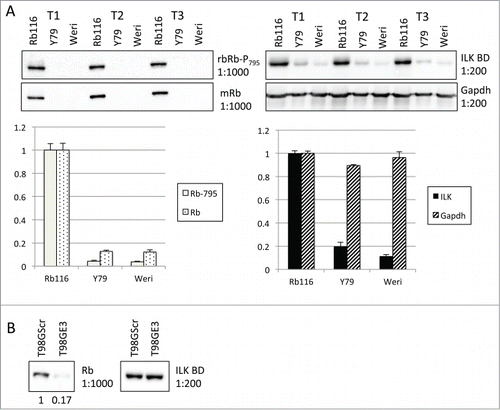
To study ILK's role in Rb-positive and -deficient cancer lines we employed human retinoblastoma and glioblastoma lines. Retinoblastoma lines grow in suspension. While traditionally, these lines are thought to carry Rb mutations, and are often negative for Rb,Citation12-15 recently, tumor cells have been shown to express a seemingly functional Rb tumor suppressor that is phosphorylated at Ser795 and immunoprecipitates with E2F.Citation12,13 The glioblastoma lines are adherent T98G cells that harbor either shScramble (T98G Scr; Rb positive) or shRb (T98G E3; Rb deficient). The Rb116 retinoblastoma cell line was recently established from an Rb-positive retinoblastoma tissueCitation12 while other lines (i.e., Y79 and Weri-Rb27) have been shown to be Rb-negative.Citation12,14,15 We confirmed these results and show here that Rb in Rb116 lines is also phosphorylated at residue Ser795 (). Interestingly, Rb116 also express high levels of ILK. ILK expression was not affected by RNA interference-mediated Rb suppression, as in T98G cells, ILK expression was not dependent on the level of Rb expressed (). Together, Western blots confirm the presence or lack of Rb expression in our cancer cell models.
Figure 2. Rb and ILK expression in retinoblastoma and glioblastoma lines. (A) Staining of additional Western blots of Rb116 cells as compared to Y79 and Weri-Rb (2 lines known to lack Rb expression). (Left Upper) Western blots of retinoblastoma cell lysates from 3 separate trials (designated T1-T3) were first probed with a rabbit antibody that recognizes Rb phosphorylated on serine 795 (phospho-Rb Ser795) and then stripped and reprobed with a mouse anti-Rb antibody. (Left Lower) Bar graph represents densitometric analysis of blots probed first for Rb and then for phospho-Rb Ser795. Rb116 cells are shown to have relatively high levels of Rb phosphorylated on serine 795 and overall Rb expression. (Right Upper) Western blots of retinoblastoma lysates, run in parallel, were probed with a mouse anti-ILK antibody and then stripped and reprobed with an anti-Gapdh antibody. (Right Lower) Bar graph represents densitometric analysis of blots probed first for ILK and then for Gapdh. (B) Lysates of T98G cells that were expressing normal levels of Rb (Scr) or were downregulated for Rb (E3) were run. Western blots probed with a mouse anti-ILK antibody and then stripped and reprobed with an anti-Gapdh antibody are shown.
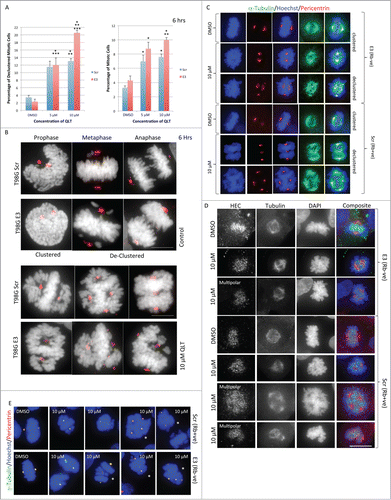
ILK inhibition increases multinucleation in Rb positive and deficient cancer cell lines
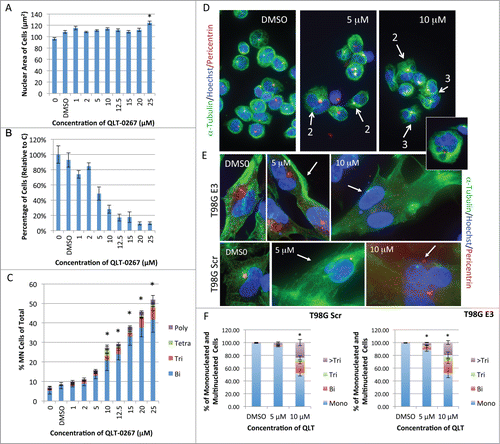
ILK inhibition was shown to compromise cytokinesis in Rb-negative retinoblastoma cells in a concentration-dependent manner.Citation8 This led to a corresponding increase in the accumulation of cells having an increased total nuclear area and increased number of nuclei. To determine whether ILK inhibition also led to multinucleation in Rb-positive retinoblastoma cells, we exposed Rb116 cells to the small molecule ILK inhibitor QLT-0267. In Rb116 retinoblastoma cells (positive for Rb), the average total nuclear area per cell did not increase significantly after a 5-day exposure to increasing concentrations of QLT-0267 (1-20 μM) (). There was however, a corresponding decrease in the total number of Rb116 cells relative to control following this exposure period (). Also, as we showed previously for the Y79 cells,Citation8 the number of multinucleated cells increased significantly at 10 μM QLT-0267 following a 5-day exposure suggesting that cytokinesis was also aberrant in these Rb positive lines. Like the Y79 cells,Citation8 the majority of multinucleated cells were binucleated (BN, blue). However, trinucleated (Tri, red), tetranucleated (Tetra, green) and polynucleated (Poly, purple) were also apparent (). Representative Rb116 cells fixed and stained for α-tubulin, pericentrin and Hoechst, following a 5-day exposure to 5 or 10 μM of QLT-0267, are also included (). Flattened stacks of Rb116 cells are shown with white arrows highlighting multinucleated cells (the number of nuclei present in these cells are also indicated). As occurred with retinoblastoma cells multinucleation also increased in T98G cells following a 5-day exposure to QLT-0267, as compared to vehicle control. ILK inhibition increased multinucleation in both T98G cell lines that were Rb positive (T98G Scr) and Rb deficient (T98G E3) as is evident from representative figures (). The percentage of cells that were mononucleated (Mono) or multinucleated following a 5-day exposure to QLT-0267 or drug vehicle (DMSO) were quantitated (). Multinucleated cells were further categorized as binucleated (Bi), trinucleated (Tri) or cells having greater than 3 nuclei (>Tri). Ten micromolar of the ILK inhibitor significantly increased multinucleation in T98G E3 and T98G Scr cells as compared to control.
