Error correction is the process by which improper kinetochore-microtubule (kt-MT) attachments are destabilized so that the cell can take another whack at getting things right before anaphase. Getting things right refers to a specific geometric configuration called biorientation in which sister kinetochores attach to dynamic microtubules (MTs) from opposite spindle poles. Every replicated chromosome must biorient to best ensure equal transmission of the genome. However, biorientation is not inherent as erroneous kt-MT attachments form frequently and must be resolved to avoid aneuploidy.
The canonical error correction pathway is mediated by the centromere-enriched aurora B kinase (ABK), which reduces the affinity of mal-oriented kinetochores for MTs by phosphorylating attachment factors such as Ndc80/HEC1. The application of opposing forces to bioriented kinetochores generates tension that stabilizes kt-MT attachments,Citation1 in part, by counteracting the attachment destabilizing activity of ABK. While the exact mechanism by which tension opposes ABK activity has not been entirely resolved, one major hypothesis called the spatial positioning model posits that tension-dependent structural changes in the kinetochore reposition attachment factors further from ABK, decreasing their likelihood of being phosphorylated and; therefore, increasing their affinity for MTs.
It has long been known that error correction often occurs near spindle poles,Citation2 but it was unclear if the polar environment somehow contributed to error correction or was merely the location where improperly attached chromosomes ended up due to force imbalances. Unlike bioriented sister kinetochores that are aligned in the mid-spindle and experience opposing forces from being attached to MTs from opposite spindle poles, erroneously attached sisters are typically pulled together toward one pole. At some point on their journey poleward, these attachments likely encounter opposing forces in a manner distinct from bioriented attachments because of polar ejection forces (PEFs) that push chromosome arms away from the pole. We had previously demonstrated that elevated PEFs stabilize erroneous kt-MT attachments,Citation3 yet the spindle poles are where PEFs are highest. Thus, spindle poles are an intriguing environment for error correction to take place.
Work by our lab and othersCitation4,5 recently characterized a conserved pole-based error correction pathway with interesting similarities and differences to the canonical CEN-based pathway (). Aurora A kinase (AAK), a close relative of ABK that shares a nearly identical consensus phosphorylation motif, is a key mediator of pole-based error correction. We identified the attachment factor Ndc80/HEC1, a well-characterized target of the CEN-based pathway, as an AAK substrate in vitro and in cells. Thus, we believe that the 2 pathways share many of the same targets. However, a major difference between the pathways is that, unlike CEN-based error correction, tension does not oppose the pole-based pathway; rather, positioning of kinetochores within the spindle relative to the poles is the primary determinant of kt-MT attachment stability. Despite this difference, at the heart of both pathways lies the central principle that spatial positioning of kinetochore substrates relative to their kinase defines kt-MT attachment stability.
Figure 1. Centromere vs. pole-based error correction. Attachment factors such as the Ndc80 complex are phosphorylated at unattached kinetochores by centromere (CEN)-based aurora B kinase (ABK). Biorientation produces tension that opposes CEN-based error correction by spatially positioning attachment factors away from the centromere and into a zone of lower ABK activity. Mal-oriented kinetochore attachments, regardless of their tension state, are phosphorylated by aurora A kinase (AAK) in the vicinity of spindle poles. Once polar kinetochores become tensionless or detached, they are likely hyper-phosphorylated by ABK and AAK working in concert.
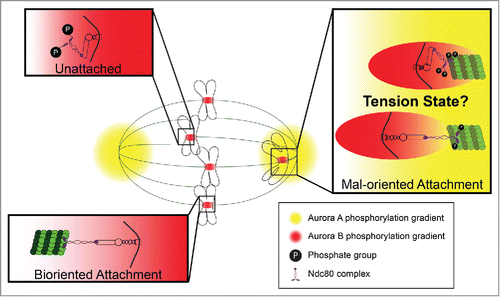
Of course it's all relative and; interestingly, the CEN and pole-based error correction machinery appear to act over distances that differ by orders of magnitude. Regarding the CEN-based pathway, tension-dependent structural changes within the kinetochore spatially position outer kinetochore substrates away from the inner kinetochore by distances in the tens of nanometers range. To the contrary, kt-MT attachment destabilization by the pole-based pathway occurs over microns as nicely evidenced by the fact that poleward moving kinetochores in mouse oocytes became unattached when approaching within ∼2–3 microns of the pole, an observation consistent with our visualization of a micron-scale, pole-centered AAK gradient in mitotic cells. The basis for such strong differences in the working distances of CEN-based ABK and pole-based AAK is unclear. Potential explanations include differences in the concentrations and turnover kinetics of each kinase at their respective locales and/or differences in the high activity “lifetimes” of each kinase either due to intrinsic properties of each kinase or their regulation via gradient amplifiers or dampeners. Alternatively, it has been suggested that the working distance of ABK is not defined solely by a diffusion-based gradient but rather by being physically tethered to the centromere by its binding partner and activator INCENP.Citation6 Recent work in chicken cells suggest that INCENP may indeed function as a so-called “dog leash” for ABK.Citation7 Importantly, the dog leash and gradient models are not mutually exclusive and both acting together could contribute to a zone of very high ABK activity in close proximity (nanometer scale) to the kinetochore.
In concluding, we propose that high fidelity error correction during cell division requires both a CEN-based pathway mediated principally by ABK and a pole-based pathway to which AAK contributes. We envision that efficient and timely error correction would be promoted by synergies between the 2 pathways, for example, via pole-localized transactivation or through the sum of ABK and AAK activities at polar tensionless kinetochores. However, because the pole-based pathway is unaffected by tension, even if erroneous kinetochores experience opposing forces near spindle poles, the attachments would still be destabilized by AAK. Once detached, the combined effects of ABK and AAK should prevent the re-establishment of improper kt-MT interactions and promote biorientation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Akiyoshi B, et al. Nature 2010; 468:576-9; PMID: 21107429; http://dx.doi.org/10.1038/nature09594
- Lampson MA, et al. Nat Cell Biol 2004; 6:232-7; PMID:14767480; http://dx.doi.org/10.1038/ncb1102
- Cane S, et al. J Cell Biol 2013; 200:203-18; PMID: 23337118; http://dx.doi.org/10.1083/jcb.201211119
- Chmatal L, et al. Curr Biol 2015; 25:1835-41; PMID: 26166779; http://dx.doi.org/10.1016/j.cub.2015.05.013
- Ye AA, et al. Curr Biol (2015) 25:1842-51; PMID: 26166783; http://dx.doi.org/10.1016/j.cub.2015.06.021
- Santaguida S, Musacchio A. EMBO J (2009) 28:2511-31; PMID:19629042; http://dx.doi.org/10.1038/emboj.2009.173
- Samejima K, et al. J Biol Chem 2015; 290:21460-72; PMID:26175154; http://dx.doi.org/10.1074/jbc.M115.645317
