ABSTRACT
The E2F transcription factors are primarily implicated in the regulation of entry and exit from the cell cycle. However, in vivo studies have established additional roles for E2Fs during organ development and homeostasis. With the goal of addressing the intestinal requirements of E2f4 and E2f5, we crossed mice carrying Vil-cre, E2f4 conditional and E2f5 germline alleles. E2f4 deletion had no detectable effect on intestinal development. However, E2f4f/f;E2f5+/−;Vil-cre males, but not E2f4f/f;Vil-cre littermates, were unexpectedly sterile. This defect was not due to defective spermatogenesis. Instead, the seminiferous tubules and rete testes showed significant dilation, and spermatozoa accumulated aberrantly in the rete testis and efferent ducts. Our data show that these problems result from defective efferent ducts, a tissue whose primary function is to concentrate sperm through fluid absorption. First, Vil-cre expression, and consequent E2F4 loss, was specific to the efferent ducts and not other reproductive tract tissues. Second, the E2f4f/f;E2f5+/−;Vil-cre efferent ducts had completely lost multiciliated cells and greatly reduced levels of critical absorptive cell proteins: aquaporin1, a water channel protein, and clusterin, an endocytic marker. Collectively, the observed testis phenotypes suggest a fluid flux defect. Remarkably, we observed rete testis dilation prior to the normal time of seminiferous fluid production, arguing that the efferent duct defects promote excessive secretory activity within the reproductive tract. Finally, we also detect key aspects of these testis defects in E2f5−/− mice. Thus, we conclude that E2f4 and E2f5 display overlapping roles in controlling the normal development of the male reproductive system.
Introduction
In mammals the E2F family of transcription factors comprises 8 genes that together regulate genes required for cell proliferation throughout the cell cycle.Citation1-3 E2Fs 1 through 6 function as heterodimers with members of the DP family of transcription factors while E2F7 and E2F8 function independently. E2Fs 1 through 3 are thought to function primarily as transcriptional activators while E2Fs 4 through 8 repressors of transcription, however, studies of mutant mice indicate that these assignments are not definitive.Citation4,5 The activity of E2Fs 1 through 5 is controlled by association with members of the pocket protein family, which includes the tumor suppressor protein pRB and its relatives p107 and p130. These can inhibit the E2Fs transcriptional activity and also form repressive complexes via recruitment of histone deacetylases (HDACs) or the DREAM complex.Citation6,7 Mitogenic signaling leads to phosphorylation of the pocket proteins by cyclin dependent kinases and complex dissociation enabling de-repression of E2F-target genes.
Analyses of mutant mice and also cell lines has demonstrated that many E2Fs function redundantly.(reviewed in ref.Citation8) For example, failure to enter the cell cycle in mouse embryo fibroblasts (MEFs) only occurs following mutation of all 3 activator E2fs, E2f1, E2f2 and E2f3, while many of the in vivo phenotypes of the single E2f1 or E2f3 mutant mice are exacerbated by double mutation of these genes.Citation9-13 Functional redundancy was thought to explain why mutation of the repressor E2f4 did not lead to E2F-target gene derepression in MEFs, despite the fact that E2F4 is the most abundant E2F in MEFs.Citation14-16 Supporting this idea, the analyses of E2f4 and E2f5 mutant MEFs showed that only double mutant MEFs fail to cell cycle arrest following expression of the cyclin dependent kinase inhibitor p16INK4a, which functions via preventing pocket proteins dissociation from E2Fs.Citation17
Despite the modest effects of E2f4 knockout on target genes, E2F4 was found to be essential for normal viability.Citation14,15 We showed that the postnatal death of the E2f4 mutant mice was due to a failure of multiciliated cell development in the airway epithelium, which results in chronic rhinitis and an increased susceptibility to opportunistic infections.Citation15,18 The loss of ciliated cells from the airway arose in the absence of detectable changes in cell proliferation suggesting that E2F4 had non-cell cycle related functions.Citation18 Interestingly this phenotype was also observed in E2f4+/−;E2f5−/− double mutants but not E2f4+/− or E2f5−/− single mutants suggesting functional redundancy between these 2 structurally related E2Fs in multiciliogenesis (P.S.D, J. Sero and J.A.L, unpublished observations). E2f4−/−;E2f5−/− double mutant animals are not viable and die in utero, reinforcing the notion that functional redundancy can occur between these 2 proteins.Citation17
