AKT (alias protein kinase B, PKB), the "grand dame" among the Serine/Threonine kinases, has been mostly studied for its roles in proliferation, (anti)-apoptosis, protein synthesis, and metabolism (). Although first reports linking AKT and stemness date back as far as nearly a decade ago,Citation1 it has been a long way until an immediate regulatory significance for pluripotency was assigned to AKT kinase, that complements its traditional roles.
Figure 1. The Ser/Thr kinase AKT is a dominant upstream mediator of various cellular effects including cell-cycle progression, protein synthesis, anti-apoptosis and metabolism. Schulze-Osthoff and team now deciphered AKT-dependent phosphorylation patterns within the key pluripotency-inducing transcription factors OCT4, SOX2 and KLF4.Citation6 Suggesting immediate regulatory significance, the identified modification sites cluster in sequence elements implicated in nuclear transport and DNA binding (blue). These results are in line with recent reports on functional roles of AKT signaling in pluripotency and stemness (red). Cytoplasm (faint gray), nucleus (dark gray).
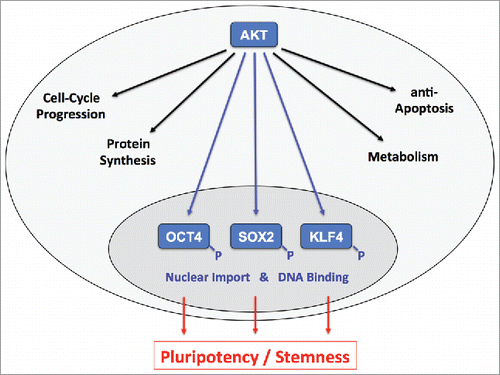
Several lines of evidence, gathered in both murine and human systems, now add a new facet to this kinase as they unravel links between AKT and the major pluripotency factors OCT4, SOX2 and KLF4. Connections are manifold and comprise functional interactions as well as altered gene expression, protein turnover and subcellular localization in response to AKT. Molecularly, some of these effects involve complex regulatory circuits, such as the recently described AKT-dependent regulation of SOX2 expression in nasopharyngeal cancer via modulation of p27 and miR-30a.Citation2 However, AKT was also shown to directly phosphorylate OCT4, SOX2 and KLF4Citation3-5 and the regulatory significance of these interactions is just slowly emerging.
Schulze-Osthoff and co-workers make a remarkable contribution to this field, as they provide a systematic AKT-dependent phosphorylation site analysis of the indicated pluripotency transcriptional triad.Citation6 By using baculovirus-transduced insect cells they establish a system where nuclear expression of OCT4, SOX2, and KLF4 proteins was derived from an organism providing for eukaryotic secondary modifications at the natural subcellular compartmentalization, which offers overt advantages over bacterial expression systems. Protein expression was achieved via GST-fusion constructs enabling a simple, one-step affinity purification with nearly homogeneous recovery of target proteins in their native conformation, as further confirmed by DNA-binding assays. Using HPLC-MS analyses, the authors identified several novel AKT phosphorylation sites in all 3 proteins. Interestingly, residues of AKT-dependent phosphorylation seemingly cluster in sequence elements either implicated in nucleo-cytoplasmatic transport or DNA-binding. Although these results await functional verification, they are in line with recent reports discussing AKT-dependent subcellular localization of SOX2 and OCT4 proteins.Citation3,7 The overt co-localization of phosphorylation sites in DNA-binding domains further has important implications since it suggests that AKT directly influences transcriptional activity of pluripotency factors.
Interestingly, a previously established Thr118 phosphorylation site within murine Sox2Citation4 (in human: Thr116) was not retrieved in this assay. This discrepancy may either reflect technical limitations of the experimental approach (e.g. deviant protein glycosylation in insect vs. human cells), or provide valuable hints toward isoform-specific phosphorylation patterns as perhaps imposed by AKT2 or AKT3, which were excluded from the present analysis. Indeed, further putative phosphorylation sites may have been missed as a result of stringent background correction (i.e. phosphor-modifications set during protein expression were not considered, but may have masked actual AKT1 sites), incomplete sequence coverage in spite of complementary MS-digests, or putative sequence masking by the affinity tag.
Of note, a deregulation of canonical AKT signaling is a common determinant in various cancers, and advanced clinical testing of several PI3K/AKT pathway blockers is currently under way. The notion that AKT kinase inhibition not only impairs growth but may also target stemness adds a novel and exciting facet to these studies. Indeed, several recent reports in different tumor entities (e.g. breast cancerCitation7 and nasopharyngeal carcinomaCitation2) underscore the potential of AKT inhibitors in the targeting of putative cancer stem cells. Even if not always confined to a stem cell compartment, expression of pluripotency factors mostly (co)-marks tumor-initiating cells and confers important oncogenic properties that could be addressed by AKT inhibitors. However, the dependence of pluripotency factor expression on AKT may depend on tissue-specific factors and therefore needs to be studied individually in specific cellular entities.Citation7
It is tempting to envision that AKT inhibitors, which were mostly brought to clinical trials in cancer patients based on their anti-proliferative and pro-apoptotic effects, may additionally target tumor cell stemness. However, inspite encouraging recent results, the role of AKT inhibition on putative cancer stem cells remains to be defined.
Taken together, research on AKT kinase currently experiences a renaissance in the context of pluripotency and stemness. By identifying a magnitude of novel phosphorylation sites, Schulze-Osthoff and coworkers highlight the importance of AKT for the regulation of transcriptional hallmarks of stemness and pluripotency, providing exciting perspectives for future studies.
References
- Watanabe S, et al. Oncogene 2006; 25(19):2697-707; PMID:16407845; http://dx.doi.org/10.1038/sj.onc.1209307
- Qin J, et al. Oncotarget 2015; 6(9):6944-58; PMID:25749514; http://dx.doi.org/10.18632/oncotarget.3128
- Lin Y, et al. Mol Cell 2012; 48(4):627-40; PMID:23041284; http://dx.doi.org/10.1016/j.molcel.2012.08.030
- Joeng CH, et al. Stem Cells 2010; 28(12):2141-50; PMID:20945330; http://dx.doi.org/10.1002/stem.540
- Chen B, et al. PLoS One 2013; 8(6):e64877; PMID:23762260; http://dx.doi.org/10.1371/journal.pone.0064877
- Malak P, et al. Cell Cycle 2015; PMID:25970831 http://dx.doi.org/10.1080/15384101.2015.1104444
- Schaefer T, et al. Oncotarget 2015; http//dx.doi.org/10.18632/oncotarget.6183.
