Abstract
We previously showed that incubation of chronic myeloid leukemia (CML) cells in very low oxygen selects a cell subset where the oncogenetic BCR/Abl protein is suppressed and which is thereby refractory to tyrosine kinase inhibitors used for CML therapy. In this study, salarin C, an anticancer macrolide extracted from the Fascaplysinopsis sponge, was tested as for its activity on CML cells, especially after their incubation in atmosphere at 0.1% oxygen. Salarin C induced mitotic cycle arrest, apoptosis and DNA damage. Salarin C also concentration-dependently inhibited the maintenance of stem cell potential in cultures in low oxygen of either CML cell lines or primary cells. Surprisingly, the drug also concentration-dependently enforced the maintenance of BCR/Abl signaling in low oxygen, an effect which was paralleled by the rescue of sensitivity of stem cell potential to IM. These results suggest a potential use of salarin C for the suppression of CML cells refractory to tyrosine kinase inhibitors
Introduction
Chronic Myeloid Leukemia (CML) is a myeloproliferative neoplasia that, especially at the onset of its chronic phase, is extremely well taken care of by treatment with Imatinib-mesylate (IM; STI571; Gleevec®) or other tyrosine kinase inhibitors (TKi), which are indeed very effective in inducing remission of disease.Citation1 However, in most cases, TKi do not prevent CML relapse after treatment withdrawal, due to the persistence of TKi-resistant minimal residual disease (MRD).Citation2-4 Leukemia stem cells (LSC) are the best candidate to sustain MRD.Citation5,6 Indeed, it has been recently reported that LSC of CML are resistant to TKi.Citation7-9 Thus, the search for drugs capable of suppressing CML cell subset/s responsible for treatment-resistant MRD is intensive.
We previously showed that incubation of CML cells in atmosphere at very low oxygen suppresses the oncogenetic BCR/Abl protein and selects a minor cell subset which is BCR/Abl-independent for persistence in culture. Thus, selected cells are refractory to the action of IM due to the lack of its molecular target. These BCR/Abl-negative cells, however, remain genetically leukemic (BCR/abl-positive), so that they are capable, once transferred into a growth-permissive environment, to reproduce a BCR/Abl-expressing population, where sensitivity to IM is rescued.Citation10-12 On this basis, we defined a novel mechanism of CML cell insensitivity to therapy: the refractoriness (primary resistance) to IM due to the lack of its molecular target.Citation13-15 This property parallels the capacity of LSC to home within the physiologically “hypoxic” stem cell niches of bone marrow.Citation16-20 Noteworthy, refractoriness does not involve BCR/abl mutations affecting the IM-binding site of BCR/Abl and conferring secondary resistance upon a CML cell subset. Thus, such a mechanism of drug insensitivity cannot be overcome by the 2nd and most probably even the next generations of TKi developed for CML therapy.Citation21 Clinical data confirmed that, in the majority of cases, relapse of disease upon IM discontinuation consists of a cell population expressing wild-type BCR/abl, indicating that MRD was sustained by refractoriness rather than acquired, mutation-driven secondary resistance. Citation22,23 On the basis of all above, cell selection in low oxygen emerged as a handy experimental model to test the effects of drugs, separately from those on cell bulk, on a CML cell subset which retains stem cell potential and is refractory to TKi.
Salarin C, an anticancer macrolide extracted from the Madagascar Fascaplysinopsis sponge,Citation24-26 inhibits growth and induces apoptosis of CML cells of the K562 stabilized line.Citation27 The study reported here was undertaken to deepen the effects of Salarin C on CML cells and in particular to establish whether the drug is active on CML cells selected in low oxygen and refractory to TKi. We determined the effects of salarin C: (a) on CML cell lines cultured in low oxygen; (b) on the maintenance of stem cell potential in cultures of cell lines as well as primary CML cells incubated in low oxygen; (c) on stem cell potential, when combined to IM. The results obtained indicated that salarin C: (a) induced mitotic cycle arrest in G2/M, apoptosis and genotoxic damage in cultures incubated in either air or low oxygen; (b) inhibited the maintenance of stem cell potential within either cell lines or primary CML cell populations incubated in low oxygen; (c) enforced the maintenance of BCR/Abl-dependent signaling in low oxygen, thereby (d) rescuing in part the sensitivity of stem cell potential to IM.
Results
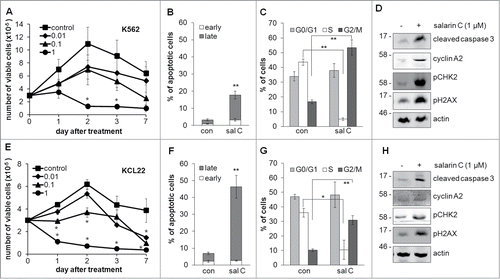
shows the overall effects of salarin C on CML cells of the K562 and KCL22 stabilized lines incubated in normoxia and treated or not from time 0 with a single drug dose. Salarin C concentration-dependently affected the kinetics of viable cell number in culture in both cell lines (). The drug concentration (1 µM) capable to reduce the number of viable cells with respect to time 0 in either cell line was then tested, at day 3 of incubation, for its capacity to induce apoptosis or to affect cell distribution across the mitotic cycle. In both cell lines, salarin C treatment markedly increased the percentage of cells in apoptosis, as determined by the annexin-V / PI assay (, Fig. S1A and C), and in the G2/M cycle phases, while decreasing that in S phase (, Fig. S1B and D). In keeping with the induction of apoptosis and G2/M accumulation, salarin C increased cleaved caspase 3 and cyclin A2, respectively, in both cell lines (). also shows that salarin C induced DNA damage, as indicated by the marked increase of CHK2 and H2AX phosphorylation with respect to untreated controls.Citation28 A link between the effects of salarin C on apoptosis and those on cell cycle distribution was established by pre-treating K562 cell cultures with lovastatin or nocodozole, inducers of G0/G1 or G2/M arrest, respectively (Fig. S2).Citation29,30 Pretreatment with lovastatin protected K562 cells from salarin C-elicited apoptosis, while nocodozole rendered the cells more sensitive to the drug. This indicates that the pro-apoptotic effects of salarin C are cell cycle phase-specific, suggesting that G2/M accumulation preludes to the induction of apoptosis by salarin C.