E2f4 mutant mice were also reported to exhibit atrophy of the intestine and several cell line studies have suggested that E2F4 plays a role in the proliferation of intestinal epithelial cells.Citation14,19,20 However, we only observed degeneration of the intestinal tract in the moribund E2f4−/− neonates, suggesting that it was an indirect consequence of dehydration and starvation of these animals.Citation15 Accordingly, E2f4−/− mice were shown to be fully viable when placed on antibiotics, to prevent respiratory infections, indicating that E2F4 is not essential for intestinal development.Citation15 However, this did not rule out the possibility that E2F4 and E2F5 exhibit functional redundancy in the intestine. With the goal of addressing this question, we intercrossed mice carrying E2f4 conditional and E2f5 germline mutant alleles with an allele that expresses Cre recombinase in the intestine (Vil-cre). We found that E2F4 is not required for normal intestinal development and homeostasis. However, because Vil-cre is also expressed in the efferent ducts of the male reproductive tract, we discovered that E2F4 and E2F5 are essential for the normal tract development and fertility.
In the reproductive system of male mammals spermatozoa arise in the seminiferous tubules of the testis, which ultimately coalesce in the rete testis. The rete testis is directly connected to the efferent ducts, which subsequently connect to the epididymis allowing sperm to move from the testis to the Vas deferens and finally the urethra. The primary function of the efferent ducts is considered to be the reabsorption of 50% to 96% of the luminal seminiferous fluid, resulting in the concentration of sperm during their passage to the epididymis.Citation21 The efferent ducts also contain multiciliated cells with motile cilia that are believed to stir the luminal fluid, rather than regulating its flow from the testis to the epididymis.Citation21 In this paper we show that mutation of E2f4 within the efferent ducts in combination with heterozygous mutation of E2f5 leads to a loss of multiciliated cells from the efferent ducts, dilation of the seminiferous tubules, dilation of the rete testis and infertility. In addition we show that homozygous mutation of E2f5 results in a partial phenotype indicating again that E2F4 and E2F5 function redundantly. These data therefore further illustrate that the E2F family of transcription factors plays critical roles in organ development in mammals.
Results
Mutation of E2f4 and E2f5 in the efferent ducts leads to male sterility
To analyze the function of E2F4 alone, and in combination with other E2Fs, in a tissue specific manner we generated a conditional (floxed) allele of E2f4 (manuscript in preparation). This allele was validated by confirming that expressing Cre recombinase throughout the early embryo recapitulated the classic defects of the E2f4 germline mutant mice (data not shown). We then initiated experiments to determine if E2F4 and E2F5 play overlapping roles in the development or homeostasis of the intestine using the Vil-cre transgene which expresses Cre recombinase throughout the intestinal epithelium from embryonic day 12.5.Citation22 We found that E2f4f/f;Vil-cre adult mice (n>10) were viable and fertile and that the intestine appeared morphologically normal (Fig. S1). Immunohistochemical (IHC) analyses confirmed that E2F4 was completely absent from the intestinal epithelium of E2f4f/f;Vil-cre mice (Fig. S1), indicating efficient mutation of E2f4. Thus, we conclude that E2F4 is not essential for development or homeostasis of the intestine in this model. We then crossed the E2f4f/f;Vil-cre mice with E2f5 germline mutant animals.Citation23 In generating these crosses, we discovered that male E2f4f/f;E2f5+/−;Vil-cre mice were sterile despite being able to plug females (n > 5). Males of all other genotypes, including E2f4f/f;E2f5+/+;Vil-cre, E2f4+/f;E2f5+/−;Vil-cre and E2f4+/f;E2f5+/+;Vil-cre, were fertile (n>5 per genotype) indicating that the levels of E2F5 expression are critical for fertility in the absence of E2F4.
