Abstract
Although Hmgn1 is involved in the regulation of gene expression and cellular differentiation, its physiological roles on the differentiation of uterine stromal cells during decidualization still remain unknown. Here we showed that Hmgn1 mRNA was highly expressed in the decidua on days 6-8 of pregnancy. Simultaneously, increased expression of Hmgn1 was also observed in the artificial and in vitro induced decidualization models. Hmgn1 induced the proliferation of uterine stromal cells and expression of Ccna1, Ccnb1, Ccnb2 and Cdk1 in the absence of estrogen and progesterone. Overexpression of Hmgn1 could enhance the expression of Prl8a2 and Prl3c1 which were 2 well-known differentiation markers for decidualization, whereas inhibition of Hmgn1 with specific siRNA could reduce their expression. Further studies found that Hmgn1 could mediate the effects of C/EBPβ on the expression of Prl8a2 and Prl3c1 during in vitro decidualization. In the uterine stromal cells, cAMP analog 8-Br-cAMP could stimulate the expression of Hmgn1 via C/EBPβ. Moreover, siRNA-mediated down-regulation of Hmgn1 could attenuate the effects of cAMP on the differentiation of uterine stromal cells. During in vitro decidualization, Hmgn1 might act downstream of C/EBPβ to regulate the expression of Cox-2, mPGES-1 and Vegf. Progesterone could up-regulate the expression of Hmgn1 in the ovariectomized mouse uterus, uterine epithelial cells and stromal cells. Knockdown of C/EBPβ with siRNA alleviated the up-regulation of progesterone on Hmgn1 expression. Collectively, Hmgn1 may play an important role during mouse decidualization.
Introduction
Embryo implantation involves the intimate interaction between an implantation-competent blastocyst and a receptive uterus.Citation1,2 Following the onset of embryo implantation, uterine stromal cells surrounding the implanting embryo undergo extensive proliferation and subsequent differentiation into Polyploid decidual cells (decidualization).Citation2,3 Proper decidualization is essential for continued embryonic development within the uterus and achieving successful pregnancy, because impaired decidualization can lead to miscarriage or even pathological pregnancy such as preeclampsia or intrauterine growth restriction.Citation2,4 Genome-wide microarray analyses have identified a number of genes that are up-regulated or down-regulated during decidualization, suggesting the presence of complex mechanisms of gene expression.Citation4 To date, accumulating data have shown that gene expression involves a change of chromatin structure which can be regulated by structural proteins such as the high mobility group nucleosomal binding (Hmgn) family.Citation5,6
Hmgn proteins are architectural nonhistone chromosomal proteins that bind specifically to nucleosome core particle, reduce the compaction of the chromatin fiber, alter the structure of chromatin and thereby affect a variety of DNA-dependent activities such as transcription, replication, recombination and DNA repair.Citation5-7 Hmgn1, previously known as Hmg14, is an abundant member of Hmgn family.Citation6,7 It can affect the expression of numerous genes and regulate cellular differentiation.Citation6,8-10 Analysis of Hmgn1-deficient cells indicated that loss of Hmgn1 led to both upregulation and downregulation of gene expression.Citation9,11,12 During brain development, Hmgn1 could promote astrocyte differentiation of neural precursor cells through modulating of the responsiveness to ciliary neurotrophic factor.Citation10 Simultaneously, Hmgn1 might also direct chondrocyte differentiation by influencing the expression of Sox9 gene.Citation8 Although our (unpublished) microarray data has revealed that Hmgn1 was strongly expressed in day 8 decidua and deciduoma under artificial decidualization compared with the uninjected uterine horn, the effects of Hmgn1 on the expression of decidualization-related genes and differentiation of uterine stromal cells during decidualization are still unknown so far.
CCAAT/enhancer-binding protein β (C/EBPβ) is a member of basic leucine zipper DNA-binding proteins and has been identified as a regulator of uterine stromal cell proliferation and differentiation.Citation13-15 C/EBPβ-null uterine stromal cells were unable to undergo proper proliferation and differentiation in response to a decidual stimulation.Citation14-16 Ablation of C/EBPβ gene in female mice resulted in infertility with a complete lack of decidual formation.Citation2,14 Although it has been proved that C/EBPβ might regulate the expression of numerous decidualization-related genes,Citation13 the relationship between C/EBPβ and Hmgn1 during decidualization remain poorly understood.
In this study, we showed that Hmgn1 was highly expressed in the decidua and decidualizing stromal cells and important for the proliferation and differentiation of uterine stromal cells during decidualization. Furthermore, our results indicated that Hmgn1 might act downstream of C/EBPβ to regulate the decidualization of uterine stromal cells.
Results
Hmgn1 mRNA expression during early pregnancy
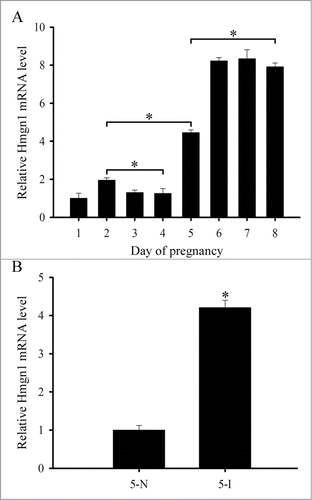
Although it has been reported that Hmgn1 was expressed in the uterus,Citation17 the detailed expression patterns of Hmgn1 in mouse uterus during early pregnancy have not been described. We thus performed in situ hybridization to localize the distribution of Hmgn1 mRNA in mouse uterus. The results showed that there was no visible Hmgn1 mRNA signal from days 1 to 4 of pregnancy (). On day 5 when embryo implanted, Hmgn1 mRNA was highly expressed in the subluminal stromal cells around implanting blasocyst at implantation sites, but not seen at inter-implantation sites (). On days 6–8 of pregnancy, Hmgn1 mRNA signal was constantly detected in the decidual cells and embryo (). However, once the DIG-labeled Hmgn1 antisense probe was replaced with DIG-labeled Hmgn1 sense probe, there was no corresponding signal in the uterus on day 7 of pregnancy (). To quantify Hmgn1 mRNA expression, real-time PCR was preformed. As expected, a high level of Hmgn1 expression was also detected on days 6–8 of pregnancy (). Compared with the inter-implantation sites, Hmgn1 mRNA expression was higher at implantation sites on day 5 of pregnancy ()
Figure 1. In situ hybridization of Hmgn1 expression in mouse uteri during early pregnancy on days 2 (B), 5 (C), 6 (D), 7 (E), and 8 (F). No hybridization signals were seen in mouse uterus on day 7 of pregnancy when DIG-labeled Hmgn1 sense probe was used to replace the antisense probe as a negative control (A). Asterisks indicate embryo. Bar = 60 μm.
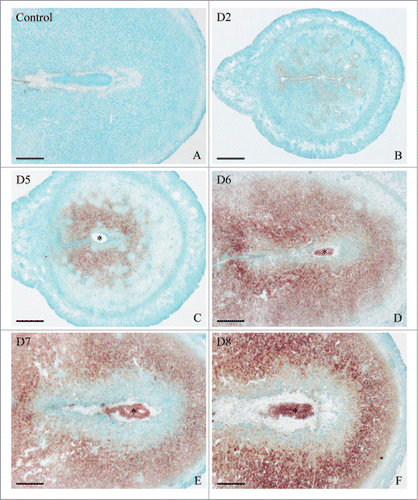
Figure 2. Hmgn1 expression in mouse uteri during early pregnancy. (A) Real-time PCR analysis of Hmgn1 expression on days 1–8 of pregnancy. (B) Real-time PCR analysis of Hmgn1 expression at the implantation sites (5-I) and inter-implantation sites (5-N) on day 5 of pregnancy. Data are shown mean ± SEM. Asterisks denote significance (P < 0.05).