Figure 3. QLT-0267 increases multinucleation in Rb positive and Rb deficient cell lines in a concentration-dependent manner. (A) Nuclear size of Rb positive retinoblastoma cells (Rb116) exposed to increasing concentrations of QLT-0267, was determined over 5 d in culture. Controls lacking drug vehicle, labeled 0 or with drug vehicle alone, labeled DMSO, were also included. The nuclear size of Rb116 cells is measured as a unit of Hoechst-stained area by Metamorph Premier software. An ANOVA followed by Dunnett's post hoc test was used to determine the significance of observed differences (*p < 0.05, QLT-0267 treated cells different from vehicle control). (B) A corresponding decrease in total Rb116 cell number with increasing QLT-0267 concentration was also observed for this same data sample. Data represent the mean ± SEM, n = 3 independent experiments with greater than 500 cells/treatment (over 5000 cells were counted in the controls). Nuclei number and nuclear area have been taken from the same data set. (C) Changes in nuclear number in retinoblastoma Rb116 cells exposed to increasing concentrations of QLT-0267 were determined over 5 d in culture. Cells were fixed and stained with Hoechst and tubulin to visualize binucleate (Bi, blue), trinucleate (Tri, red), tetranucleate (Tetra, green) and polynucleate (Poly or greater than 4 nuclei, purple). Data for the percentage of multinucleated cells are represented as the mean ± SEM of 4 independent experiments. An ANOVA followed by Dunnett's post hoc test was used to determine the significance of observed differences (*p < 0.05, different from vehicle control; vehicle control and nonvehicle control were not statistically different). (D) Shown are representative Rb116 cells after they were stained for α-tubulin, pericentrin and Hoechst following a 5-day exposure to 5 or 10 μM QLT-0267 or DMSO control. White arrows depict cells that are multinucleated as determined by scanning through individual z-stacks. Associated numbers indicate the number of nuclei that is present. (Inset) A quadrinucleated cell is represented following a 5-day exposure to 5 or 10 μM QLT-0267 or DMSO control. Centrosome amplification is evident in both mononucleated and multinucleated cell populations. (E) Representative T98G cells are shown following a 5-day exposure to 5 or 10 μM QLT-0267 or DMSO control. Multinucleation was evident after cells were stained for α-tubulin, pericentrin and DAPI. White arrows depict cells that are multinucleated following analysis of z-stacks. Multinucleation increased in both QLT-0276 treated T98G cell lines (those with stably integrated shRb (T98G E3) and those with shScramble (T98G Scr)). (F) The percentage of cells that were mononucleated (Mono) or multinucleated following a 5-day exposure to QLT-0267 or DMSO control were quantitated. Multinucleated cells were further categorized as binucleated (Bi), trinucleated (Tri) or cells having greater than 3 nuclei (>Tri). *p < 0.05, percentage of multinucleated cells different from vehicle control within the same cell line. Data are mean ± SEM from 3 independent experiments. Significance was determined using an ANOVA and Fisher's (LSD) test.
Treatment with the ILK inhibitor results in early aberrant mitosis effecting apoptosis and G2+M-phase arrest in an Rb-dependent manner
A comparison of early mitotic changes in glioblastoma cell lines was undertaken to determine whether or not Rb expression altered the sensitivity of glioblastoma cells to early molecular changes induced by the proposed anti-mitotic chemotherapeutic, QLT-0267. We exposed T98G cell lines to QLT-0267 or vehicle control for 6 hours and then stained cells for α-tubulin, pericentrin and Hoechst. QLT-0267 increased the proportion of cells exhibiting aberrant mitotic spindles, centrosome declustering and multipolar division in both lines at 5 and 10 μM, relative to control.
In both lines, the percentage of cells undergoing cell division increased over 2-fold when unsynchronized cells were exposed to 10 μM of QLT-0267 for 6 hours. Glioblastoma cells deficient for Rb (T98G E3) were more sensitive to the chromosomal anti-clustering effects at the highest concentration of QLT-0267 (10 μM). In particular, T98G E3 cells treated with the inhibitor resulted in 21% declustered mitotic cells versus 2% vehicle control while in the Rb expressing cells (T98G Scr), ILK inhibition increased declustering to 13% vs. 4% vehicle control. A wide range of aberrant mitotic figures were observed in both T98G lines and these included both clustered (mitotic cells with bipolar spindles) and declustered cells (mitotic cells with more than 2 spindle poles) (). Declustered mitotic cells included those that appeared to have chromosomes lining up along 2 “metaphase plates” bearing 3 mitotic organizing centers (MTOC) (see T98G Scr, 10 μM QLT in far right panel) and 4 spindle poles (see panel 5).
Both T98G cell lines demonstrated a wide variety of chromosomal arrangements when undergoing mitosis. Most representative mitotic figures shown were observed in both Rb backgrounds (). However, in the Rb-positive background, the multipolar cells sometimes exhibit a uniform number of chromosomes at the midzones versus non-paired chromosomes at the poles. This is in contrast to the mitotic structures observed in the Rb-negative background in which the multipolar spindles are more disorganized and the chromosomes less equalized in distribution (). To uncover any potential differences in mitotic processes observed between Rb lines treated with the ILK inhibitor, kinetochore distribution and nucleation sites were examined. Highly Expressed in Cancer protein 1 (Hec1) staining was performed to observe kinetochore distribution, defects in which could produce chromosome segregation errors. Hec1 is of particular interest as disruption of Rb function has been shown to lead to mitotic defects through Hec1 overexpression in other cell lines.Citation30 While some variability in Hec1 staining intensity between mitotic cells was observed, we did not observe qualitative differences in Hec1 staining between the Rb-positive and Rb-deficient cells (). Hec1 staining was most intense in areas of high chromatin condensation and treatment of cells with QLT-0267 for 6 hours did not appear to alter Hec's localization to the kinetochores. This was true for bipolar and multipolar mitotic cells in both Rb backgrounds (). Gamma tubulin staining was also performed, as this protein is required for microtubule nucleation and centrosome function.Citation31,32 Pericentrin and γ-tubulin colocalized in mitotic cells regardless of treatment with the ILK inhibitor and this was true for both bipolar and multipolar mitotic cells () indicating that this chemotherapeutic did not affect microtubule nucleation in these 2 cell lines.