Figure 1. Salarin C inhibits cell proliferation and induces apoptosis and DNA damage in CML cell lines. K562 (A) or KCL22 (E) cells were plated at 3×105 cells/ml and after 24 hours (time 0) were treated or not (control) with a single dose of salarin C at the indicated final concentration (µM); cells were then incubated in normoxia and trypan blue-negative cells were counted at the indicated timepoints; values are averages ± SEM of data from 3 independent experiments; significant differences are indicated (Student's t test for independent samples; *: p< 0.05. K562 (B, C) or KCL22 (F, G) cells were incubated as above in the presence (sal C) or not (con) of 1 µM salarin C and subjected to Annexin V / propidium iodide assay to determine the percentages of cells in early or late apoptosis (B, F) or labeled with propidium iodide alone (C, G) to determine cell cycle phase distribution. Analysis was performed by flow cytometry at day 3 of incubation; values are averages ± SEM of data from 3 independent experiments; significant differences are indicated (Student's t test for independent samples; *: p< 0.05, **: p< 0.01). K562 (D) or KCL22 (H) cells were lysed at day 3 of incubation and lysates subjected to immuno-blotting with antibodies raised against the indicated proteins; anti-actin antibody was used to verify equalization of protein loading; one representative experiment out of 3 is shown.
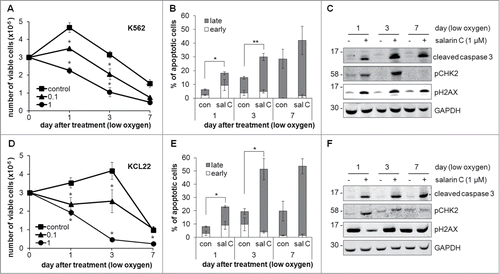
We previously showed that BCR/Abl is suppressed in CML cells incubated in low oxygen, which are thereby refractory to IM,Citation10,11 making the search for drugs that target BCR/Abl-negative cells selected in low oxygen of high therapeutic interest (see Introduction). Thus, we tested the effects of salarin C on K562 or KCL22 cells incubated at 0.1% oxygen (). Under these conditions, control K562 or KCL22 cells, as expected according to our previous results,Citation11 underwent an initial, limited numerical increase, followed by a fall to cell numbers well below the time 0 level. SalarinC concentration-dependently suppressed this increase or reduced the number of viable cells with respect to time 0 (), thus yielding results similar to those obtained in normoxia (see ). Accordingly, salarin C induced a 2-fold increase of the percentage of apoptotic cells (, Fig. S3 and markedly enhanced caspase 3 cleavage (). Salarin C also increased CHK2 and H2AX phosphorylation (). The effects of salarin C on cell cycle distribution and cyclin A2 expression in low oxygen were negligible (data not shown). When the results reported in are compared with the corresponding data of , day 3, it emerged that, for either cell line, the pro-apoptotic effects of salarin C in low oxygen were similar to those in normoxia, although the apoptosis levels in untreated control cultures were higher at 0.1% oxygen than in normoxia.
Figure 2. Salarin C induces apoptosis and DNA damage in CML cell lines incubated in low oxygen. K562 (A) or KCL22 (D) cells were plated at 3×105 cells/ml and after 24 hours (time 0) treated or not (control) with a single dose of salarin C at the indicated concentration (µM); cells were then incubated at 0.1% oxygen for the indicated times and trypan blue-negative cells were counted. Values in graphs are averages ± SEM of data from 3 independent experiments; significant differences are indicated (Student's t test for independent samples; *: p< 0.05). K562 (B) or KCL22 (E) cells were incubated as above in the presence (sal C) or not (con) of 1 µM salarin C and subjected to Annexin V/propidium iodide assay at the indicated timepoints to determine the percentages of cells in early or late apoptosis; values are averages ± SEM of data from 3 independent experiments; significant differences are indicated (Student's t test for independent samples; *: p< 0.05, **: p< 0.01). K562 (C) or KCL22 (F) cells treated as above were lysed in Laemmli buffer at the indicated times and lysates subjected to immuno-blotting with antibodies raised against the indicated proteins; anti-GAPDH antibody was used to verify equalization of protein loading; one representative experiment out of 3 is shown.