Hmgn1 mRNA expression during delayed implantation and activation
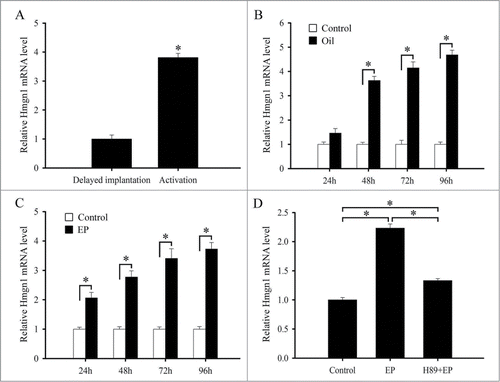
To determine whether Hmgn1 expression was dependent on the presence of an active blastocyst, we examined its expression in the delayed-implantation uterus or activated implantation sites and found that there was no obvious Hmgn1 mRNA signal in the delayed uterus (). After estrogen activated the dormant blastocyst to implant, a high level of Hmgn1 mRNA signal was detected in the subluminal stromal cells surrounding the implanting embryo (). Simultaneously, a significantly high level of Hmgn1 expression was observed in the activated implantation sites rather than in the delayed uterus by real-time PCR ()
Figure 3. In situ hybridization of Hmgn1 expression in mouse uteri. Delay, delayed implantation. Activation, activation of delayed implantation by estrogen. Con, uninjected uterine horn of control. Oil, oil-induced decidualization. Bar = 60 μm.
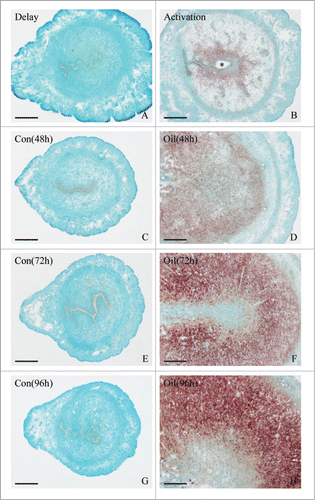
Figure 4. Real-time PCR analysis of Hmgn1 expression in mouse uteri. (A) Hmgn1 expression in delayed and activated uteri. (B) Hmgn1 expression under artificial decidualization. (C) Hmgn1 expression during in vitro decidualization. (D) Hmgn1 expression after uterine stromal cells were induced for decidualization with estrogen plus progesterone in the presence of H89. EP, estrogen plus progesterone.
Hmgn1 mRNA expression during decidualization
Because Hmgn1 was highly expressed in the decidual cells, we also tested its expression under artificially induced decidualization in vivo and found that no visible signal was detected in the uninjected control uterus (). After the pseudopregnant uterine horn was induced to be artificially decidualized by injecting sesame oil into the uterine lumen, Hmgn1 mRNA signal was obviously seen in the decidualizing stromal cells (). By real-time analysis, elevated expression levels of Hmgn1 were observed in the uterine horns undergoing artificially stimulated decidualization with a time-dependent increase and reached a maximum at 96 h after sesame oil injection ().
Our previous study has confirmed that primary stromal cells isolated from mouse uteri on day 4 of pregnancy were treated with the combination of estrogen and progesterone to induce in vitro decidualization.Citation18 As the progression of decidualization was increasing, Hmgn1 expression was gradually enhanced and reached a peak at 96 h (). It has previously established that protein kinase A (PKA) signal transduction pathway is involved in the process of decidualization.Citation19 Thus we treated the uterine stromal cells with PKA inhibitor H89, then added estrogen and progesterone and monitored the expression of Hmgn1. The results found that H89 could evidently inhibit the expression of Hmgn1 in the uterine stromal cells undergoing decidualization ().
Effects of Hmgn1 on decidualization
Decidualization involves extensive proliferation and differentiation of uterine stromal cells.Citation2,3 Since stromal cell proliferation is the first step of decidualization, we first investigated the effects of Hmgn1 on stromal cell proliferation. After three different Hmgn1 siRNA duplexes were transfected in the uterine stromal cells, expression of Hmgn1 mRNA was determined by real-time PCR. The results showed that Hmgn1 siRNA 1, 2, or 3 could suppress the expression of Hmgn1 mRNA in the stromal cells compared with scrambled siRNA, and Hmgn1 siRNA 2 was the most effective among them (). Therefore, Hmgn1 siRNA 2 was chosen for further experiments. After transfection with Hmgn1 siRNA, proliferation of uterine stromal cells displayed a significant decline compared with control (). In contrast, overexpression of Hmgn1 could raise the expression of Hmgn1 in the stromal cells and proliferation activity of stromal cells (). Interestingly, neither overexpression nor inhibition for Hmgn1 had any distinct impact on the proliferation of stromal cells in the presence of estrogen and progesterone (). To understand the molecular basis for the proliferative role of Hmgn1, we subsequently analyzed the influences of Hmgn1 on the expression of cyclin A1 (Ccna1), Ccnb1, Ccnb2, Ccnd1, Ccnd3, Ccne1, cyclin-dependent kinase 1 (Cdk1), Cdk2, Cdk4 and Cdk6. Overexpression of Hmgn1 could strengthen the expression of Ccna1, Ccnb1, Ccnb2 and Cdk1 in the uterine stromal cells, while silencing of Hmgn1 reduced the expression of Ccna1, Ccnb1, Ccnb2 and Cdk1 (). After stromal cells were transfected with Hmgn1 overexpression plasmid or siRNA and then treated with estrogen and progesterone, the expression of Ccna1, Ccnb1, Ccnb2 and Cdk1 did not show any obvious change (data not shown). Similarly, Hmgn1 could not direct the expression of Ccnd1, Ccnd3, Ccne1, Cdk2, Cdk4 and Cdk6 in the uterine stromal cells which were untreated or treated with a combination of estrogen and progesterone ()
Figure 5. Effects of Hmgn1 on the proliferation of uterine stromal cells. (A) Effects of Hmgn1 siRNAs on Hmgn1 mRNA expression in the uterine stromal cells. After transfection with control siRNA, Hmgn1 siRNA 1, siRNA 2 or siRNA 3, Hmgn1 mRNA expression was determined by real-time PCR. (B) Effects of Hmgn1 siRNA on the proliferation of uterine stromal cells. After transfection with Hmgn1 siRNA, stromal cells were analyzed by MTS assay in the absence or presence of estrogen and progesterone. (C) Hmgn1 expression after uterine stromal cells were transfected with Hmgn1 overexpression plasmid. (D) Effects of Hmgn1 overexpression on the proliferation of uterine stromal cells. After transfection with Hmgn1 overexpression plasmid, stromal cells were analyzed by MTS assay in the absence or presence of estrogen and progesterone. (E) Effects of Hmgn1 siRNA on the expression of Ccna1, Ccnb1, Ccnb2, Ccnd1, Ccnd3 and Ccne1 in the stromal cells in the absence of estrogen and progesterone. (F) Effects of Hmgn1 overexpression on the expression of Ccna1, Ccnb1, Ccnb2, Ccnd1, Ccnd3 and Ccne1 in the absence of estrogen and progesterone. (G) Effects of Hmgn1 siRNA on the expression of Cdk1, Cdk2, Cdk4 and Cdk6 in the stromal cells in the absence of estrogen and progesterone. (H) Effects of Hmgn1 overexpression on the expression of Cdk1, Cdk2, Cdk4 and Cdk6 in the absence of estrogen and progesterone. NC, control siRNA duplex; siHmgn1, Hmgn1 siRNA; Con, empty pcDNA3.1 vector; Hmgn1, Hmgn1 overexpression plasmid.