To examine the later effects of ILK inhibition on cell cycle progression, we stained fixed retinoblastoma and glioblastoma cells with propidium iodide and performed fluorescence-activated cell cycle analysis. Later effects of QLT-0267 on cell cycle progression were compared between Rb-positive and Rb-deficient lines following a 5 day exposure for the relatively slow growing and suspended, retinoblastoma lines (as previously performed).Citation8 Faster growing, adherent T98G cell lines were compared following a one day drug exposure. As has been shown previously for Rb-negative retinoblastoma cell lines, a 5-day exposure to QLT-0267 significantly increased the fraction of Y79 cells in G2+M-phase. The percentage of cells in G2+M-phase was 39.6 ± 5% as compared to 24.6 ± 2.1% in vehicle control (see ). As the percentage of cells in G2+M-phase increased, the percentage of cells in G1/G0-phase decreased, while the proportion of cells in S-phase was not significantly different. Furthermore, the sub-G1 peak, indicative of apoptotic cells did not change significantly. Conversely, Rb positive Rb116 cells exposed to the inhibitor, did not arrest in G2+M-phase. Instead there was a significant increase in the sub-G1 peak when cells were treated with 10 μM QLT-0267 and a corresponding decrease in the proportion of cells in S-phase. To confirm whether or not there was indeed a difference in sensitivity between these lines to the apoptotic effects of QLT-0267, TUNEL positive retinoblastoma cells were quantitated following a 5-day exposure to increasing concentrations of QLT-0267. A significant increase in the percent of TUNEL positive cells was observed for Rb116 at QLT-0267 concentrations greater than or equal to 10 μM while a significant increase in TUNEL positive Y79 cells was not observed. T98G cell lines were exposed to 5 and 10 μM QLT-0267 or drug vehicle for one day. In order to analyze the entire cell population, suspended cells were collected and separated from adherent populations. Fluorescent-activated cell sorting analysis was performed on both populations separately. The ILK inhibitor induced a significantly higher sub-G1 peak in T98G cells expressing normal levels of Rb (T98G Scr) than those downregulated for Rb (T98G E3) at 10 μM. In adherent populations, T98G Scr exhibited a G2+M-phase arrest. However, G2+M-phase arrest was only observed in suspended populations of T98G E3 cells (downregulated for Rb). Although changes in cell viability were observed following a one-day exposure (as measured by sub-G1 peak differences), changes in viability were not observed at an earlier time frame. Specifically, an increase in phospho-histone H2A.X staining (indicative of a DNA damage response) was not observed in either T98G Scr or T98G E3 cell lines, exposed to 5 and 10 μM QLT-0267 relative to control ().
Figure 5. ILK Inhibition effects apoptosis and G2+M-phase arrest in an Rb-dependent manner. (A) Rb116 and Y79 cells were exposed to 5 and 10 μM QLT-0267 or drug vehicle for 5 d. Cells were then stained with propidium iodide for the fluorescence-activated cell sorting analysis. The percentage of cells in sub-G1, G0/G1-, S- and G2+M-phase is expressed as an average ± SEM of independent platings (n = 2−5) for both QLT-0267 and vehicle treated groups. A marked increase in the percentage of Y79 cells in the G2+M-phase was observed in QLT-0267 treated cells as compared to vehicle control. The ILK inhibitor induces a significant sub-G1 peak in Rb positive Rb116 cell lines but not Rb negative Y79 cells following a long-term exposure to QLT-0267. Rb116 cells do not show any significant mitotic arrest, but show a significant sub-G1 peak indicative of apoptosis at 10 μM QLT-0267. Data are represented as the mean ± SEM, n = 3−6 independent experiments. *p < 0.10, different from vehicle control and **p < 0.10, different from vehicle control and lower QLT-0267 concentration, as determined using an ANOVA and Fisher's (LSD) test. (B) TUNEL positive retinoblastoma cells (Y79 and Rb116) exposed to increasing concentrations of QLT-0267 were determined following 5 d in culture. A significant increase in the percent of TUNEL positive cells was observed for Rb116 at QLT-0267 concentrations greater than or equal to 10 μM while a significant increase in TUNEL positive Y79 cells was not observed. Data are represented as the mean ± SEM, n = 3−6 independent experiments. *p < 0.05, different from vehicle control as determined using an ANOVA and Fisher's (LSD) test; vehicle control and nonvehicle control were not statistically different. (C) T98G cells expressing normal levels of Rb (labeled Scr) and those having Rb downregulated (labeled E3) were exposed to 5 and 10 μM QLT-0267 or drug vehicle for one day (1D). Nonadherent or suspended cells (lower panel) were separated from adherent populations (upper panel) and fluorescent-activated cell sorting analysis was performed. The percentage of cells in sub-G1, G0/G1-, S- and G2+M-phase (G2/M) is expressed as an average ± SEM of 3 independent platings. The ILK inhibitor induces a significantly higher sub-G1 peak in T98G cells expressing normal levels of Rb (T98G Scr) than those downregulated for Rb (T98G E3). In adherent populations, T98G Scr exhibited a G2+M-phase arrest. However, G2+M-phase arrest was only observed in suspended populations of T98G E3 cells (downregulated for Rb). *p < 0.05, different from vehicle control and ** different from other QLT-0267 treatments as determined using an ANOVA and Fisher's (LSD) test. (D) Early DNA damage response is not apparent in T98G cells following a 6 hour exposure to QLT-0267. Increased phospho-histone H2A.X staining was not observed in T98G Scr and T98G E3 cell lines, exposed to 5 and 10 μM QLT-0267 as compared to vehicle control.