E2f5−/− mutant mice develop hydrocephalus and show early lethality.Citation23 In our hands, these animals require euthanasia by 3 weeks of age (data not shown). Thus, to further investigate the infertility phenotype we analyzed males derived from crossing E2f4+/f;E2f5+/−;Vil-cre males with E2f4f/f females. Previous studies had reported that Villin, in addition to being expressed in the intestine, is expressed in the efferent ducts and fallopian tube epithelium.Citation24 Since the efferent ducts connect the rete testis to the epididymis,Citation21 it seemed likely that defects within the efferent duct epithelium were causing the observed infertility. To validate the expression of Vil-cre in the male reproductive tract, we generated E2f4+/f;E2f5+/−;Vil-cre males carrying a reporter allele which expresses the fluorescent protein ZsGreen1 following Cre mediated recombination.Citation25 We detected strong ZsGreen1 expression in the efferent ducts of the male reproductive system, but no significant expression in the testis or the epididymis, demonstrating that the Cre recombinase was active in cells giving rise to or constituting the efferent ducts (Fig. S2). Villin is also expressed in the ciliated epithelium of the fallopian tube Citation24 but the Vil-cre transgene did not express efficiently in the fallopian tube epithelium of female mice (data not shown), precluding analysis of the role of E2f4 and E2f5 in this setting.
Having established that Vil-cre was expressed in the efferent ducts we next analyzed the efficacy of recombination at the conditional E2f4 locus in this epithelium by examining the expression of E2F4 by IHC staining. In control mice E2F4 is expressed sporadically in most cells of the adult efferent duct epithelium at varying levels, with particularly high expression occurring in nuclei furthest from the basement membrane (Fig. S3). E2F4 expression was absent from the efferent duct epithelium in E2f4f/f;Vil-cre adult mice indicating efficient Vil-cre-mediated recombination of the locus (Fig. S3). The efferent duct epithelium of E2f4f/f;Vil-cre mice was morphologically indistinguishable from wild-type epithelium (n>10) demonstrating that loss of E2F4 alone does not significantly compromise efferent duct development.
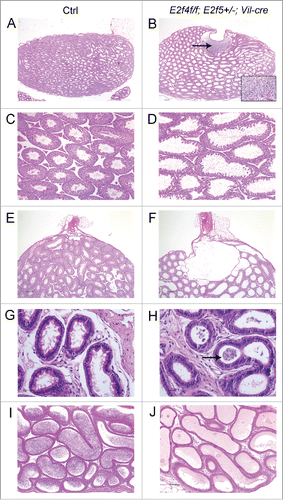
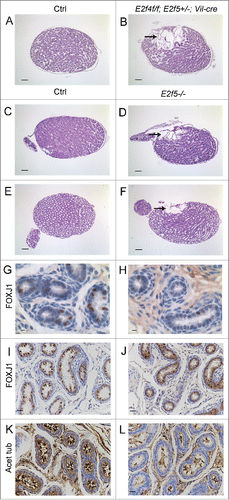
To ascertain the reason for the infertility, we conducted histological analyses of the reproductive organs of littermate controls (E2f4+/f;E2f5+/+ or E2f4+/f;E2f5+/− mice with or without Vil-cre, as well any genotype that lacked Vil-cre) and E2f4f/f;E2f5+/−;Vil-cre adult male littermates between 2 and 6 months of age. In some crosses mice also carried the ZsGreen1 Cre-reporter allele. This analysis showed several major pathological features in the E2f4f/f; E2f5+/−;Vil-cre adult males, and not the controls, that were outside the efferent ducts. First, the seminiferous tubules of the testis were dilated in all E2f4f/f;E2f5+/−;Vil-cre animals (n > 12 ; ), relative to littermate controls (). In many tubules, the lumen was dilated at the expense of the epithelium, which exhibited atrophy. Measurements showed a statistically significantly decrease in the total epithelium height of the tubule, relative to the total tubule diameter, at both 2 months (controls, 67+/−10.6% v mutants, 39.3+/−12.6%, p<0 .0001) and 4 months (controls, 66.3+/−9.8% v mutants, 40+/−15.1%, p<0 .0001) of age (Fig. S4). Despite this disruption of seminiferous tubule morphology, histological examination