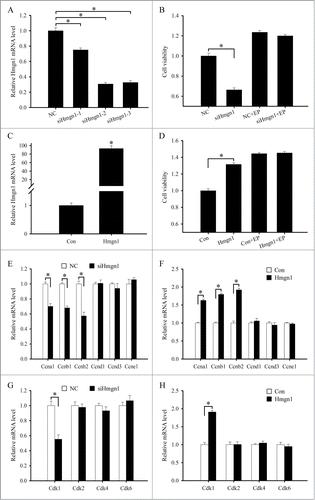
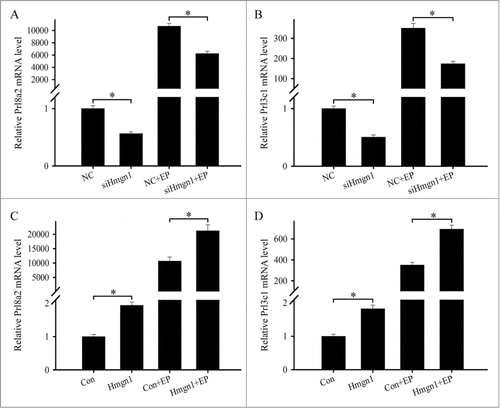
To explore the effects of Hmgn1 on the differentiation of uterine stromal cells, we monitored the effects of Hmgn1 on the expression of prolactin family 8, subfamily a, member 2 (Prl8a2) and prolactin family 3, subfamily c, member 1 (Prl3c1) which were 2 well-known differentiation markers for decidualization.Citation20 After stromal cells were transfected with Hmgn1 siRNA, the expression levels of Prl8a2 and Prl3c1 were markedly reduced (). During the induction of in vitro decidualization with estrogen and progesterone, knockdown of Hmgn1 could also decrease the expression of Prl8a2 and Prl3c1 ( and B). On the contrary, overexpression of Hmgn1 could apparently enhance the expression of Prl8a2 and Prl3c1 in the uterine stromal cells in the absence or presence of estrogen and progesterone ()
Figure 6. Effects of Hmgn1 on the differentiation of uterine stromal cells. (A and B) Effects of Hmgn1 siRNA on the expression of Prl8a2 and Prl3c1. After transfection with Hmgn1 siRNA, the expression of Prl8a2 and Prl3c1 was determined by real-time PCR in the absence or presence of estrogen and progesterone. (C and D) Effects of Hmgn1 overexpression on the expression of Prl8a2 and Prl3c1. After transfection with Hmgn1 overexpression plasmid, the expression of Prl8a2 and Prl3c1 was determined by real-time PCR in the absence or presence of estrogen and progesterone.
Hmgn1 mediates the effects of C/EBPβ on the differentiation of uterine stromal cells
It is well known that C/EBPβ is critical for the differentiation of uterine stromal cells and regulates the expression of a number of decidualization-related genes.Citation13,14 Because C/EBPβ was also expressed in the decidualized cells and overlapped with Hmgn1, we assumed that there was a relationship between C/EBPβ and Hmgn1 during decidualization. To elucidate the relationship, we first studied the regulation of Hmgn1 on C/EBPβ. The results evidenced that neither overexpression nor inhibition of Hmgn1 could impact the expression of C/EBPβ in the stromal cells undergoing decidualization (). We next investigated whether C/EBPβ could modulate the expression of Hmgn1 during in vitro decidualization. Three different C/EBPβ siRNA duplexes were transfected in the uterine stromal cells to choose the most effective siRNA. Real-time PCR result showed that each siRNA could significantly down-regulate the expression of C/EBPβ mRNA in the stromal cells, and C/EBPβ siRNA 3 exhibited obvious inhibition effect (). Thus, C/EBPβ siRNA 3 was selected to knock down C/EBPβ gene in the following study. After transfection with C/EBPβ siRNA along with the addition of estrogen and progesterone, the expression of Hmgn1 was noticeably declined compared with control (). Conversely, overexpression of C/EBPβ could augment the expression of Hmgn1 and C/EBPβ during in vitro decidualization ()
Figure 7. Hmgn1 mediates the effects of C/EBPβ on the differentiation of uterine stromal cells. (A) Effects of Hmgn1 siRNA or overexpression on the expression of C/EBPβ. (B) C/EBPβ expression after uterine stromal cells were transfected with C/EBPβ siRNAs. (C) Effects of C/EBPβ siRNA on the expression of Hmgn1. (D) C/EBPβ expression after uterine stromal cells were transfected with C/EBPβ overexpression plasmid. (E) Effects of C/EBPβ overexpression on the expression of Hmgn1. (F and G) Hmgn1 siRNA abrogated the effects of C/EBPβ overexpression on the expression of Prl8a2 and Prl3c1. After co-transfection with C/EBPβ overexpression plasmid and Hmgn1 siRNA, the expression of Prl8a2 and Prl3c1 was determined by real-time PCR in the presence of estrogen and progesterone. (H and I) Overexpression of Hmgn1 improved the effects of C/EBPβ siRNA on the expression of Prl8a2 and Prl3c1.
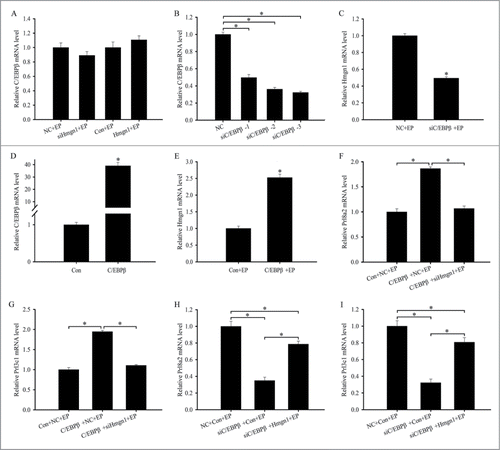
To test whether Hmgn1 was necessary for the stromal cells differentiation induced by C/EBPβ overexpression, the uterine stromal cells were co-transfected with C/EBPβ overexpression plasmid and Hmgn1 siRNA and then subjected to in vitro decidualization in presence of estrogen and progesterone. The results found that knockdown of Hmgn1 could prevent the C/EBPβ-induced up-regulation of Prl8a2 and Prl3c1 in the stromal cells undergoing decidualization (). We next assessed whether the effects of C/EBPβ siRNA on the stromal cells differentiation could be rescued by Hmgn1 overexpression. After co-transfection with C/EBPβ siRNA and Hmgn1 overexpression plasmid, the expression of Prl8a2 and Prl3c1 was evidently enhanced in the uterine stromal cells induced for in vitro decidualization ().