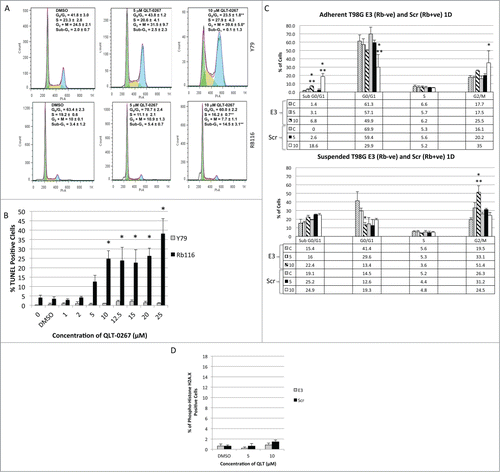
ILK inhibition and knockdown effects senescence in an Rb-dependent manner
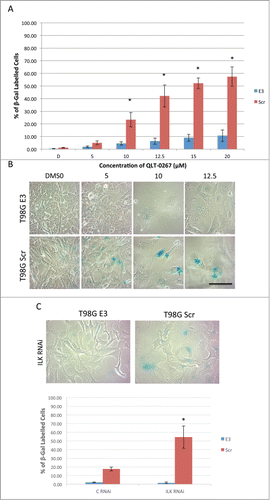
Although tumor cells can have various mutations that allow them to resist apoptosis, these cells can still be driven into growth arresting phenotypes such as senescence. Cellular senescence is more than just growth arrest and is characterized by mitotic infidelity in the presence of growth stimulation.Citation18,19 Cytochemical determination of senescence is associated with increased expression levels of ß-galactosidase at pH 6.0 and is called senescence-associated ß-galactosidase (SA-ß-gal). To determine if Rb expression influences whether or not cancer cells succumb to senescence following anti-ILK therapies, retinoblastoma lines were stained for this classic marker of senescence after a 5-day exposure to QLT-0267 or DMSO control. A concentration-dependent increase in cellular senescence was observed in Rb116 cells while comparatively little senescence was observed in Y79 cells, even at the highest QLT-0267 concentrations (). In Rb116 cells, SA-ß-gal activity was often seen in enlarged cells having one or more enlarged prominent nuclei. Although an increase in cell size was observed in Y79 cells with increasing drug concentrations, evidence of SA-ß-gal activity was not (see ). To address the possibility that ILK inhibition is the result of off-target effects, that alter signaling independent of ILK kinase activity,Citation33 ILK was functionally downregulated in retinoblastoma cells by another means. Specifically, retinoblastoma cells were treated twice with control (C RNAi) or ILK siRNA (ILK RNAi). Three days following the last siRNA treatment, cells were stained for SA-ß-gal activity. ILK knockdown significantly increased SA-ß-gal activity in Rb116 cells as compared to control (). This effect was not observed in Y79 cells. QLT-0267-induced senescence was significantly higher in T98G Rb positive cells (T98G Scr) as compared to T98G Rb deficient cells (T98G E3) (). Representative figures used in the analysis of SA-ß-gal activity in T98G cells exposed to 5, 10 or 12.5 µM QLT-0267 or drug vehicle alone indicate a higher level of senescence in Rb positive cells (T98G Scr) as compared to Rb deficient cells (T98G E3). With increasing concentration of QLT-0267, morphological changes indicative of senescence were observed (). These include an increase in cells that were flattened and enlarged having a prominent nucleus and increased cytoplasmic granularity. Additionally, increased SA-ß-gal activity staining within the perinuclear compartment was observed in cells having undergone senescence. Representative photographs of T98G Scr or T98G E3 cells following ILK knockdown are shown above the histograms and indicate that ILK siRNA treatment increased SA-ß-gal activity in Rb positive cells but not Rb deficient cells (). Likewise, histograms of the % SA-ß-gal stained cells indicate that ILK knockdown resulted in a significantly higher level of senescence in T98G cells expressing normal levels of Rb (T98G Scr) as compared those cells having Rb knocked down (T98G E3).
Figure 6. ILK inhibition and knockdown effects senescence in an Rb-dependent manner in retinoblastoma cells. (A) Retinoblastoma lines that were either Rb-ve (Y79) or Rb+ve (Rb116) were stained for SA-β-gal activity, a classical marker of senescence after a 5-day exposure to QLT-0267 or DMSO control. A concentration-dependent increase in cellular senescence was observed in Rb116 cells while comparatively little senescence was observed in Y79 cells, even at the highest QLT-0267 concentrations. Data are represented as the mean ± SEM, n = 3 −5 independent experiments. *p < 0.05, different from vehicle control; **p < 0.05, different from the other cell line at the same drug concentration and ***p < 0.05 different from the same cell line treated with lower QLT-0267 concentrations. Significance was determined using an ANOVA and Fisher's (LSD) test (B) Representative figures used in the analysis of SA-β-gal staining is shown. An Rb+ve (Rb116) and Rb-ve (Y79) retinoblastoma cell line was exposed to increasing QLT-0267 indicates that in Rb116 cells increased SA-β-gal activity staining within the perinuclear compartment was observed with QLT-0267 concentrations ≥ 5 µM. In Rb116 cells, SA-β-gal staining was often observed within the perinuclear compartment of enlarged cells having one or more enlarged prominent nuclei. Although an increase in cell size was observed in Y79 cells with increasing drug concentration, evidence of SA-β-gal activity was not. (C) Retinoblastoma lines were treated twice with control (C RNAi) or ILK siRNA (ILK RNAi). Three days following the last siRNA treatment, cells were stained for SA-β-gal activity. Histograms of the % SA-β-gal stained cells indicate that ILK knockdown resulted in a significantly higher level of senescence in Rb116 cells. *p < 0.05, different from control and all other groups as determined using a one-way ANOVA and Fisher's (LSD) test. (D) Representative figures used in the analysis of SA-ß-gal staining are shown. SA-β-gal staining is seen here within the perinuclear compartment of enlarged and normal sized cells.
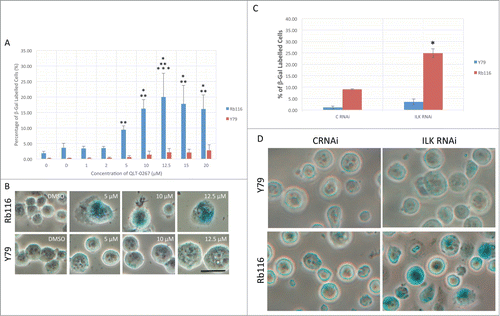
Figure 7. ILK Inhibition and Knockdown Effects Senescence in an Rb-Dependent Manner in Glioblastoma Cells. (A) QLT-0267-induced senescence was significantly higher in T98G Rb+ve cells (T98G Scr) as compared T98G Rb-ve cells (T98G E3), n = 4−5 *p < 0.05, different from vehicle control and from T98G E3 cells treated with the same concentration of QLT-0267 as determined using an ANOVA and Fisher's (LSD) test. (B) Representative figures used in the analysis of SA-β-gal activity in T98G cells are shown. T98G cells (Scr and E3) exposed to 5, 10 or 12.5 µM QLT-0267 or drug vehicle alone indicate a higher level of senescence in T98G Scr cell as compared to T98G E3 cells. With increasing concentration of QLT-0267 morphological changes indicative of senescence include an increase in cells that are flattened and enlarged having a prominent nucleus and increased cytoplasmic granularity. Additionally, increased SA-β-gal activity staining within the perinuclear compartment was observed. (C) T98G cells were treated twice with control (C RNAi) or ILK siRNA (ILK RNAi). Three days following the last siRNA treatment, cells were stained for SA-β-gal activity. (Upper) Representative photographs of T98G Scr or T98G E3 cells following ILK knockdown are shown above the histograms. (Lower) Histograms of the % SA-β-gal stained cells indicate that ILK knockdown resulted in a significantly higher level of senescence in T98G cells expressing normal levels of Rb (T98G Scr) as compared those cells with Rb knocked down (T98G E3). n = 3. *p < 0.05, different from all other groups as determined using a one-way ANOVA and Fisher's (LSD) test.