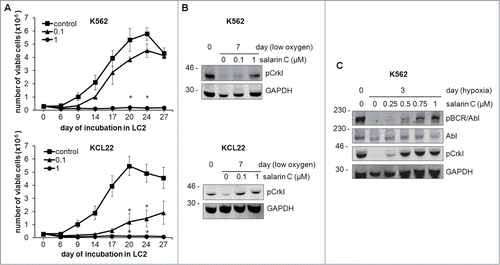
We had also previously found that in low oxygen, under conditions where BCR/Abl is suppressed, the stem cell potential of CML cell populations is maintained independently of BCR/Abl protein expression.Citation10,11 This maintenance was confirmed in control cultures of the experiments of , which shows the repopulation ability (LC2) of K562 and KCL22 cells rescued from cultures incubated for 7 days at 0.1% oxygen (LC1), an indicator of the maintenance of stem cell potential in LC1 (CRA assay). Salarin C concentration-dependently reduced the maintenance of stem cell potential, KCL22 cells being more sensitive to the drug than K562 cells; at 1 µM, however, salarin C completely suppressed stem cell potential in either cell line. These results, taken together with those of , led to conclude that salarin C is active on both CML cell bulk and stem cell potential. The effects of salarin C on BCR/Abl signaling at day 7 of incubation in low-oxygen LC1, when the cells were transferred to normoxic LC2 in the experiments of , are addressed in the experiments of , which shows the level of phosphorylation of the BCR/Abl substrate Crkl, the standard criterion for the monitoring of BCR/Abl kinase activity.Citation31,32 Incubation in low oxygen, as expected, suppressed Crkl phosphorylation in either K562 or KCL22 cells, while salarin C concentration-dependently antagonized this suppression. shows in better detail this effect of salarin C on Crkl and BCR/Abl phosphorylation as well as BCR/Abl expression, in K562 cells incubated in low oxygen for 3 days, i.e. upon BCR/Abl suppression in control cells. Taken together, the results of indicated that the detrimental action of salarin C on the maintenance of CML stem cell potential in low oxygen may be linked to the enforced persistence of BCR/Abl signaling, which is usually suppressed in low oxygen.
Figure 3. Salarin C inhibits Culture Repopulation Ability of CML cell lines and rescues BCR/Abl signaling in CML cell lines incubated in low oxygen. (A) K562 or KCL22 cells were treated or not (control) at time 0 with a single dose of salarin C at the indicated concentration (μM) and incubated at 0.1% oxygen (LC1). On day 7 of LC1, cells were transferred to secondary cultures (LC2) established in the absence of salarin C and incubated in normoxia. Trypan blue-negative cells were counted at the indicated timepoints of LC2; the graphs represent average ± SEM of data from 3 independent experiments; significant differences are indicated (Student's t test for independent samples; *: p< 0.05). (B, C) Cells were treated or not at time 0 with the indicated concentrations of salarin C and incubated at 0.1% oxygen for the indicated times. Cells were lysed in Laemmli buffer and lysates subjected to immuno-blotting with antibodies for the indicated proteins. Due to the marked MW difference, anti-phospho-Abl antibody made it possible to detect phospho-BCR/Abl. Anti-GAPDH antibody was used to verify equalization of protein loading. One representative experiment out of 3 is shown.
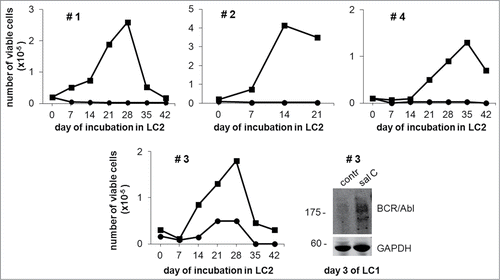
Given the potential relevance of the results of to design therapeutic protocols, we tested the effects of salarin C on the maintenance of stem cell potential in low oxygen of primary bone marrow cells explanted from CML patients ().Treatment with 1 µM salarin C suppressed the maintenance of stem cell potential of CML patient-derived cells, fully confirming the results obtained with CML cell lines in the experiments of . In the case of patient #3, the antagonistic effect of Salarin C on BCR/Abl suppression in low oxygen is also shown.
Figure 4. Salarin C inhibits Culture-Repopulation Ability of primary CML cells incubated in low oxygen. Bone marrow cells explanted from 4 CML patients (#) were treated (circle) or not (square) at time 0 with a single dose of 1 µM salarin C and incubated at 0.1% oxygen (LC1). On day 7 of LC1, cells were transferred to secondary cultures (LC2) established in the absence of salarin C and incubated therein in normoxia. Trypan blue-negative cells were counted at the indicated timepoints of LC2. The graphs represent data from one experiment. In the case of patient # 3, cells treated or not (contr) at time 0 with 1 µM salarin C (sal C) and incubated at 0.1% oxygen for 3 days were lysed in Laemmli buffer and lysates subjected to immuno-blotting with anti-Abl antibody, which, due to the marked MW difference, made it possible to detect BCR/Abl. Anti-GAPDH antibody was used to verify equalization of protein loading
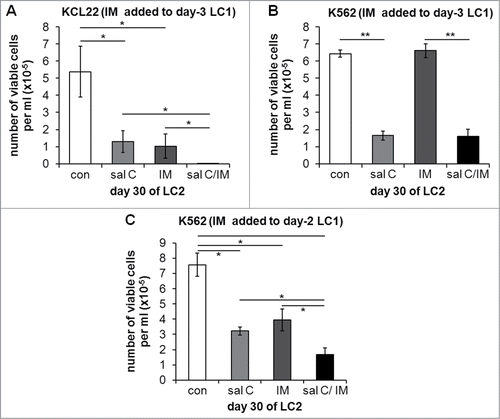
The persistence of BCR/Abl signaling in low oxygen under salarin C treatment () prompted us to test the effects of the combination of salarin C with IM on the maintenance of stem cell potential (). In these experiments, in order to better appreciate the effects of the combination, we added salarin C (at time 0) at relatively low concentrations (i.e., 0.5 and 0.1 μM for K562 or KCL22 cells, respectively), yet effective in rescuing some BCR/Abl signaling (see ). IM was then added to low-oxygen LC1 at day 2 or 3 (see below) and cells were transferred at day 7 from LC1 to LC2, to be incubated therein in normoxia for 30 days (when the peak of expansion in LC2 was reached). Low-concentration salarin C significantly reduced stem cell potential of either K562 or KCL22 cell cultures (), in keeping with the results of . The treatment with IM at day 3 was effective on KCL22 cells (), but ineffective on K562 cells (), as expected due to the complete suppression of BCR/Abl at day 3 in these cells when incubated in low oxygen (). However, IM was effective on K562 cells too when added to cultures at day 2 (). The fact that, in order to obtain data comparable with those of KCL22 cells (), K562 cells needed to be treated with IM one day earlier than KCL22 cells depends on the faster suppression of BCR/Abl signaling in low oxygen in K562 cells. This suppression well explains the complete ineffectiveness of IM shown in . The slower response to low oxygen and the higher sensitivity to IM of KCL22 when compared to K562 cells () are well-known (previous unpublished results). When IM was effective alone (), its combination with salarin C significantly enhanced the suppression of stem cell potential in low oxygen.