Hmgn1 acts downstream of C/EBPβ to mediate the effects of cAMP on the differentiation of uterine stromal cells
Because cAMP is important for differentiation of uterine stromal cells into decidual cells,Citation19 we treated the stromal cells with cAMP analog 8-Bromoadenosine-cAMP (8-Br-cAMP, 500 μM). The results found that the expression of Hmgn1 was significantly elevated from 3 to 24 h after 8-Br-cAMP treatment (). As a ubiquitous second messenger, cAMP prominently activates the PKA signal transduction pathway.Citation19 After stromal cells were pretreated with PKA inhibitor H89, 8-Br-cAMP-induced Hmgn1 expression was clearly dropped (). To ascertain whether Hmgn1 could mediate the effects of cAMP on the differentiation of uterine stromal cells, we transfected the stromal cells with Hmgn1 siRNA, then add 8-Br-cAMP and analyzed the expression of Prl8a2 and Prl3c1. The results showed that siRNA-mediated downregulation of Hmgn1 in the stromal cells led to a notable reduction in the expression of differentiation markers Prl8a2 and Prl3c1 ().
Figure 8. Hmgn1 acts downstream of C/EBPβ to mediate the effects of cAMP on the differentiation of uterine stromal cells. (A) Hmgn1 expression in the uterine stromal cells after 8-Br-cAMP treatment. (B) Hmgn1 expression after uterine stromal cells were treated with 8-Br-cAMP, or both 8-Br-cAMP and H89. (C and D) Effects of cAMP on the expression of Prl8a2 and Prl3c1 through Hmgn1. After transfection with Hmgn1 siRNA and addition of 8-Br-cAMP, the expression of Prl8a2 and Prl3c1 was determined by real-time PCR. (E) C/EBPβ expression in the uterine stromal cells after 8-Br-cAMP treatment. (F) C/EBPβ expression after uterine stromal cells were treated with 8-Br-cAMP, or both 8-Br-cAMP and H89. (G and H) Effects of cAMP on the expression of Prl8a2 and Prl3c1 through C/EBPβ. (I) Effects of cAMP on the expression of Hmgn1 via C/EBPβ.
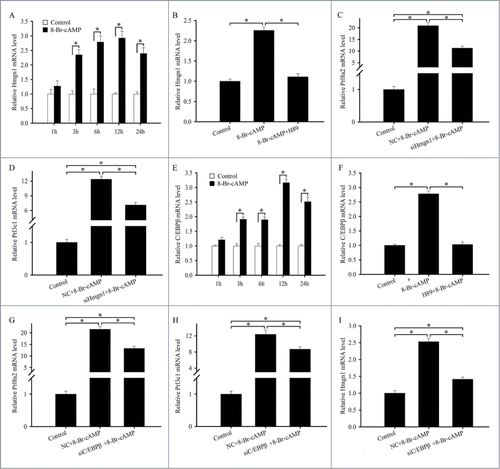
It has been well documented that cAMP could stimulate the expression of C/EBPβ in human endometrial stromal cells.Citation21 Similarly, administration of 8-Br-cAMP to the mouse stromal cells resulted in a marked increase in the expression of C/EBPβ (). H89 pretreatment dramatically abrogated the 8-Br-cAMP-induced expression of C/EBPβ (). Knockdown of C/EBPβ with specific siRNA could obviously attenuate the stimulation of 8-Br-cAMP on the expression of Prl8a2 and Prl3c1 (). We next investigated whether the effects of cAMP on the expression of Hmgn1 was mediated by C/EBPβ. After the stromal cells were transfected with C/EBPβ siRNA and then added cAMP analog 8-Br-cAMP, the expression of Hmgn1 was significantly diminished ().
Hmgn1 mediates the effects of C/EBPβ on the expression of Cox-2, mPGES-1 and Vegf
Because Hmgn1 was involved in transcriptional regulation of numerous genes,Citation6 we examined the effects of Hmgn1 on the expression of cyclooxygenase-2 (Cox-2), microsomal prostaglandin E synthase 1 (mPGES-1), vascular endothelial growth factor (Vegf), whose expression in the decidual bed overlapped with that of Hmgn1. During in vitro decidualization, overexpression of Hmgn1 could up-regulate the expression of Cox-2, mPGES-1 and Vegf, while inhibition of Hmgn1 with specific siRNA could down-regulate the expression of Cox-2, mPGES-1 and Vegf ().
Figure 9. Hmgn1 mediates the effects C/EBPβ on the expression of Cox-2, mPGES-1 and Vegf. (A) Effects of Hmgn1 siRNA on the expression of Cox-2, mPGES-1 and Vegf. (B) Effects of Hmgn1 overexpression on the expression of Cox-2, mPGES-1 and Vegf. (C) Effects of C/EBPβ siRNA on the expression of Cox-2, mPGES-1 and Vegf. (D) Effects of C/EBPβ overexpression on the expression of Cox-2, mPGES-1 and Vegf. (E) The expression of Cox-2, mPGES-1 and Vegf after stromal cells were co-transfected with C/EBPβ overexpression plasmid and Hmgn1 siRNA. (F) The expression of Cox-2, mPGES-1 and Vegf after stromal cells were co-transfected with C/EBPβ siRNA and Hmgn1 overexpression plasmid.
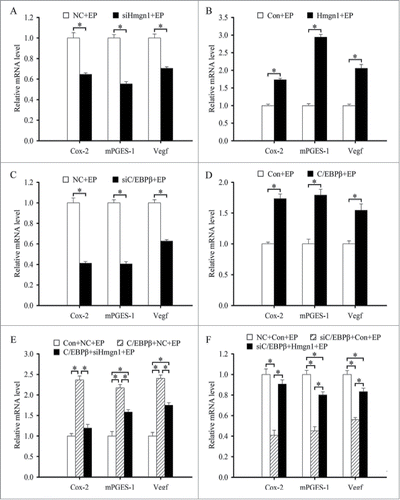
As demonstrated above, we have verified that C/EBPβ regulated the expression of Hmgn1 which directed the expression of Cox-2, mPGES-1 and Vegf during in vitro decidualization. We next asked whether Hmgn1 might mediate the regulation of C/EBPβ on the expression of Cox-2, mPGES-1 and Vegf. To confirm the possibility, we first studied the effects of C/EBPβ on the expression of Cox-2, mPGES-1 and Vegf in the uterine stromal cells induced for in vitro decidualization. The results found that treatment with siRNA targeted to C/EBPβ mRNA led to a marked reduction in the expression of Cox-2, mPGES-1 and Vegf, whereas overexpression of C/EBPβ could augment the expression of these genes (). We next explored whether Hmgn1 could mediate the effects of C/EBPβ on the expression of Cox-2, mPGES-1 and Vegf in the uterine stromal cells which were subjected to in vitro decidualization in presence of estrogen and progesterone. As expected, overexpression of Hmgn1 improved the expression of Cox-2, mPGES-1 and Vegf in the C/EBPβ siRNA-transfected stromal cells (). Conversely, siRNA-mediated down-regulation of Hmgn1 inhibited the expression of Cox-2, mPGES-1 and Vegf in the uterine stromal cells transfected with C/EBPβ overexpression plasmid ().