Conclusion
Centrosome clustering is an essential mechanism for the survival of cancer cellsCitation34-36 permitting cells with an amplified number of centrosomes to divide in a pseudo-bipolar fashion. Should this clustering fail, spindle multipolarity results, predisposing the cell to either mitotic arrest or death. The catastrophic nature of multipolar division is a proposed endpoint for some anti-mitotic chemotherapeutic drugs, including those that target ILK.Citation17 In theory, these drugs would be cancer cell-selective targeting only those cells with an abnormal centrosome complement and a propensity toward multipolar division.Citation2,3 The present studies were undertaken to follow up on earlier work in retinoblastoma lines showing that anti-ILK therapies prevented successful bipolar division.Citation8 In contrast to earlier reports on cells that were wild-type for the Rb gene,Citation2 in these Rb deficient cancer cell lines, ILK inhibition decreased death following unsuccessful multipolar division (multipolar division where the polarity of division during anaphase was greater than the number of cells produced). This led to an increase in the accumulation of multinucleated cells over time. The present work was undertaken to: 1) determine the ultimate fate of cells that had survived anti-ILK therapies and 2) determine whether or not Rb expression altered the outcome of these cells. Our findings indicate that in Rb expressing cancer lines, ILK inhibition or downregulation increases aberrant mitosis that results in apoptosis and senescence. In contrast, although anti-ILK therapies increase aberrant mitosis in Rb deficient cells, these cells are comparatively more resistant to apoptosis and to cellular senescence, becoming mitotically arrested instead.
For these studies we compared retinoblastoma lines grown in suspension and adherent glioblastoma lines. We chose retinoblastoma lines that are Rb positive (i.e., Rb116) or Rb negative (i.e., Y79) and T98G cells that harbor either shScramble (T98G Scr; Rb positive) or shRb (T98G E3; Rb deficient). A comparison of retinoblastoma cell lines based on their expression of Rb is complicated by the fact that these lines often carry additional mutations. For example, many non-familial retinoblastomas that express Rb and lack RB1 mutations had high-level MYCN oncogene amplification.Citation13 It is often difficult to reexpress Rb into tumor cell lines with mutations in the RB1 gene as this often leads to cell cycle arrest and growth suppression. This is true even of cells with inducible Rb constructs.Citation37 Therefore, we chose to also compare anti-ILK therapies in T98G cell lines that expressed normal levels or markedly reduced levels of Rb. Interestingly, Rb-dependent effects of anti-ILK strategies were found to be similar in both of these quite different cellular paradigms.
Following ILK downregulation, if some cells irrespective of Rb expression levels are able to “cheat death” following chromosomal segregation to 2 or more “daughter” nuclei, without cytokinesis, then multinucleated cells would be expected to increase. Indeed we observed an increase in multinucleated cells following anti-ILK therapies in all retinoblastoma cells and T98G cells. Multinucleated cells have only been observed in a single retinoblastoma patient sample and in an irradiated model of retinoblastoma grown in nude mice. Both were thought to be undergoing tumor regression.Citation38 Therefore, increased multinucleation observed when ILK is downregulated may be a unique anticancer mechanism ultimately leading to mitotic arrest, decreased proliferation, the gradual elimination of multinucleated senescent cancer cells and to tumor regression. In cellular senescence cell cycle profiling typically show a decrease in the number of cells in S phase and an increase in the number of cells in G2+M.Citation25 Indeed, this was true in Rb116 (an Rb positive) retinoblastoma line grown in suspension following a 5-day exposure to the ILK inhibitor QLT-0267, while in the Rb negative Y79 line, the number of cells in G2+M increased without a corresponding decrease in S phase cells. Similarly, an increase in G2+M was observed for both T98G lines following a one-day exposure, however changes in the number of cells in S phase was not observed at this early time-point. However, cell cycle arrest is not yet senescence.Citation18,19 Senescence is a cellular phenotype characterized by a cell cycle blockage in the presence of growth stimulation with cells having characteristic morphological and cytochemical changes.Citation18,19 These cells have lost replicative capacity. An argument for senescence can be made more strongly for those cells positive for SA-β-gal staining that were seen to increase following anti-ILK therapies in Rb-expressing lines. Although Rb-deficient cells appeared to exhibit morphological changes indicative of cellular senescence (including a flattened appearance, growth in size and an increase in ratio of nucleus to cytoplasm) it is unknown whether or not these cells have undergone an irreversible loss of replicative capacity. It is encouraging to note however that these cells in spite of deficiencies in Rb, a key tumor suppressor responsible for executing proliferative arrest, become mitotically arrested with anti-ILK therapies. It is possible that this proliferative arrest is also irreversible and that senescence has only been delayed in Rb deficient tumor cells. Further long-term studies will be required to unravel the ultimate outcome of these Rb deficient cells.
In retinoblastoma, chemoreduction therapy has emerged as an important initial approach to retinoblastoma treatment and adjuvant chemotherapy is used for eyes with optic nerve invasion.Citation39 These treatments including carboplatin, etoposide, and vincristine have been shown to shrink the size of the tumor allowing focal treatments (i.e., cryotherapy, focal laser therapy, or thermotherapy).Citation29,40 This sequence is sometimes preferred in order to avoid enucleation.Citation29,40 Although many cancers have at least partially lost the ability to undergo apoptosis or senescence, these responses can still be reactivated with chemotherapy drugs.Citation41 Senescence inducing drugs may be a desirable outcome for those tumor cells that are resistant to apoptosis based therapies.Citation41 Moreover, this antitumour strategy may be more desirable because it is effective at lower concentrations thereby bypassing untoward side effects. The prognostic impact of the chemotherapy-induced senescence is largely unknown. Nevertheless, senescence remains a potential cytostatic anticancer strategy for many types of cancer including retinoblastoma.