Figure 5. Effects of salarin C, Imatinib-mesylate or their combination on Culture Repopulation Ability of CML cell lines incubated in low oxygen. KCL22 (A) or K562 (B, C) cells were incubated at 0.1% oxygen (LC1) and treated or not (con) at time 0 with a single dose of 0.1 µM (A) or 0.5 µM (B, C) salarin C (sal C), and/or of 1 µM Imatinib-mesylate (IM) on day 3 (A, B) or day 2 (C). On day 7 of LC1, cells were transferred to drug-free secondary cultures (LC2) and incubated therein in normoxia until peak of numerical expansion was reached in control untreated cultures (day 30). Trypan blue-negative cells were then counted. Values are averages ± SEM of data from 3 independent experiments; significant differences are indicated (Student's t test for independent samples; *: p< 0.05; **: p<0.005)
Discussion
This paper deepened the characterization of the effects of salarin C on CML cells, addressing in particular those on cells where BCR/Abl-dependent signaling is suppressed following incubation in low oxygen. The results obtained indicated that salarin C exerts on CML cell bulk an overall pro-apoptotic effect, which may be linked to the induction of DNA damage, as the marked increase of CHK2 and H2AX phosphorylation in drug-treated cultures seems to indicate. While H2AX is a histone which is phosphorylated following DNA damage, CHK2 phosphorylation and activation is responsible for the phosphorylation and stabilization of p53.Citation33 On the other hand, the proapoptotic effect of salarin C seems linked to cell distribution through the mitotic cycle, as suggested by the effects of K562 cell pre-treatment with lovastatin or nocodozole. Indeed, G0/G1 arrest determined by lovastatin protected from salarin C-induced apoptosis, while G2/M accumulation determined by nocodozole sensitized to the effects of the drug. This suggests that an early block of cell cycling antagonizes, while cycle progression favors, the induction of apoptosis by salarin C. The relationship of cell cycle phase to CHK2 and H2AX phosphorylation with respect to the induction of apoptosis remains to be investigated. All the effects of salarin C on CML cell bulk were confirmed when the drug was added at time 0 to cultures incubated at 0.1% oxygen.
The effects of salarin C were then tested using a stem cell assay. At 0.1% oxygen, BCR/Abl-dependent signaling is progressively suppressed in function of incubation time, so that the stem cell potential of the culture becomes refractory to the effects of IM and most probably of TKi in general.Citation13-15 Salarin C inhibited the maintenance of stem cell potential in either KCL22 or K562 cell culture incubated in low oxygen. Thus, salarin C was active not only on CML cell bulk undergoing BCR/Abl-dependent growth, but also on the BCR/Abl-independent stem cell potential. Of relevance under the translational point of view is that identical results were obtained using primary cells explanted from CML patients. These data indicated that salarin C may be suitable to overcome the refractoriness to TKi of LSC which most likely sustain MRD.
We previously hypothesized that BCR/Abl signaling provides a selective advantage under growth-permissive environmental conditions (relatively high tissue oxygen tension), but is detrimental to the maintenance of LSC responsible for MRD within the stem cell niches (low oxygen).Citation15 On this basis, the observation that salarin C was active on stem cell potential commanded to deepen the effects of the drug on BCR/Abl-dependent signaling in low oxygen. Salarin C concentration-dependently induced the maintenance in low oxygen of Crkl phosphorylation through day 7 and of BCR/Abl phosphorylation at least until day 3 on the basis of what observed in K562 cells. Thus, salarin C apparently antagonized the selection of BCR/Abl-independent CML cells. This led to speculate that, in the presence of salarin C, the reduced maintenance of stem cell potential in low oxygen was linked at least in part to the extended persistence of BCR/Abl signaling under conditions where it should be suppressed. The effects of lovastatin and nocodozole suggest that this persistence may push LSC to G0>G1 progression, which would not be affordable in low oxygen, resulting in the induction of apoptosis in coincidence with G2/M accumulation. The mechanism determining the persistence of BCR/Abl signaling in salarin C-treated cultures remains to be elucidated. On the basis of all above, salarin C is likely to exhibit a dual suppressive effect on stem cell potential, inducing DNA damage in LSC as much as in the bulk of cell population and preventing their adaptation to the low-oxygen stem cell niche via the maintenance of growth-promoting signaling.Citation5,8,21,23,34,35 The latter issue makes salarin C worth to be characterized not only just as a potential anti-leukemic agent, but also as a tool to deepen the antagonism between signals driving LSC clonal expansion and those enforcing LSC maintenance.
