In naive T lymphocytes, the outcome of TCR stimulation is regulated by the simultaneous binding of CD28 to its natural ligand, expressed on APCs. Zynda et al. found that a mild, sustained increase in temperature not only enhances IL-2 production by CD4+ T cells receiving the canonical two 2 activation signals, but it also enhances IL-2 production under conditions of insufficient co-stimulation of CD28. Since co-stimulation via CD28 is considered necessary for full activation and IL-2 production at 37°C, these data suggest that mild hyperthermia may be able to mimic at least some of the responses elicited by CD28-mediated co-stimulation.
Numerous studies show that optimal T cell activation involves reorganization of the plasma membrane, both in the order of membrane lipids and membrane cortex associated proteins, such as actin, spectrin, and vimentin.Citation1,2 The authors provide evidence that reversible changes in membrane fluidity occur immediately after exposing cells to mild hyperthermia and were able to duplicate these effects using a known membrane fluidizer, 0.05% ethanol. They also demonstrated that co-stimulation through CD28 affects membrane fluidization in an identical manner. Collectively, these data suggest the intriguing possibility that heat can replace or mimic the co-stimulation via CD28 through its ability to alter membrane fluidity (see ). The observed effects of fever-range hyperthermia on T-cell stimulation persist over hours indicating that the changes in lipid fluidity likely modulate the molecular order in the membrane.
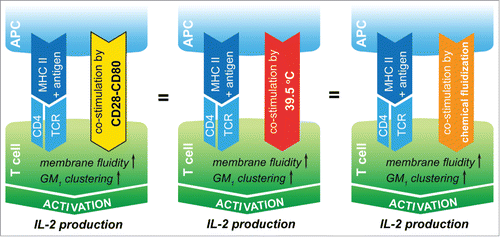
Figure 1. Comparing co-stimulation, temperature elevation and isothermal membrane fluidization effects on T cell activation threshold. CD4+ T cells need co-stimulation via CD28 receptors for sufficient survival, activation and IL-2 production. Fever-range heat and isothermal membrane fluidization by 0.05% EtOH induce similar membrane alterations as CD28 ligation, including GM1 clustering and fluidization in the membrane of T lymphocytes. Both mild heat and EtOH exposures are sufficient to result in enhanced T cell activation via their membrane perturbing effect.
Under resting conditions, distinct sphingomyelin and cholesterol containing microdomains with selective confinement of signaling molecules occur in the plasma membrane of T cells, but generally, these individual membrane domains are only a few nanometers in diameter and difficult to distinguish by microscopy.Citation3 However, when T cells are activated using TCR/CD28-receptors at 37°C, the nanoscale membrane domains appear to cluster into larger aggregates that can be visualized. The authors observed that CD4+ T lymphocytes exposed to mild heating alone exhibit significant, yet reversible, clustering of plasma membrane GM1 enriched membrane domains. Similarly, it has previously been shown that CD28 mediated co-stimulation enhances the clustering of macromolecular signaling platforms in the plasma membrane, and that CD28s function is actually dependent on this membrane reorganization. In this way, perhaps mild hyperthermia and CD28 can each lower the T cell activation threshold.
Whether mild heating acts as a co-stimulator itself, or through the enhancement of a signaling pathway downstream of CD28 signaling is an important question. The data of Zynda et al. show that the greatest amount of IL-2 is produced when both heat and CD28 co-stimulation are used, suggesting that there may be some non-overlapping or additive effects of heat and CD28 ligation in addition to observed similarities. The authors, using CD4+ T cells, demonstrated that mild heating and ethanol (both of which increased membrane fluidity) caused an identical rearrangement of ankyrin and spectrin. In addition to the spectrin and ankyrin cytoskeletal systems, actin polymerization has also been shown to play a prominent role in cholesterol dependent signal domain aggregation and synapse formation during T cell activation.Citation4,5 The authors also found that actin polymerization was necessary for enhancement of IL-2 production by mild heating and ethanol. These data provide additional support for the hypothesis that thermal signals influence cytoskeletal reorganization in order to lower the threshold for T-cell activation.
These findings fit perfectly with the “Membrane Sensor Hypothesis” which postulates that subtle changes in the fluidity and/or organization of cell membranes can be the most upstream event of temperature-sensing and signaling during mild heat shock.Citation6 Hyperfluidization by mild heat is coupled with specific reorganization of membrane domains and results in heat shock protein expression, similar to isothermal hyperfluidization gained by the administration of alcoholsCitation6 or with lipid interacting drugs.Citation7
The central role that membrane fluidity and reorganization of the plasma membrane microdomains play in T cell activation strongly supports a fundamental role for heat sensitive changes at the plasma membrane to mediate many different functional effects of temperature. Finally, the possibility that a sustained increase in temperature could reduce the activation threshold of T lymphocytes may help to explain multiple studies that demonstrate a significant survival benefit associated with fever following infection in multiple vertebrates.
References
- Janes PW, et al. Semin Immunol 2000 12:23-34; PMID:10723795; http://dx.doi.org/10.1006/smim.2000.0204.
- He HT, et al. Semin Immunol 2005 17:23-33; PMID:15582486; http://dx.doi.org/10.1016/j.smim.2004.09.001.
- Schade AE, Levine AD. J Immunol 2002 168:2233-9; PMID:11859110; http://dx.doi.org/10.4049/jimmunol.168.5.2233.
- Pradhan D, Morrow JS. Immunity 2002 17:303-15; PMID:12354383; http://dx.doi.org/10.1016/S1074-7613(02)00396-5.
- Kumari S, et al. Biochim Biophys Acta 2014 1838: 546-56; PMID:23680625; http://dx.doi.org/10.1016/j.bbamem.2013.05.004.
- Török Z, et al. Biochim Biophys Acta 2014 1838:1594-618; PMID:24374314; http://dx.doi.org/10.1016/j.bbamem.2013.12.015.
- Vigh L, et al. Trends Biochem Sci 2007 32:357-63; PMID:17629486; http://dx.doi.org/10.1016/j.tibs.2007.06.009.
