Immune memory, generally considered to be a characteristic of the adaptive immune system, is crucially important for resistance to reinfection by specific pathogens. On the other hand, recent experimental and epidemiological studies indicate that the innate immune system also has functions similar to immune memory. For example, plants and invertebrates such as insects, which lack adaptive immune systems, still exhibit immune memory-like behavior. Furthermore, BCG (Bacille-Calmette-Guerin) vaccination in childhood in West Africa reduces overall mortality by enhancing resistance to multiple pathogens other than tuberculosis. Although accumulating evidence suggests that innate immune memory (also known as “trained immunity”) exists, the issue remains controversial within the field, in part because its molecular mechanism is unknown.
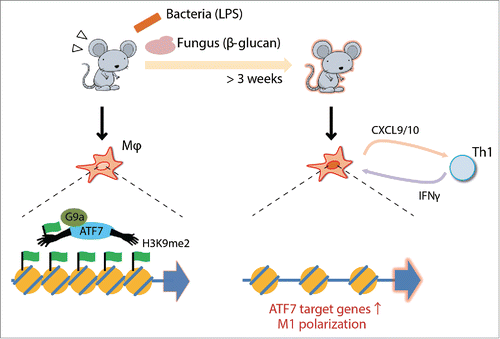
We found that the transcription factor ATF7 is a key regulator of epigenetic memory induced by pathogen infection, which contributes to innate immune memoryCitation1 (). ATF7 belongs to the ATF2 subfamily, which plays a critical role in epigenetic memory.Citation2 In peritoneal macrophages at steady state, ATF7 binds to a group of innate immunity-related genes and silences their expression by introducing a repressive epigenetic mark, H3K9me2, via recruitment of the histone dimethyltransferase G9a. Following LPS (lipopolysaccharide) treatment, ATF7 is phosphorylated by p38 and released from chromatin, leading to disruption of heterochromatin and a reduction in the level of H3K9me2. Once ATF7 is released from chromatin, it is not entirely recruited back to the original binding sites, contributing to maintenance of partially disrupted heterochromatin. Such long-term maintenance of epigenetic changes in macrophages enhances resistance to pathogens.
Figure 1. Schematic view of ATF7-mediated innate immune memory in macrophages. The ATF7-G9a complex forms heterochromatic structures by introducing H3K9me2. The complex is released from chromatin upon bacterial or fungal infection, thereby reducing the level of H3K9me2 and disrupting heterochromatin. Macrophages primed by infection express higher levels of ATF7 target genes, including M1 marker genes that affect Th1 activity.
A recent study showed that pre-treatment of human monocytes with β-glucan during in vitro cultivation increases the level of H3K27Ac and H3K4me3 on a set of innate immune genes in the resultant differentiated macrophages,Citation3 suggesting that TLR ligands induce epigenetic changes during differentiation from monocytes into macrophages. On the other hand, although all types of macrophages were once believed to arise from monocytes circulating in the blood, a series of studies in the last decade showed that 2 subsets of tissue macrophage exist, and that they originate from different lineagesCitation4: one population is derived from monocytes in adult blood, and the other is established during embryonic development and stably maintained until adulthood, independently of monocytes. Around 90% of peritoneal macrophages are of the latter type, suggesting that pathogen infection induces epigenetic changes primarily in macrophages of embryonic origin. Furthermore, generation of H3K27Ac and H3K4me3 is stimulated by the transcription-coupled recruitment of the coactivator CBP (CREB Binding Protein) and ubiquitination of histone H2B, respectively. These findings suggest that elevation of H3K27Ac and H3K4me3 levels might be due to transcriptional induction caused by the sustained reduction in H3K9me2 on ATF7 target genes.
We found that treatment with some TLR ligands that mimic infection by Gram-positive and -negative bacteria, fungi, and viruses can also induce innate immune memory. Administration of LPS (TLR4 ligand from virulent E. coli), β-glucan (TLR2 and Dectin-1 ligand from C. albicans), and peptidoglycan (TLR2 from B. subtilis) maintained the elevated expression of the ATF7-regulated genes in macrophages, whereas administration of imiquimod, which has a structure similar to that of single-stranded viral RNA, did not. This observation suggests that ATF7 mediates bacteria- and fungi-induced innate memory, but not virus-induced innate memory. After priming with LPS or β-glucan, elevated expression of genes associated with infectious disease and immunity were maintained for long periods, consistent with the maintenance of enhanced resistance to various pathogens. Because M. tuberculosis produces various macrophage-activating factors, including ligands for TLR and dectin-1, BCG-induced innate immune memory may also be mediated by ATF7.
Macrophages treated with LPS or β-glucan maintain the phenotypic signature of M1-type polarization in vivo, and STAT1 appears to play a key mode in this regulation. This observation is consistent with the fact that IRF/STAT pathway is critical for M1 macrophage polarization.Citation5 The M1-marker chemokines, CXCL9/10, whose expression is most strongly induced by LPS administration, promote recruitment of TH1 cells, making TH1 predominant over TH2. Maintained expression of such chemokines may explain why bacterial infection during infancy continues to activate TH1 cells. According to the “hygiene hypothesis,” this diminishes susceptibility to allergy in adulthood,Citation6 suggesting that genes whose upregulation is maintained for long periods by innate immune memory could be used to monitor susceptibility to allergy.
These findings regarding innate immune memory also force us to reconsider the mechanism of adjuvants, which activate innate immunity and are required for effective vaccines. Although activation of innate immunity by adjuvants was previously thought to last only a few days,Citation7 it is now widely believed that adjuvants continue to activate innate immunity for longer periods. This information may improve screening methods used to identify more effective adjuvants, e.g., by monitoring the expression levels of genes whose upregulation is maintained for long period by innate immune memory. Thus, understanding of the mechanism of innate immune memory is important not only for fundamental research on the immune system but also for potential clinical applications.
Disclosure of potential conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Yoshida K, et al. Nat Immunol 2015; 16:1034-43. PMID:26322480; http://dx.doi.org/10.1038/ni.3257
- Seong KH, et al. Cell 2011; 145:1049-61. PMID:21703449; http://dx.doi.org/10.1016/j.cell.2011.05.029
- Saeed S, et al. Science 2014; 345:1251086. PMID:25258085; http://dx.doi.org/10.1126/science.1251086
- Epelman S, et al. Immunity 2014; 41:21-35. PMID:25035951; http://dx.doi.org/10.1016/j.immuni.2014.06.013
- Wang N, et al. Front Immunol 2014; 5:614. PMID:25506346; http://dx.doi.org/10.3389/fimmu.2014.00614
- Yazdanbakhsh M, et al. Science 2002; 296:490-4. PMID:11964470; http://dx.doi.org/10.1126/science.296.5567.490
- Benn CS, et al. Trends Immunol 2013; 34:431-9. PMID:23680130; http://dx.doi.org/10.1016/j.it.2013.04.004