The salarin C-induced persistence of BCR/Abl signaling in low oxygen led to predict an enhanced sensitivity of stem cell potential to TKi. This possibility was addressed by adding IM at time points (i.e. when BCR/Abl protein was still expressed) selected in order to enable IM to inhibit alone the maintenance of stem cell potential. The response of the 2 cell lines tested was slightly different, BCR/Abl signaling and thereby sensitivity to IM being suppressed faster in K562 cells. However, under conditions where either cell line was sensitive to IM alone, low concentrations of salarin C enhanced the effects of IM, indicating that salarin C rescued part of the sensitivity of stem cell potential to IM. The fact that salarin C alone or IM alone exhibited quantitatively similar effects led to hypothesize that, within the time window of sensitivity of stem cell potential to IM alone, salarin C and IM targeted overlapping cell subsets.
The interplay between the effects of very low oxygen, salarin C and IM falls within the general issue of the development of cell resistance to growth-limiting conditions.Citation36 Once this resistance is acquired, the persistence of growth-promoting stimuli in a non-growth-permissive environment, far from being useful, is most likely detrimental to stem cell survival.Citation14,15 On the other hand, the suppression of such stimuli is believed to represent a main issue in the selection of cancer stem cells. This concept is suitable to be applied not only to the irreversible genetic selection of mutant cancer subclones, but also to the reversible phenotypic shift we found enforced upon CML cells in low oxygen. The latter case is surely compatible, as we discussed extensively elsewhere,Citation13,15 with tumor progression.Citation37 Thus, LSC of CML reversibly selected in low oxygen would exhibit, on one hand, refractoriness to therapy (IM and TKi) like genetically-selected cancer stem cells, on the other hand, sensitivity to any treatment, such as salarin C, capable to rescue growth-promoting stimuli in a non-growth-permissive environment.
The results presented in this paper are of interest in view of the design of experimental therapeutic strategies based on the salarin C/IM combination. As TKi alone are very effective in CML debulking and inducing remission, little advantage is to be expected using their combination with salarin C from the action of salarin C on cell bulk we observed. On the other hand, a treatment with the salarin C / TKi combination at the onset of disease is liable to induce a “deeper” remission, where stem cell potential is targeted directly by salarin C and to some extent also TKi, as well as by TKi following salarin C action. Finally, a treatment with salarin C following successful response to TKi may “sensitize” MRD to further TKi cycles carried out within the post-remission follow-up; salarin C could indeed antagonize the adaptation of LSC to the low-oxygen environment of stem cell niches where BCR/Abl signaling is suppressed and MRD is most probably long-term maintained. In this respect, it is worth pointing out that salarin C treatment should be always combined to that with TKi, in order to counteract any possible stimulus to CML expansion due to the persistence of BCR/Abl signaling in salarin C-primed LSC.
Materials and Methods
Cells and culture conditions
K562 and KCL22 cells are stabilized CML cell lines derived from primary cells of blast crisis CML patients. Maintenance cultures were established in RMPI-1640 medium (Lonza, cat. no. BE12-167F) supplemented with 10% fetal bovine serum (FBS; Euro Clone, cat. no. ECSO18OL), 1% L-glutamine and 1% penicillin/streptomycin, and incubated at 37°C in water-saturated air additioned with 5% CO2 (standard atmosphere). Primary cells were collected from bone marrow aspirates of CML patients following informed consent and under the approval of the Ethics Committee of Azienda Ospedaliero-Universitaria Careggi (AOUC/University Hospital) at the Division of Hematology (Authorization number 520/10 issued on 18/10/2010 by the Ethical Committee of AOUC). To determine the number of viable cells in culture, cells were diluted 1:1 with a 0.04% w/v trypan blue solution in PBS (buffered saline solution containing NaCl 80 g/l, KCl 2.5 g/l, Na2HPO4 2H2O 18 g/l, KH2PO4 2.5 g/l) and the resulting cell suspension was counted in a Bürker's hemocytometer.
Cell incubation at low oxygen tension
Cells from maintenance cultures were plated at 5×105/ml (“intermediate” passage), incubated for 24 hours under standard atmosphere and then replated at 3×105/ml (liquid culture 1; LC1). LC1 were incubated at 37°C at very low oxygen tension (water-saturated atmosphere containing 0.1% O2, 5% CO2, 94.9% N2) in a gas-tight incubator/manipulator (Don Whitley Scientific DG250 anaerobic workstation). At different times of incubation in low oxygen, cells were recovered from LC1, washed, plated at 3×104/ml (liquid culture 2; LC2) and incubated under standard oxygen-containing atmosphere (“normoxia”).
Drug treatment
Salarin C was administered at time 0 to cultures incubated at 0.1% oxygen or in normoxia, at concentrations ranging from 0.01 to 1 μM, in a single dose.Citation27 Lovastatin was administered as a single 60 μM dose, at time 0, to cultures incubated in normoxia. Nocodozole was administered as a single 0.1 μM dose, at time 0, to cultures incubated in normoxia. IM was administered as a single 1 μM dose on day 2 or 3 of incubation at 0.1% oxygen.