Steroid hormonal regulation of Hmgn1 expression
As estrogen and progesterone were essential for embryo implantation and decidualization,Citation1,2 ovariectomized mice were used to examine whether ovarian steroid hormones could regulate the expression of Hmgn1. In the uteri of ovariectomized mice, a weak hybridization signal was observed in the luminal and glandular epithelium (). After ovariectomized mice were treated with estrogen for 24 h, Hmgn1 mRNA was highly expressed in the uterine luminal epithelium (). Progesterone could stimulate the expression of Hmgn1 in the luminal epithelium, glandular epithelium and stromal cells (). After a combined injection of estrogen and progesterone, the pattern of Hmgn1 expression in uterus was similar to that of progesterone treatment alone but at much higher levels (). By real-time PCR analysis, both estrogen and progesterone could induce the expression of Hmgn1 in the uteri of ovariectomized mice (). Based on above observations, the time course of Hmgn1 expression was further examined at 1, 3, 6, 12 and 24 h after estrogen or progesterone treatment. By in situ hybridization, Hmgn1 expression was gradually heightened in the luminal and glandular epithelium after injection of estrogen and reached the highest level at 6 and 12 h (). Similarly, Hmgn1 mRNA was also detected in the luminal and glandular epithelium, and its expression was high at 6 h after injection of progesterone (). Meanwhile, progesterone could induce the expression of Hmgn1 mRNA in the uterine stromal cells at 6, 12 and 24 h (). Real-time PCR results showed that Hmgn1 expression was strongly detected at 6 h after estrogen treatment even if it was higher in the uteri of ovariectomized mice treated with estrogen for 3, 6, 12 and 24 h compared with that in the control uteri (). Progesterone injection could result in an increase in uterine Hmgn1 mRNA levels, which reached a peak at 6 h ().
Figure 10. In situ hybridization of Hmgn1 expression after ovariectomized mice were treated with sesame oil (Control), estrogen, progesterone or a combination of estrogen and progesterone. E, estrogen; P, progesterone. Bar = 60 μm.
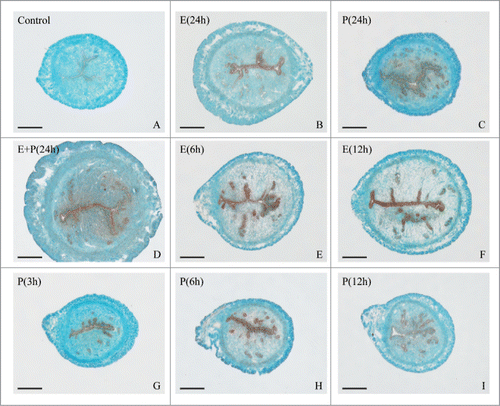
Figure 11. Hormonal regulation of Hmgn1 expression. (A) Real-time PCR analysis of Hmgn1 expression after ovariectomized mice were treated with sesame oil, estrogen, progesterone or a combination of estrogen and progesterone for 24 h. (B) Real-time PCR analysis of Hmgn1 expression in ovariectomized mouse uteri after injection of estrogen for 1, 3, 6, 12 and 24 h. (C) Real-time PCR analysis of Hmgn1 expression in ovariectomized mouse uteri after injection of progesterone for 1, 3, 6, 12 and 24 h. (D) Real-time PCR analysis of Hmgn1 expression after uterine epithelial cells were treated with estrogen or both estrogen and ICI 182,780. (E) Real-time PCR analysis of Hmgn1 expression after uterine epithelial cells were treated with progesterone or both progesterone and RU486. (F) Real-time PCR analysis of Hmgn1 expression in uterine stromal cells treated with progesterone for 1, 3, 6, 12 and 24 h. (G) Real-time PCR analysis of Hmgn1 expression after uterine stromal cells were treated with progesterone or both progesterone and RU486. (H) Effects of progesterone on the expression of Hmgn1 via C/EBPβ.
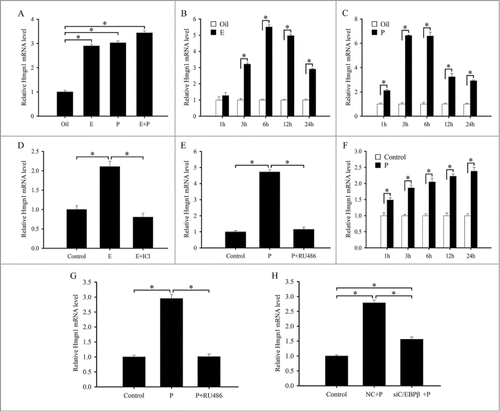
To further evaluate the effects of these hormones on the expression of Hmgn1, uterine epithelial and stromal cells were treated with estrogen or progesterone. In the in vitro cultured uterine epithelial cells, Hmgn1 mRNA expression was remarkably upregulated at 6 h after treatment with estrogen or progesterone (). However, the upregulation was abrogated by a pretreatment with estrogen receptor (ER) antagonist ICI 182,780 or progesterone receptor (PR) antagonist RU486, respectively (). In the uterine stromal cells, progesterone could stimulate the expression of Hmgn1 at 1, 3, 6, 12 and 24 h, respectively. The highest level was reached at 24 h after progesterone treatment (). RU486 pretreatment could dramatically attenuate the progesterone-stimulated Hmgn1 expression ().
C/EBPβ mediates the effects of progesterone on the expression of Hmgn1
Previous studies have tested that progesterone could induce the expression of C/EBPβ in the uterine stromal cells.Citation14 As demonstrated above, we have found that C/EBPβ and progesterone also regulated the expression of Hmgn1 in the stromal cells. We next asked whether C/EBPβ could mediate the effects of progesterone on the expression of Hmgn1. To verify the hypothesis, we treated the stromal cells with C/EBPβ siRNA, then added progesterone and analyzed the expression of Hmgn1. The results showed that siRNA-mediated attenuation of C/EBPβ could efficiently block the induction of progesterone on the expression of Hmgn1 in the uterine stromal cells ().
Discussion
In this study, we investigated the expression and regulation of Hmgn1 in mouse uterus during pre-implantation and then focused our attention on analyzing the physiological role of Hmgn1 in regulating the proliferation and differentiation of uterine stromal cells during decidualization. The results showed that Hmgn1 mRNA was highly expressed in the subluminal stromal cells around implanting blasocyst at implantation sites, but not seen at inter-implantation sites or in the delayed uterus, implying that Hmgn1 might be important for embryo implantation and its expression was dependent upon the presence of active blastocyst. After blastocyst attachment to the uterine luminal epithelium, uterine stromal cells surrounding the implanting blastocyst undergo extensive proliferation and subsequent differentiation into decidual cells.Citation2,3 With the progression of decidualization, the expression of Hmgn1 was gradually enhanced. Overexpression of Hmgn1 was effective to raise the proliferation of uterine stromal cells while inhibition of Hmgn1 with specific siRNA could reduce the proliferation of stromal cells. It is well established that the proliferation of animal cells proceeds through various stages of the cell cycle, G1, S, G2 and M, and is controlled by cyclins and Cdks.Citation15 In the uterine stromal cells, Hmgn1 could modulate the expression of Ccna1, Ccnb1, Ccnb2 and Cdk1, implying that Hmgn1 was involved in regulating cell cycle progression at the G2-M phase transition. Indeed, Hmgn1-deficient mouse embryonic fibroblasts failed to arrest properly in G2-M phase of the cell cycle after ionizing radiation treatment which was known to activate the G2-M cell cycle checkpoint.Citation22 However, Hmgn1 did not affect the proliferation of stromal cells and the expression of Ccna1, Ccnb1, Ccnb2 and Cdk1 after uterine stromal cells were induced for in vitro decidualization. These results suggest that Hmgn1 is important for the proliferation of uterine stromal cells during the initiation of decidualization. After this marked proliferation, stromal cells begin to terminally differentiate into decidual cells with acquisition of polyploidy.Citation2,23 The present study showed that Hmgn1 could stimulate the expression of Prl8a2 and Prl3c1 which were 2 well-known markers for decidual cell differentiation. Taken together, these results revealed an important role of Hmgn1 in maintaining the proliferation and differentiation of uterine stromal cells during decidualization.