Materials and Methods
Cell culture and drug exposure
Human retinoblastoma cell lines Y79, Weri-Rb and Rb116 were grown in suspension (unless stated otherwise) and cultured using DMEM+Glutamax (GIBCO), with 10% fetal bovine serum and gentamicin (50 μg/ml). All cell lines were originally derived from primary tumor explants recovered from the enucleated eye of a patient. Cells were incubated at 37°C and 5% CO2. High-density retinoblastoma cultures were split 1:2 every one to 2 d while low-density cultures were split 1:10 once a week. T98G Scr and T98G E3 cells as previously describedCitation37 were grown in the same media with 2 μg/ml puromycin to select for cells stably integrated with shScramble (T98G Scr; Rb positive) or shRb (T98G E3; Rb deficient).
Cells were treated with a selective, small molecule inhibitor for ILK that belongs to the K15792 class of the pharmacor family (QLT-0267, Valocor Therapeutics, Inc.). Stock QLT-0267 was suspended in DMSO at a concentration of 25 mM and stored at −20°C. Cells were exposed to QLT-0267 (or vehicle control) in complete media containing 10% fetal bovine serum.
Immunohistochemistry
Prior to undertaking these studies, ethical approval was obtained from the University of British Columbia Ethical Review Board and from the Trinity Western University Ethical Review Board for the use of human paraffin embedded tissue. Paraffin embedded sections were deparaffinized and subjected to a citrate buffer (pH 6.0) antigen retrieval step. Hydrated sections were then blocked in 3% milk and solubilized in 0.2% Triton X-100 in PBS. We then performed immunohistochemistry with a primary monoclonal antibody to ILK (1:400; Santa Cruz Biotechnology, http://www.scbt.com/datasheet-20019-ilk-65-1-antibody.html). Sections were incubated in primary ILK antibody overnight or an IgG control at 4°C. Staining was achieved using biotin-free techniques including MACH 4 Universal (Biocare Medical) and treatment with a Nova Red substrate (as previously reported Citation42).
Transfections (siRNA) and senescence β-Gal
ILK-specific and control siRNA constructs (5′-GACGCTCAGCAGACATGTGGA-3′) were purchased from Qiagen and used with Qiagen's HiPerFect reagent (http://www.qiagen.com/ca/products/catalog/assay-technologies/transfection-reagents/hiperfect-transfection-reagent) to transfect T98G cells according to the manufacturer's protocol and previously published manuscripts.Citation2,4,8 T98G cells were plated onto PDL coated coverslips and allowed to adhere overnight. Cell were transfected with siRNA in Opti-MEM media (Life Technologies Inc.) according to the manufacturer's instructions. Cells were re-transfected 2 d after the initial transfection, and analyzed 5 d after the initial transfection for senescence. Staining was done according to manufacturer's instructions as outlined in the Senescence β-Gal Staining Kit (Cell Signaling Technology).
Terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end (TUNEL) labeling
After a 5 day drug treatment, DNA fragmentation was measured in cell lines using the In Situ Cell Death Detection Kit (Roche, Fluorescein, http://lifescience.roche.com/shop/products/in-situ-cell-death-detection-kit-fluorescein) as previously described.Citation42 Cells were then washed in PBS and mounted in Vectashield mounting media containing DAPI (Vector Laboratories). The number of TUNEL-positive cells was calculated as a percentage of total cell number (DAPI positive cells).
Western blotting
Western blotting was used to confirm Rb deficiency in retinoblastoma (Rb116) and T98G E3 cells. Cells were lysed in K4IP buffer containing protease inhibitors (Complete-protease inhibitor tablets; Roche Applied Science), 1 mM PMSF, 2mM NaF and 1 mM Na3VO4 and tip sonicated on ice. Lysates were spun for 5 minutes at 13 000 rpm on a table-top microcentrifuge to remove the insoluble fraction and Western blots were run as previously described.Citation8,43 Western blots were probed first with a rabbit phospho-Rb Ser795 antibody (1:1000; Cell Signaling Technologies, http://media.cellsignal.com/pdf/9969.pdf) and then stripped and reprobed with a mouse anti-Rb antibody (1:1000; Cell Signaling Technologies, http://media.cellsignal.com/pdf/9969.pdf ). Western blots of lysates run in parallel were probed with a mouse anti-ILK antibody (1:400; BD Biosciences #611802) and then stripped and reprobed with a rabbit anti-Gapdh antibody (1:200; Santa Cruz, http://www.scbt.com/datasheet-25778-gapdh-fl-335-antibody.html) as a control for total protein expression. Blots were stripped using Restore™ buffer (Thermo Fisher Scientific) as previously described.Citation44 The Proteins were detected using Biorad Clarity™ Western ECL substrate and visualized by Biorad ChemiDoc XRS+ with Image Lab™ Software (Mississauga).
Immunocytochemistry and analysis of mitotic cells
Y79 and Rb116 cells grown in suspension were adhered to poly-D-lysine coated 12-well slides before fixation. Retinoblastoma cells or glioblastoma cells were fixed for 10 minutes at room temperature in 4% paraformaldehyde. Cells were then permeabilized using 0.2% Triton/TBS and blocked with 5% NGS in 0.1% BSA/TBS-Tween. Cells were then incubated overnight at 4°C with antibodies against α-tubulin (Sigma-Aldrich, mouse, https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Datasheet/t6199dat.pdf, 1:1000), pericentrin (Abcam, rabbit, http://www.abcam.com/pericentrin-antibody-centrosome-marker-ab4448.html, 1:1000) hec1 (Abcam, rabbit, http://www.abcam.com/hec1-antibody-9g3-ab3613.html, 1:500) or β-tubulin (Cell Signaling, rabbit, 1:200) as previously described.Citation8 Cells co-stained for γ-tubulin (Abcam, mouse, http://www.abcam.com/gamma-tubulin-antibody-tu-30-ab27074.html 1:500) and pericentrin were fixed in methanol for 10 minutes followed by 6 minutes in acetone at −20°C. Cells were then stained for Hoechst 33342 or DAPI, washed and mounted using Vectashield mounting media (Vector Laboratories). Cells that were considered declustered were those having greater than 2 spindle poles. Mitotic cells were those cells having chromosomal condensation and spindle formation as determined microscopically.