Analysis of apoptosis and cell cycle
Apoptosis and cell cycle analysis was performed as previously described.Citation34 In brief, to quantify apoptosis, cells were centrifuged, resuspended in the buffer supplied within the Annexin V-fluos staining kit (Roche Diagnostics, Basel, Switzerland) and incubated with FITC-labeled Annexin V and propidium iodide (PI) for 15 min at room temperature (RT) in the dark. Flow cytometry was performed using a FACSCanto (Beckton & Dickinson, San Josè, CA, USA). The percentages of Annexin V+/PI− or Annexin V+/PI+ cells are considered to reflect “early” or “late” apoptosis, respectively. To determine cell-cycle phase distribution, cells were centrifuged and pellet resuspended in 1 ml of PI solution (50 mg/ml PtdIns, 0.1% trisodium citrate, 0.1% NP40). After 30 min of incubation at RT in the dark, nuclei were analyzed by flow cytometry and data processed using the FlowJo® software.
Evaluation of stem cell potential by the Culture Repopulation Ability (CRA) assay
The CRA assay, an in vitro procedure for the estimate of the stem potential contained within a cell population, yields results comparable to those obtainable via the corresponding Marrow Repopulation Ability assay in vivo.Citation38 The CRA assay is based indeed on the transfer of the cell population to be assayed into growth-permissive cultures rather than on its transplantation into syngeneic animals.Citation38-40 This prompted the adaptation of CRA assay to the study of human leukemia cell populations of various types.Citation10,41 In brief, cells were incubated for different times under selective conditions (incubation in low oxygen, drug treatment) in LC1 (where the experiment actually occurs) and then transferred to non-selective LC2 (where their stem cell potential is exploited) incubated in normoxia and in the absence of drugs. In LC2, LC1 cells undergo clonal expansion, being the peak value of LC2 repopulation and the time taken to reach the peak considered indicators of their stem cell potential (CRA).
Cell lysis and western blotting
Cell aliquots were harvested while keeping the cultures inside the anaerobic workstation, transferred into 15 ml-tubes previously kept in ice and centrifuged for 5 minutes at 1300 rpm and 4°C. Cell lysis and immunoblotting were performed as previously reported.Citation34 BCR/Abl expression, phosphorylation and activity was determined using rabbit polyclonal antibodies raised against c-Abl, p-Abl or p-CRLK (Cell Signaling Technology, cat. no. 2861, 2862 and 3181, respectively). Apoptosis was determined using a mouse anti-caspase 3 monoclonal antibody (Santa Cruz Biotechnology, cat. no. Sc-7272) and a rabbit polyclonal antibody specific for a product of caspase 3 activation (Cell Signaling Technology, cat. no. 9661) and the cleavage of PARP-1 using rabbit polyclonal antibodies against either PARP-1 or cleaved PARP (Cell Signaling Technology, cat. no. 9542 or 9541, respectively). H2AX or CHK2 phosphorylation was detected with a rabbit monoclonal antibody anti-p-H2AX or a rabbit polyclonal antibody anti-p-CHK2 (Cell Signaling, cat. no. 9718 or 2661, respectively). A goat anti-GAPDH antibody (Santa Cruz, cat. no. Sc-20 357) was used to check equalization of sample loading. The primary antigen-antibody complexes were detected with secondary fluorochrome-conjugated antibodies: goat anti-rabbit, goat anti-mouse or donkey anti-goat (IRDye®800CW or IRDye®680, LI-COR). When serial immunoblotting was performed on the same membrane, the antibodies used first were removed by incubating the membranes for 30 minutes at 50°C in stripping buffer (Tris/HCl, 0.25 mM at pH 6.7, 100 mM 2-mercaptoethanol, SDS 2%) and shaking the membranes every 10 minutes. The membranes were then washed in PBS-T (3 washes of 10 minutes each on a shaker/rocker), incubated again in blocking buffer and finally subjected to the second immunoblotting.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
1078029_supplemental_files.zip
Download Zip (4.4 MB)Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Funding
Research was supported by: (PDS) Istituto Toscano Tumori; Ministero della Salute (grant no. RF-TOS-2008–1163728); Regione Toscana – Programma per la Ricerca in Materia di Salute; Associazione Italiana per la Ricerca sul Cancro (grants no. IG5220 and IG13466); Associazione Italiana per la Lotta contro le Leucemie e i Linfomi (Sezione di Prato); Fondazione Oretta Bartolomei-Corsi; (DN) US-Israel Binational Science Foundation (BSF). DN and PDS were members of the Managing Committee of the HypoxiaNet network (action no. TD0901) of the Cooperation in Science & Technology (COST) agency of EU.
References
- Abruzzese E, Breccia M, Latagliata R. Second-generation tyrosine kinase inhibitors in first-line treatment of chronic myeloid leukaemia (CML). BioDrugs 2014; 28: 17-26; PMID:24043361; http://dx.doi.org/10.1007/s40259-013-0056-z
- Tang M, Foo J, Gönen M, Guilhot J, Mahon FX, Michor F. Selection pressure exerted by imatinib therapy leads to disparate outcomes of imatinib discontinuation trials. Haematologica 2012; 97: 1553-61; PMID:22419579; http://dx.doi.org/10.3324/haematol.2012.062844
- Cortes J, O'Brien S, Kantarjian H. Discontinuation of imatinib therapy after achieving a molecular response. Blood 2004; 104: 2204-05; PMID:15377577; http://dx.doi.org/10.1182/blood-2004-04-1335
- Breccia M, Alimena G. Discontinuation of tyrosine kinase inhibitors and new approaches to target leukemic stem cells: treatment-free remission as a new goal in chronic myeloid leukemia. Cancer Lett 2014; 347: 22-8; PMID:24508029; http://dx.doi.org/10.1016/j.canlet.2014.01.033
- Graham SM, Jørgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, Holyoake TL. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood 2002; 99: 319-25; PMID:11756187; http://dx.doi.org/10.1182/blood.V99.1.319
- Hu Y, Swerdlow S, Duffy TM, Weinmann R, Lee FY, Li S. Targeting multiple kinase pathways in leukemic progenitors and stem cells is essential for improved treatment of Ph+ leukemia in mice. Proc Natl Acad Sci USA 2006; 103: 16870-875.
- Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest 2011; 121: 396-409; PMID:21157039; http://dx.doi.org/10.1172/JCI35721
- Hamilton A, Helgason GV, Schemionek M, Zhang B, Myssina S, Allan EK, Nicolini FE, Müller-Tidow C, Bhatia R, Brunton VG, et al. Chronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survival. Blood 2012; 119: 1501-10; PMID:22184410; http://dx.doi.org/10.1182/blood-2010-12-326843
- Zhang H, Li S. Molecular mechanisms for survival regulation of chronic myeloid leukemia stem cells. Protein Cell 2013; 4: 186-96; PMID:23483480; http://dx.doi.org/10.1007/s13238-013-2115-0
- Giuntoli S, Rovida E, Barbetti V, Cipolleschi MG, Olivotto M, Dello Sbarba P. Hypoxia suppresses BCR/Abl and selects Imatinib-insensitive progenitors within clonal CML population. Leukemia 2006; 20: 1291-93; PMID:16710305; http://dx.doi.org/10.1038/sj.leu.2404224
- Giuntoli S, Tanturli M, Di Gesualdo F, Barbetti V, Rovida E, Dello Sbarba P. Glucose availability in hypoxia regulates the selection of chronic myeloid leukemia progenitor subsets with different resistance to imatinib-mesylate. Haematologica 2011; 96: 204-12; PMID:21071498; http://dx.doi.org/10.3324/haematol.2010.029082
- Giuntoli S, Barbetti V, Rovida E, Dello Sbarba P. Hypoxia selects bortezomib-resistant stem cells of chronic myeloid leukemia. PLoS-One 2011; 6(2): e17008; PMID:21347297.
- Cipolleschi MG, Rovida E, Dello Sbarba P. The Culture-Repopulating Ability assays and incubation in low oxygen: a simple way to test drugs on leukemia stem or progenitor cells. Curr Pharm Des 2013; 19: 5374-83; PMID:23394087; http://dx.doi.org/10.2174/1381612811319300006
- Rovida E, Marzi I, Cipolleschi MG, Dello Sbarba P. One more stem cell niche: how the sensitivity of chronic myeloid leukemia cells to imatinib-mesylate is modulated within a “hypoxic” environment. Hypoxia 2014; 2: 1-10.
- Rovida E, Peppicelli S, Bono S, Bianchini F, Tusa I, Cheloni G, Marzi I, Cipolleschi MG, Calorini L, Dello Sbarba P. The metabolically-modulated stem cell niche: a dynamic scenario regulating cancer cell phenotype and resistance to therapy. Cell Cycle 2014; 13:3169-75; PMID:25485495; http://dx.doi.org/10.4161/15384101.2014.964107
- Ivanovic Z. Hypoxia or in situ normoxia: The stem cell paradigm. J Cell Physiol 2009; 219: 271-75; PMID:19160417; http://dx.doi.org/10.1002/jcp.21690
- Winkler IG, Barbier V, Wadley R, Zannettino AC, Williams S, Lévesque JP. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood 2010; 116: 375-85; PMID:20393133; http://dx.doi.org/10.1182/blood-2009-07-233437
- Lévesque JP, Winkler IG. Hierarchy of immature hematopoietic cells related to blood flow and niche. Curr Opin Hematol 2011; 18: 220-25; ; http://dx.doi.org/10.1097/MOH.0b013e3283475fe7
- Nombela-Arrieta C, Pivarnik G, Winkel B, Canty KJ, Harley B, Mahoney JE, Park SY, Lu J, Protopopov A, Silberstein LE. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol 2013; 15: 533-44; PMID:23624405; http://dx.doi.org/10.1038/ncb2730
- Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, Zaher W, Mortensen LJ, Alt C, Turcotte R, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 2014; 508: 269-73; PMID:24590072; http://dx.doi.org/10.1038/nature13034
- Konig H, Holtz M, Modi H, Manley P, Holyoake TL, Forman SJ, Bhatia R. Enhanced BCR-ABL kinase inhibition does not result in increased inhibition of downstream signaling pathways or increased growth suppression in CML progenitors. Leukemia 2008; 22: 748-55; PMID:18273048; http://dx.doi.org/10.1038/sj.leu.2405086
- Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F, Legros L, Charbonnier A, Guerci A, Varet B, et al. Intergroupe Français des Leucémies Myéloïdes Chroniques. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre STopIMatinib (STIMtrial. Lancet Oncol 2010; 11: 1029-35; PMID:20965785; http://dx.doi.org/10.1016/S1470-2045(10)70233-3
- Modi H, McDonald T, Chu S, Yee JK, Forman SJ, Bhatia R. Role of BCR/ABL gene-expression levels in determining the phenotype and imatinib sensitivity of transformed human hematopoietic cells. Blood 2007; 109: 5411-21; PMID:17347407; http://dx.doi.org/10.1182/blood-2006-06-032490
- Bishara A, Rudi A, Aknin M, Neumann D, Ben-Califa N, Kashman Y. Salarin C, a new cytotoxic sponge-derived nitrogenous macrolide. Tetrahedron Lett 2008; 49: 4355-58; http://dx.doi.org/10.1016/j.tetlet.2008.05.038
- Bishara A, Rudi A, Goldberg I, Aknin M, Neumann D, Ben-Califa N, Kashman Y. Tausalarin C: a new bioactive marine sponge-derived nitrogenous bismacrolide. Org Lett 2009; 11: 3538-41; PMID:19627102; http://dx.doi.org/10.1021/ol9011019
- Zur LG, Bishara A, Aknin M, Neumann D, Ben-Califa N, Kashman Y. Derivatives of salarin A, salarin C and tulearin A–Fascaplysinopsis sp. metabolites. Mar Drugs 2013; 11: 4487-509.