Previous studies have evidenced that the process of decidualization were accompanied by an increase of intracellular cAMP level which could initiate the differentiation of uterine stromal cells prominently via the PKA signal transduction pathway.Citation19,24 PKA inhibitor H89 could block the expression of Hmgn1 in the uterine stromal cells undergoing decidualization and the stimulation of cAMP on Hmgn1 expression. The siRNA-mediated downregulation of Hmgn1 in the stromal cells could attenuate the effects of cAMP on the expression of differentiation markers Prl8a2 and Prl3c1, indicating that Hmgn1 is a critical regulator of cAMP-induced stromal differentiation during decidualization. Further analysis revealed that the effect of cAMP on Hmgn1 expression was mediated by C/EBPβ which was essential for decidualization and controlled the differentiation of stromal cells during decidualization.Citation14,15 In the uterine stromal cells undergoing decidualization, C/EBPβ might induce the expression of Hmgn1. Moreover, overexpression of Hmgn1 could improve the expression of Prl8a2 and Prl3c1 in the C/EBPβ siRNA-transfected stromal cells, whereas knockdown of Hmgn1 prevented the C/EBPβ-induced up-regulation of Prl8a2 and Prl3c1. These results provide convincing evidence that Hmgn1 is the downstream target of regulation by C/EBPβ during uterine stromal differentiation.
It has been well established that Hmgn1 was involved in transcriptional regulation of numerous genes.Citation6 In the uterine stromal cells treated with estrogen plus progesterone, Hmgn1 could stimulate the expression of Cox-2 which was a rate-limiting enzyme in the biosynthesis of prostaglandins (PGs) and implicated in mouse decidualization.Citation25,26 Targeted disruption of Cox-2 led to defective decidualization.Citation25 Under artificially induced decidualization, Cox-2 inhibitor nimesulide diminished the production of uterine PGE2 which was important for decidualization.Citation26,27 mPGES-1 might catalyze the conversion of PGH2 to PGE2 and was also modulated by Hmgn1 during in vitro decidualization.Citation26 Cumulative evidences have declared that deficiency of Cox-2 or mPGES-1 caused a reduction in uterine Vegf mRNA level.Citation28,29 Vegf was a well-known angiogenesis promoter and could profoundly influence the uterine angiogenesis which was crucial to decidualization.Citation28,30 In fact, Hmgn1 could also direct the expression of Vegf in the uterine stromal cells undergoing decidualization. Collectively, these results suggest that Hmgn1 may impact the uterine decidualization by affecting the expression of Cox-2, mPGES-1 and Vegf genes. Previous studies have tested that silencing of C/EBPβ with siRNA weakened the expression of Vegf in the process of decidualization of human endometrial stromal cells.Citation13 The evidence presented in this study demonstrated that C/EBPβ might regulate the expression of Cox-2, mPGES-1 and Vegf genes during decidualization of mouse stromal cells. Furthermore, Hmgn1 might act downstream of C/EBPβ to regulate the expression of Cox-2, mPGES-1 and Vegf genes during in vitro decidualization.
It has long been recognized that progesterone was essential for the initiation and maintenance of decidualization.Citation2,3 The actions of progesterone were executed by its receptor PR.Citation2,3 In this study, progesterone could induce the expression of Hmgn1 in the ovariectomized mouse uterus, uterine epithelial cells and stromal cells. Moreover, the induction was abrogated by PR antagonist RU486, suggesting PR requirement for this induction. It has previously been reported that C/EBPβ was a critical downstream target of progesterone during decidualization.Citation14 In C/EBPβ-transfected stromal cells, stimulation of progesterone on Hmgn1 expression was obviously attenuated, indicating that C/EBPβ mediates the expression of Hmgn1 in response to progesterone. Additionally, Hmgn1 expression in the ovariectomized mouse uterus and uterine epithelial cells was also induced by estrogen which was required for embryo implantation and decidualization.
In summary, Hmgn1 may act downstream of C/EBPβ and function via Cox-2, mPGES-1 and Vegf to regulate the decidualization of uterine stromal cells. Progesterone and cAMP regulate the expression of Hmgn1 through C/EBPβ.
Materials and Methods
Animals
Matured mice (Kunming white strain) were caged in a controlled environment with a cycle of 14L:10D. All animal procedures were approved by the Institutional Animal Care and Use Committee of Jilin University. To confirm reproducibility of results, at least 3 mice per group were used in each stage or treatment in this study.
Pregnancy and pseudopregnancy
Adult female mice were mated with fertile or vasectomized males of the same strain to induce pregnancy or pseudopregnancy by co-caging, respectively (day 1=day of vaginal plug). On days 1–4, pregnancy was confirmed by recovering embryos from the oviducts or uterus. The implantation sites on day 5 were identified by intravenous injection of 0.1 ml of 1% Chicago blue (Sigma, St. Louis, MO) in 0.85% sodium chloride.
Delayed implantation and activation
To induce delayed implantation, pregnant mice were ovariectomized under ether anesthesia at 08:30–09:00h on day 4 of pregnancy. Progesterone (1 mg/mouse; Sigma) was injected subcutaneously to maintain delayed implantation from days 5 to 7. Estradiol-17β (25 ng/mouse, Sigma) was given to progesterone-primed delayed-implantation mice to activate blastocyst implantation. The mice were sacrificed to collect uteri at 24 h after estrogen treatment. The implantation sites were identified by intravenous injection of Chicago blue solution. Delayed implantation was confirmed by flushing the blastocysts from the uterus.
Artificial induced decidualization
Artificial decidualization was induced by intraluminally infusing 25 µl of sesame oil into one uterine horn on day 4 of pseudopregnancy, while the contralateral uninjected horn served as a control. The mice were killed to collect uteri at 24, 48, 72 or 96 h after artificial induced decidualization. Decidualization was confirmed by weighing the uterine horn and histological examination of uterine sections.
Steroid hormonal treatments
Mature female mice were ovariectomized and, after 2 weeks, given a single injection of estradiol-17β (100 ng/mouse), progesterone (2 mg/mouse) or a combination of the same doses of estradiol-17β and progesterone, respectively. All steroids were dissolved in sesame oil and injected subcutaneously. Control mice received the vehicle only (sesame oil, 0.1 ml/mouse). Mice were sacrificed at different time to collect uteri after the hormonal injections.
Uterine epithelial and stromal cells from day 4 of pregnancy were isolated and cultured as previously described.Citation31 Cultured epithelial and stromal cells were also treated with 1 μM of progesterone or 0.1 nM of estradiol-17β, respectively. For further studies, cells were pretreated with RU486 (1 μM) for 2 h or ICI 182,780 (100 nM) before the addition of progesterone or estrogen, respectively. Progesterone and antagonist were dissolved in ethanol. Controls received the vehicle only.
In situ hybridization
Total RNA from the mouse uteri was reverse-transcribed and amplified with Hmgn1 primers. Hmgn1 forward primer 5′- ATACTGAATCAGGAGAGGCT and reverse primer 5′-CAGGACTTCCATCTTTCACA were designed according to Mus musculus high mobility group nucleosomal binding domain 1 gene (Genbank accession number NM_008251). The amplified fragment (291 bp) of Hmgn1 was cloned into pGEM-T plasmid (pGEM-T Vector System 1, Promega, Madison, WI) and verified by sequencing. Hmgn1-containing plasmid was amplified with the primers for T7 and SP6 to prepare templates for labeling. Digoxigenin (DIG)-labeled antisense and sense cRNA probes were transcribed in vitro using a DIG RNA labeling kit (Roche Diagnostics GmbH, Mannheim, Germany).