Flow cytommetry
Cells were rinsed with PBS and fixed with ice cold 70% ethanol. Cells were subsequently stained with a propidium iodide staining buffer (0.1 mg/ml RNase A, 0.05% Triton-X-100 and 50 μg/ml in PBS) for 1 hour. Samples were analyzed using a BD FACS Canto II flow cytometer and BD FACSDiva or Flo Jo software.
Microscopy
Cells were viewed under an Olympus IX81 inverted microscope equipped with the Olympus DSU (Disk Scanning Unit) spinning disk confocal. Images taken using the Olympus IX81 inverted microscope, were analyzed using ImageJ and Metamorph Premier software. Nuclear size was analyzed using wide-field imaging while nuclear number was determined by analyzing z-stacks of fluorescent images.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We gratefully acknowledge the award from the Murdock College Research Program for Life Sciences that made this research possible. Dr. Valerie A. White, Department of Pathology and Laboratory Medicine, University of British Columbia is gratefully acknowledged for her provision of the retinoblastoma tissue for these studies and Dr. Nicholas J. Dyson, Department of Medicine, Massachusetts General Hospital Cancer Centre and Harvard Medical School for his provision of the T98G cell lines along with his technical advice. We also thank Dr. Gail M. Seigel, Department of Ophthalmology, University at Buffalo, for her provision of the retinoblastoma cell lines used in this study.
Funding
A Murdock General Science Grant as a match to the CFI Leaders Opportunity Fund Grant enabled the purchase of the Olympus DSU spinning disk confocal used in this study. The Molecular and Cell Biology laboratory and Cell Culture laboratory in which these studies took place were made possible by a grant from Murdock Trust as a match to the Industry Canada Knowledge Infrastructure Program Award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Akhtar N, Streuli CH. An integrin-ILK-microtubule network orients cell polarity and lumen formation in glandular epithelium. Nat Cell Biol 2012; 15(1):17-27.
- Fielding AB, Lim S, Montgomery K, Dobreva I, Dedhar S. A critical role of integrin-linked kinase, ch-TOG and TACC3 in centrosome clustering in cancer cells. Oncogene 2011; 30:521-34; PMID:20838383; http://dx.doi.org/10.1038/onc.2010.431
- Fielding AB, Dobreva I, McDonald PC, Foster LJ, Dedhar S. Integrin-linked kinase localizes to the centrosome and regulates mitotic spindle organization. J Cell Biol 2008; 180:681-9; PMID:18283114; http://dx.doi.org/10.1083/jcb.200710074
- Fielding AB, Dobreva I, Dedhar S. Beyond focal adhesions: integrin-linked kinase associates with tubulin and regulates mitotic spindle organization. Cell Cycle 2008; 7:1899-906; PMID:18604167; http://dx.doi.org/10.4161/cc.7.13.6204
- Lim S, Kawamura E, Fielding AB, Maydan M, Dedhar S. Integrin-linked kinase regulates interphase and mitotic microtubule dynamics. PloS one 2013; 8:e53702; PMID:23349730; http://dx.doi.org/10.1371/journal.pone.0053702
- Wickstrom SA, Lange A, Hess MW, Polleux J, Spatz JP, Kruger M, Pfaller K, Lambacher A, Bloch W, Mann M, et al. Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae. Dev Cell 2010; 19:574-88; PMID:20951348; http://dx.doi.org/10.1016/j.devcel.2010.09.007
- Fielding AB, Dedhar S. The mitotic functions of integrin-linked kinase. Cancer Metastasis Rev 2009; 28:99-111; PMID:19153670; http://dx.doi.org/10.1007/s10555-008-9177-0
- Sikkema WK, Strikwerda A, Sharma M, Assi K, Salh B, Cox ME, Mills J. Regulation of mitotic cytoskeleton dynamics and cytokinesis by integrin-linked kinase in retinoblastoma cells. PLoS One 2014; 9:e98838; PMID:24911651; http://dx.doi.org/10.1371/journal.pone.0098838
- Chen Z, Yang A, Xu C, Xing Y, Gong W, Li J. c-Jun N-terminal kinase is involved in the regulation of proliferation and apoptosis by integrin-linked kinase in human retinoblastoma cells. Graefes Arch Clin Exp Ophthalmol 2011; 249:1399-407; http://dx.doi.org/10.1007/s00417-010-1607-3
- Gagne D, Groulx JF, Benoit YD, Basora N, Herring E, Vachon PH, Beaulieu JF. Integrin-linked kinase regulates migration and proliferation of human intestinal cells under a fibronectin-dependent mechanism. J Cell Physiol 2010; 222:387-400; PMID:19885839; http://dx.doi.org/10.1002/jcp.21963
- Radeva G, Petrocelli T, Behrend E, Leung-Hagesteijn C, Filmus J, Slingerland J, Dedhar S. Overexpression of the integrin-linked kinase promotes anchorage-independent cell cycle progression. J Biol Chem 1997; 272:13937-44; PMID:9153256; http://dx.doi.org/10.1074/jbc.272.21.13937
- Bejjani A, Choi MR, Cassidy L, Collins DW, O'Brien JM, Murray T, Ksander BR, Seigel GM. RB116: an RB1+ retinoblastoma cell line expressing primitive markers. Mol Vis 2012; 18:2805-13; PMID:23233783
- Rushlow DE, Mol BM, Kennett JY, Yee S, Pajovic S, Thériault BL, Prigoda-Lee NL, Spencer C, Dimaras H, Corson TW, et al. Characterisation of retinoblastomas without RB1 mutations: genomic, gene expression, and clinical studies. Lancet Oncol 2013; 14:327-34; PMID:23498719; http://dx.doi.org/10.1016/S1470-2045(13)70045-7
- Reid TW, Albert DM, Rabson AS, Russell P, Craft J, Chu EW, Tralka TS, Wilcox JL. Characteristics of an established cell line of retinoblastoma. J Natl Cancer Inst 1974; 53:347-60; PMID:4135597
- Sery TW, Lee EY, Lee WH, Bookstein R, Wong V, Shields JA, Augsburger JJ, Donoso LA. Characteristics of two new retinoblastoma cell lines: WERI-Rb24 and WERI-Rb27. J Pediatr Ophthalmol Strabismus 1990; 27:212-7; PMID:2391623