- Ben-Califa N, Bishara A, Kashman Y, Neumann D. Salarin C, a member of the salarin superfamily of marine compounds, is a potent inducer of apoptosis. Invest New Drugs 2012; 30: 98-104; PMID:20734109; http://dx.doi.org/10.1007/s10637-010-9521-4
- Fernandez-Capetillo O, Chen HT, Celeste A, Ward I, Romanienko PJ, Morales JC, Naka K, Xia Z, Camerini-Otero RD, Motoyama N, et al. DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nat Cell Biol 2002; 4: 993-97; PMID:12447390; http://dx.doi.org/10.1038/ncb884
- Jin Z, Dicker DT, El-Deiry WS. Enhanced sensitivity of G1 arrested human cancer cells suggests a novel therapeutic strategy using a combination of simvastatin and TRAIL. Cell Cycle 2002; 1: 82-9; PMID:12429913; http://dx.doi.org/10.4161/cc.1.1.104
- Clark SS, Perman SM, Sahin MB, Jenkins GJ, Elegbede JA. Antileukemia activity of perillyl alcohol (POH): uncoupling apoptosis from G0/G1 arrest suggests that the primary effect of POH on Bcr/Abl-transformed cells is to induce growth arrest. Leukemia 2002; 16: 213-22; PMID:11840288; http://dx.doi.org/10.1038/sj.leu.2402369
- Sattler M, Salgia R, Okuda K, Uemura N, Durstin MA, Pisick E, Xu G, Li JL, Prasad KV, Griffin JD. The proto-oncogene product p120CBL and the adaptor proteins CRKL and c-CRK link c-ABL, p190BCR/ABL and p210BCR/ABL to the phosphatidylinositol-3′ kinase pathway. Oncogene 1996; 12: 839-46; PMID:8632906.
- Feller SM. Crk family adaptors-signalling complex formation and biological roles. Oncogene 2001; 20: 6348-71; PMID:11607838; http://dx.doi.org/10.1038/sj.onc.1204779
- Sedelnikova OA, Rogakou EP, Panyutin IG, Bonner WM. Quantitative detection of (125)IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat Res 2002; 158: 486-92; PMID:12236816; http://dx.doi.org/10.1667/0033-7587(2002)158%5b0486:QDOIID%5d2.0.CO;2
- Barbetti V, Tusa I, Cipolleschi MG, Rovida E, Dello Sbarba P. AML1/ETO sensitizes via TRAIL acute myeloid leukemia cells to the pro-apoptotic effects of hypoxia. Cell Death Dis 2013; 4: e536; PMID:23492767; http://dx.doi.org/10.1038/cddis.2013.49
- Kumari A, Brendel C, Hochhaus A, Neubauer A, Burchert A. Low BCR-ABL expression levels in hematopoietic precursor cells enable persistence of chronic myeloid leukemia under imatinib. Blood 2012; 119: 530-9; PMID:22101898; http://dx.doi.org/10.1182/blood-2010-08-303495
- Blagosklonny MV. Oncogenic resistance to growth-limiting conditions. Nat Rev Cancer 2002; 2: 221-5; PMID:11990858; http://dx.doi.org/10.1038/nrc743
- Blagosklonny MV. Antiangiogenic therapy and tumor progression. Cancer Cell 2004; 5: 13-7; PMID:14749122; http://dx.doi.org/10.1016/S1535-6108(03)00336-2
- Cipolleschi MG, Rovida E, Ivanović Z, Praloran V, Olivotto M, Dello Sbarba P. The expansion of murine bone marrow cells preincubated in hypoxia as an in vitro indicator of their marrow repopulating ability. Leukemia 2000; 14: 735-39; PMID:10764163; http://dx.doi.org/10.1038/sj.leu.2401744
- Ivanović Z, Belloc F, Faucher JL, Cipolleschi MG, Praloran V, Dello Sbarba P. Hypoxia maintains and interleukin-3 reduces the precolony-forming cell potential of dividing CD34(+ murine bone marrow cells. Exp Hematol 2002; 30: 67-73; PMID:11823039; http://dx.doi.org/10.1016/S0301-472X(01)00765-2
- Ivanović Z, Bartolozzi B, Bernabei PA, Cipolleschi MG, Rovida E, Milencović P, Praloran V, Dello Sbarba P. Incubation of bone marrow cells in hypoxia ensures the maintenance of marrow-repopulating ability together with the expansion of committed progenitors. Br J Haematol 2000; 108: 424-29; PMID:10691876; http://dx.doi.org/10.1046/j.1365-2141.2000.01842.x
- Giuntoli S, Rovida E, Gozzini A, Barbetti V, Cipolleschi MG, Olivotto M, Dello Sbarba P. Severe hypoxia defines heterogeneity and selects highly immature progenitors within clonal erythroleukemia cells. Stem Cells 2007; 25: 1119-25; PMID:17255519; http://dx.doi.org/10.1634/stemcells.2006-0637