Frozen sections (10 μm) were mounted on 3-aminopropyltriethoxy-silane (Sigma)-coated slides and fixed in 4% paraformaldehyde solution in PBS. Hybridization was performed as described previously.Citation18 Sections were counterstained with 1% methyl green. The positive signal was visualized as a dark brown color. The sense probe was also hybridized and served as a negative control. There was no detectable signal from sense probes.
Real-time PCR
Total RNAs from mouse uteri or cultured cells were isolated using TRIPURE reagent according to the manufacturer's instructions (Roche) and reverse-transcribed into cDNA using M-MLV reverse-transcriptase (Promega). Reverse transcriptase was performed at 42°C for 60 min with 2 μg total RNA in 25 μl volume. For real-time PCR, cDNA was amplified using FS Universal SYBR Green Real Master (Roche) on BIO-RAD CFX96TM Real Time Detection System. The conditions used for real-time PCR were as follows: 95°C for 3 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. All reactions were run in triplicate. The result was analyzed using CFX Manager Software. After analysis using the 2−ΔΔCt method, data were normalized to Gapdh expression. Primer sequences for real-time PCR were listed in
Table 1. Primers for real-time PCR
In vitro decidualization
Mouse in vitro decidualization was performed as previously described with modification.Citation18 Briefly, uterine stromal cells were induced for in vitro decidualization with fresh medium supplemented with progesterone (1 μM) and estradiol-17β (10 nM) in DMEM-F12 with 2% charcoal-treated FBS (Biological Industries Ltd., Kibbutz Beit Hemeek, Israel).
Plasmid Construction and Transfection
Full-length Hmgn1 and C/EBPβ cDNA fragments were amplified by PCR from mouse uterus using the following primers: Hmgn1 (5′-GAATTC (EcoRI) ATGCCCAAGAGGAAGGTTAG and 5′- GATATC (EcoRV) GAGACAGTCACAGCCTCTCC) and C/EBPβ (5′- GAATTC (EcoRI) ATGGAAGTGGCCAACTTCTACT and 5′- CTCGAG (XhoI) CTAGCAGTGGCCCGCCGA). The amplified products were purified and cloned into pGEM-T vector. The pGEM-T-Hmgn1, pGEM-T-C/EBPβ and pcDNA3.1 vector were cut by EcoRI/EcoRV or EcoRI/XhoI (TaKaRa, Dalian, China) at 37°C for 1 h, and then the fragments were ligated into pcDNA3.1 with T4 ligase (Promega) at 4°C overnight to construct pcDNA3.1-Hmgn1 and pcDNA3.1-C/EBPβ, respectively. An empty pcDNA3.1 expression vector was served as control.
Transfection of uterine stromal cells was performed according to the manufacturer's protocol for lipofectamine 2000 (Invitrogen). After transfection with control plasmid (empty pcDNA3.1 vector) or overexpression plasmids, stromal cells were collected or induced for in vitro decidualization for 48 h.
RNA interference
The small-interfering RNA (siRNA) duplexes for targeting Hmgn1 and C/EBPβ as well as a scrambled sequence (control siRNA duplex, negative control) were designed and synthesized by GenePharma. The sequences were shown as follows: 5′-GGGAAAGGAUAAAGCAUCATT and 5′-UGAUGCUUUAUCCUUUCCCTT (Hmgn1 siRNA 1); 5′-GUGCAUUCGUGUCU GAAAUTT and 5′-AUUUCAGACACGAAUGCACTT (Hmgn1 siRNA 2); CCUGUCAGAAC UGUGAUGUTT and 5′-ACAUCACAGUUCUGACAGGTT (Hmgn1 siRNA 3); 5′-GCGACG AGUACAAGAUGCGTT and 5′-CGCAUCUUGUACUCGUCGCTT (C/EBPβ siRNA 1); 5′-ACAAGGCCAAGAUGCGCAATT and 5′-UUGCGCAUCUUGGCCUUGUTT (C/EBPβ siRNA 2); 5′-GGAACUUGUUCAAGCAGCUTT and 5′-AGCUGCUUGAACAAGUUCCTT (C/EBPβ siRNA 3); 5′-UUCUCCGAACGUGUCACGUTT and 5′-ACGUGACACGUUCGGAGAATT (nonspecific scrambled siRNA, negative control). Transfections for siRNA were performed according to Lipofectamine 2000 protocol. After transfection with Hmgn1 siRNAs or C/EBPβ siRNAs, uterine stromal cells were collected or induced for in vitro decidualization for 48 h.
Cell proliferation
Proliferation assays were performed using MTS reagent (Promega) according to the manufacturer's directions. Uterine stromal cells were seeded at a density of 1×105/well in 96-well plates and cultured in the DMEM/F12 medium containing 2% heat-inactivated FBS. After transfection with Hmgn1 overexpression plasmid or siRNA, stromal cells were cultured or induced for in vitro decidualization for 48 h. Finally, 20 µl of MTS reagent was added to each well and incubated for 4h. Absorbance was measured at 490 nm using a 96-well plate reader. Every experiment was performed in triplicate.
Statistics
All the experiments were independently repeated at least 3 times. The significance of difference was analyzed by one-way ANOVA or Independent-Samples T Test using the SPSS software program (SPSS Inc., Chicago). The differences were considered significant at P < 0.05.
Disclosure of Potential Conflict of interest
No potential conflicts of interest were disclosed.
Funding
This work was financially supported by Special Funds for Scientific Research on Public Causes (201303119) and National Natural Science Foundation of China (31472158 and 31372390).