- Vitale I, Galluzzi L, Castedo M, Kroemer G. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol 2011; 12:385-92; http://dx.doi.org/10.1038/nrm3115
- Galimberti F, Thompson SL, Ravi S, Compton DA, Dmitrovsky E. Anaphase catastrophe is a target for cancer therapy. Clin Cancer Res 2011; 17:1218-22; PMID:21288923; http://dx.doi.org/10.1158/1078-0432.CCR-10-1178
- Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging (Albany NY) 2012; 4:159-65; PMID:22394614
- Blagosklonny MV. Geroconversion: irreversible step to cellular senescence. Cell Cycle 2014; 13:3628-35; PMID:25483060; http://dx.doi.org/10.4161/15384101.2014.985507
- Manning AL, Dyson NJ. RB: mitotic implications of a tumour suppressor. Nat Rev Cancer 2012; 12:220-6; PMID:22318235
- Margolis RL, Lohez OD, Andreassen PR. G1 tetraploidy checkpoint and the suppression of tumorigenesis. J Cell Biochem 2003; 88:673-83; PMID:12577301; http://dx.doi.org/10.1002/jcb.10411
- Vitale I, Galluzzi L, Senovilla L, Criollo A, Jemaà M, Castedo M, Kroemer G. Illicit survival of cancer cells during polyploidization and depolyploidization. Cell Death Differ 2011; 18:1403-13; PMID:21072053; http://dx.doi.org/10.1038/cdd.2010.145
- Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev 2010; 24:2463-79; PMID:21078816; http://dx.doi.org/10.1101/gad.1971610
- Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res 2003; 63:2705-15; PMID:12782571
- Ewald JA, Desotelle JA, Wilding G, Jarrard DF. Therapy-induced senescence in cancer. J Natl Cancer Inst 2010; 102:1536-46; PMID:20858887; http://dx.doi.org/10.1093/jnci/djq364
- Saretzki G. Cellular senescence in the development and treatment of cancer. Curr Pharm Des 2010; 16:79-100; PMID:20214620; http://dx.doi.org/10.2174/138161210789941874
- Chen Z, Yang A, Xu C, Xing Y, Gong W, Li J. c-Jun N-terminal kinase is involved in the regulation of proliferation and apoptosis by integrin-linked kinase in human retinoblastoma cells. Graefes Arch Clin Exp Ophthalmol 2011; 249(9):1399-407.
- Wippold FJ, Perry A. Neuropathology for the neuroradiologist: rosettes and pseudorosettes. AJNR Am J Neuroradiol 2006; 27:488-92; PMID:16551982
- Grossniklaus HE. Retinoblastoma. Fifty Years of Progress. The LXXI Edward Jackson Memorial Lecture. Am J Ophthalmol 2014; 158(5):875-91.
- Ferretti C, Totta P, Fiore M, Mattiuzzo M, Schillaci T, Ricordy R, Di Leonardo A, Degrassi F. Expression of the kinetochore protein Hec1 during the cell cycle in normal and cancer cells and its regulation by the pRb pathway. Cell Cycle 2010; 9:4174-82; PMID:20948316; http://dx.doi.org/10.4161/cc.9.20.13457
- O'Toole E, Greenan G, Lange KI, Srayko M, Müller-Reichert T. The role of γ-tubulin in centrosomal microtubule organization. PLoS One 2012; 7:e29795; PMID:22253783; http://dx.doi.org/10.1371/journal.pone.0029795
- Lüders J, Stearns T. Microtubule-organizing centres: a re-evaluation. Nat Rev Mol Cell Biol 2007; 8:161-7; PMID:17245416; http://dx.doi.org/10.1038/nrm2100
- Wickstrom SA, Lange A, Montanez E, Fassler R. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J 2010; 29:281-91; PMID:20033063; http://dx.doi.org/10.1038/emboj.2009.376
- Ogden A, Rida PC, Aneja R. Let's huddle to prevent a muddle: centrosome declustering as an attractive anticancer strategy. Cell Death Differ 2012; 19:1255-67; PMID:22653338; http://dx.doi.org/10.1038/cdd.2012.61
- Kwon M, Godinho SA, Chandhok NS, Ganem NJ, Azioune A, Thery M, Pellman D. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev 2008; 22:2189-203; http://dx.doi.org/10.1101/gad.1700908
- Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature 2009; 460:278-82; PMID:19506557; http://dx.doi.org/10.1038/nature08136
- Nicolay BN, Gameiro PA, Tschöp K, Korenjak M, Heilmann AM, Asara JM, Stephanopoulos G, Iliopoulos O, Dyson NJ. Loss of RBF1 changes glutamine catabolism. Genes Dev 2013; 27:182-96; PMID:23322302; http://dx.doi.org/10.1101/gad.206227.112
- Howard MA, Dryja TP, Walton DS, Albert DM. Identification and significance of multinucleate tumor cells in retinoblastoma. Arch Ophthalmol 1989; 107:1025-30; PMID:2751457; http://dx.doi.org/10.1001/archopht.1989.01070020087037
- Chintagumpala M, Chevez-Barrios P, Paysse EA, Plon SE, Hurwitz R. Retinoblastoma: review of current management. Oncologist 2007; 12:1237-46; PMID:17962617; http://dx.doi.org/10.1634/theoncologist.12-10-1237
- Antoneli CB, Ribeiro KC, Steinhorst F, Novaes PE, Chojniak MM, Malogolowkin M. Treatment of retinoblastoma patients with chemoreduction plus local therapy: experience of the AC Camargo Hospital, Brazil. J Pediatr Hematol Oncol 2006; 28:342-5; PMID:16794500; http://dx.doi.org/10.1097/00043426-200606000-00004
- Vergel M, Marin JJ, Estevez P, Carnero A. Cellular senescence as a target in cancer control. J Aging Res 2010; 2011:725365; PMID:21234095
- Mills J, Niewmierzycka A, Oloumi A, Rico B, St-Arnaud R, Mackenzie IR, Mawji NM, Wilson J, Reichardt LF, Dedhar S. Critical role of integrin-linked kinase in granule cell precursor proliferation and cerebellar development. J Neurosci 2006; 26:830-40; PMID:16421303; http://dx.doi.org/10.1523/JNEUROSCI.1852-05.2006
- Miller TM, Moulder KL, Knudson CM, Creedon DJ, Deshmukh M, Korsmeyer SJ, Johnson EM Jr. Bax deletion further orders the cell death pathway in cerebellar granule cells and suggests a caspase-independent pathway to cell death. J Cell Biol 1997; 139:205-17; PMID:9314540; http://dx.doi.org/10.1083/jcb.139.1.205
- Sikkema. In: Strikwerda, ed., 2014.