References
- Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med 2012; 18:1754-67; PMID:23223073; http://dx.doi.org/10.1038/nm.3012
- Zhang S, Lin H, Kong S, Wang S, Wang H, Wang H, Armant DR. Physiological and molecular determinants of embryo implantation. Mol Aspects Med 2013; 34:939-80; PMID:23290997; http://dx.doi.org/10.1016/j.mam.2012.12.011
- Large MJ, DeMayo FJ. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol Cell Endocrinol 2012; 358:155-65; PMID:21821095; http://dx.doi.org/10.1016/j.mce.2011.07.027
- Tamura I, Ohkawa Y, Sato T, Suyama M, Jozaki K, Okada M, Lee L, Maekawa R, Asada H, Sato S, et al. Genome-wide analysis of histone modifications in human endometrial stromal cells. Mol Endocrinol 2014; 28:1656-69; PMID:25073104; http://dx.doi.org/10.1210/me.2014-1117
- Furusawa T, Cherukuri S. Developmental function of HMGN proteins. Biochim Biophys Acta 2010; 1799:69-73; PMID:20123069; http://dx.doi.org/10.1016/j.bbagrm.2009.11.011
- Kugler JE, Deng T, Bustin M. The HMGN family of chromatin-binding proteins: dynamic modulators of epigenetic processes. Biochim Biophys Acta 2012; 1819:652-6; PMID:22326857; http://dx.doi.org/10.1016/j.bbagrm.2012.01.013
- González-Romero R, Eirín-López JM, Ausió J. Evolution of high mobility group nucleosome-binding proteins and its implications for vertebrate chromatin specialization. Mol Biol Evol 2015; 32:121-31; http://dx.doi.org/10.1093/molbev/msu280
- Furusawa T, Lim JH, Catez F, Birger Y, Mackem S, Bustin M. Downregulation of nucleosomal binding protein HMGN1 expression during embryogenesis modulates Sox9 expression in chondrocytes. Mol Cell Biol 2006; 26:592-604; PMID:16382150; http://dx.doi.org/10.1128/MCB.26.2.592-604.2006
- Deng T, Zhu ZI, Zhang S, Leng F, Cherukuri S, Hansen L, Mariño-Ramírez L, Meshorer E, Landsman D, Bustin M. HMGN1 modulates nucleosome occupancy and DNase I hypersensitivity at the CpG island promoters of embryonic stem cells. Mol Cell Biol 2013; 33:3377-89; PMID:23775126; http://dx.doi.org/10.1128/MCB.00435-13
- Nagao M, Lanjakornsiripan D, Itoh Y, Kishi Y, Ogata T, Gotoh Y. High mobility group nucleosome-binding family proteins promote astrocyte differentiation of neural precursor cells. Stem Cells 2014; 32:2983-97; PMID:25069414; http://dx.doi.org/10.1002/stem.1787
- Belova GI, Postnikov YV, Furusawa T, Birger Y, Bustin M. Chromosomal protein HMGN1 enhances the heat shock-induced remodeling of Hsp70 chromatin. J Biol Chem 2008; 283:8080-8; PMID:18218636; http://dx.doi.org/10.1074/jbc.M709782200
- Rochman M, Taher L, Kurahashi T, Cherukuri S, Uversky VN, Landsman D, Ovcharenko I, Bustin M. Effects of HMGN variants on the cellular transcription profile. Nucleic Acids Res 2011; 39:4076-87; PMID:21278158; http://dx.doi.org/10.1093/nar/gkq1343
- Wang W, Taylor RN, Bagchi IC, Bagchi MK. Regulation of human endometrial stromal proliferation and differentiation by C/EBPβ involves cyclin E-cdk2 and STAT3. Mol Endocrinol 2012; 26:2016-30; http://dx.doi.org/10.1210/me.2012-1169
- Mantena SR, Kannan A, Cheon YP, Li Q, Johnson PF, Bagchi IC, Bagchi MK. C/EBPβ is a critical mediator of steroid hormone-regulated cell proliferation and differentiation in the uterine epithelium and stroma. Proc Natl Acad Sci U S A 2006; 103:1870-5; PMID:16439483; http://dx.doi.org/10.1073/pnas.0507261103
- Wang W, Li Q, Bagchi IC, Bagchi MK. The CCAAT/enhancer binding protein β is a critical regulator of steroid-induced mitotic expansion of uterine stromal cells during decidualization. Endocrinology 2010; 151:3929-40; PMID:20501671; http://dx.doi.org/10.1210/en.2009-1437
- Ramathal C, Wang W, Hunt E, Bagchi IC, Bagchi MK. Transcription factor CCAAT enhancer-binding protein β (C/EBPβ) regulates the formation of a unique extracellular matrix that controls uterine stromal differentiation and embryo implantation. J Biol Chem 2011; 286:19860-71; PMID:21471197; http://dx.doi.org/10.1074/jbc.M110.191759
- Lefèvre PL, Palin MF, Beaudry D, Dobias-Goff M, Desmarais JA, Llerena VEM, Murphy BD. Uterine signaling at the emergence of the embryo from obligate diapause. Am J Physiol Endocrinol Metab 2011; 300:E800-8; http://dx.doi.org/10.1152/ajpendo.00702.2010
- Guo B, Tian XC, Li DD, Yang ZQ, Cao H, Zhang QL, Liu JX, Yue ZP. Expression, regulation and function of Egr1 during implantation and decidualization in mice. Cell Cycle 2014; 13:2626-40; PMID:25486203; http://dx.doi.org/10.4161/15384101.2014.943581
- Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol 2003; 178:357-72; PMID:12967329; http://dx.doi.org/10.1677/joe.0.1780357
- Clementi C, Tripurani SK, Large MJ, Edson MA, Creighton CJ, Hawkins SM, Kovanci E, Kaartinen V, Lydon JP, Pangas SA, DeMayo FJ, Matzuk MM. Activin-like kinase 2 functions in peri-implantation uterine signaling in mice and humans. PLoS Genet 2013; 9:e1003863; PMID:24244176; http://dx.doi.org/10.1371/journal.pgen.1003863
- Pohnke Y, Kempf R, Gellersen B. CCAAT/enhancer-binding proteins are mediators in the protein kinase A-dependent activation of the decidual prolactin promoter. J Biol Chem 1999; 274:24808-18; PMID:10455153; http://dx.doi.org/10.1074/jbc.274.35.24808
- Birger Y, Catez F, Furusawa T, Lim JH, Prymakowska-Bosak M, West KL, Postnikov YV, Haines DC, Bustin M. Increased tumorigenicity and sensitivity to ionizing radiation upon loss of chromosomal protein HMGN1. Cancer Res 2005; 65:6711-8; PMID:16061652; http://dx.doi.org/10.1158/0008-5472.CAN-05-0310
- Sroga JM, Gao F, Ma X, Das SK. Overexpression of cyclin D3 improves decidualization defects in Hoxa-10(−/−) mice. Endocrinology 2012; 153:5575-86; PMID:23008516; http://dx.doi.org/10.1210/en.2012-1528
- Huang Z, Wang TS, Qi QR, Zuo RJ, Liang XH, Zhao XY, Yang ZM. Progesterone regulates secretin expression in mouse uterus during early pregnancy. Reprod Sci 2014; 21:724-32; PMID:24336673; http://dx.doi.org/10.1177/1933719113512527
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 1997; 91:197-208; PMID:9346237; http://dx.doi.org/10.1016/S0092-8674(00)80402-X
- Kennedy TG, Gillio-Meina C, Phang SH. Prostaglandins and the initiation of blastocyst implantation and decidualization. Reproduction 2007; 134:635-43; PMID:17965253; http://dx.doi.org/10.1530/REP-07-0328
- Pakrasi PL, Jain AK. Cyclooxygenase-2 derived PGE2 and PGI2 play an important role via EP2 and PPARdelta receptors in early steps of oil induced decidualization in mice. Placenta 2008; 29:523-30; PMID:18407349; http://dx.doi.org/10.1016/j.placenta.2008.03.001
- Matsumoto H, Ma WG, Daikoku T, Zhao X, Paria BC, Das SK, Trzaskos JM, Dey SK. Cyclooxygenase-2 differentially directs uterine angiogenesis during implantation in mice. J Biol Chem 2002; 277:29260-7; PMID:12034746; http://dx.doi.org/10.1074/jbc.M203996200
- Numao A, Hosono K, Suzuki T, Hayashi I, Uematsu S, Akira S, Ogino Y, Kawauchi H, Unno N, Majima M. The inducible prostaglandin E synthase mPGES-1 regulates growth of endometrial tissues and angiogenesis in a mouse implantation model. Biomed Pharmacother 2011; 65:77-84; PMID:21247731; http://dx.doi.org/10.1016/j.biopha.2010.12.008
- Klauber N, Rohan RM, Flynn E, D'Amato RJ. Critical components of the female reproductive pathway are suppressed by the angiogenesis inhibitor AGM-1470. Nat Med 1997; 3:443-6; PMID:9095179; http://dx.doi.org/10.1038/nm0497-443
- Zhao YC, Chi YJ, Yu YS, Liu JL, Su RW, Ma XH, Shan CH, Yang ZM. Polyamines are essential in embryo implantation: expression and function of polyamine-related genes in mouse uterus during peri-implantation period. Endocrinology 2008; 149:2325-32; PMID:18202119; http://dx.doi.org/10.1210/en.2007-1420