indicated that all the stages of spermatogenesis were present. Thus, spermatogenesis was not compromised in these mutant animals (Fig. S5). Second, the rete testis was also substantially dilated in E2f4f/f;E2f5+/−;Vil-cre males (n = 6/6) and often contained many spermatozoa (). Spermatozoa were detected in the efferent ducts of 11/13 E2f4f/f;E2f5+/−;Vil-cre males indicating that there was not a physical block in the tubules preventing the transition of spermatozoa from the rete testis into the efferent ducts (). However, the presence of spermatozoa in the ducts was aberrant, as spermatozoa should not accumulate in the efferent ducts due to the normally rapid transit time. Thus, the passage of sperm from the efferent ducts into the epididymis was compromised in the E2f4f/f;E2f5+/−;Vil-cre males. Third, no spermatozoa were observed in the epididymis of 9/12 E2f4f/f;E2f5+/−;Vil-cre males (), and very few were seen in the remaining 3/12 males. In addition, the lumen of the epididymis was often filled with material that stains pink following hematoxylin and eosin staining, which is typically observed when spermatozoa fail to reach the epididymis.Citation26,27 In contrast, spermatozoa were detected in the epididymis of littermate males of all control genotypes (n>12 ; ). The primary function of the efferent ducts is considered to be the reabsorption of luminal fluid, with estimates of the amount of seminiferous fluid being reabsorbed ranging from 50% to 96%.Citation21 Given the above defects, our findings suggest that defective efferent duct function impedes the flow of seminiferous tubule luminal fluid, causing it to back up in the testis and induce seminiferous tubule and rete testis dilation, and impair passage of spermatozoa into the epididymis, yielding infertility.
Figure 1. Mutation of E2f4 and E2f5 leads to seminiferous tubule and rete testis dilation and a failure of spermatozoa to reach the epididymis. (A) Sagittal sections of adult testis from control (Ctrl) and (B) E2f4f/f;E2f5+/−;Vil-cre littermates showing dilation of the seminiferous tubules and rete testis as well as spermatozoa within the rete testis (arrow), and inset image in the mutant. (C) Seminiferous tubules from control and (D) mutant testis showing tubule dilation. (E) Section through the rete testis of control and (F) mutant testis showing rete testis dilation. (G) The efferent ducts of control and (H) mutant testis showing spermatozoa within the efferent ducts of the mutant (arrow). (I) The epididymis of control adults contains spermatozoa in contrast with the mutants (J) where none are detected. All tissue sections were stained with hematoxylin and eosin. Scale bars in A and B, 250 μm; E and F, 200 μm; C, D, I and J, 100 μm; G and H, 20 μm.
The efferent duct epithelium of infertile males lacks multiciliated cells
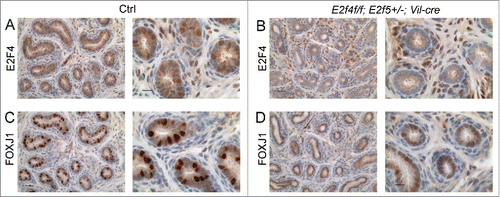
The efferent duct epithelium is comprised of absorptive cells and multiciliated cells with motile cilia.Citation21 Since we showed previously that E2F4 is required for multiciliogenesis in the airway,Citation18 we began by assessing the development of the multiciliated cells in the mutants. As indicated above, spermatozoa were detected in the efferent ducts of most mutant animals indicating that the ducts were patent, but the entire efferent duct epithelium in all adult E2f4f/f;E2f5+/−;Vil-cre males lacked ciliated cells (n>10 ; ). This was verified by IHC staining for acetylated α-tubulin, a component of motile cilia (n = 4/4; ). IHC analysis verified that E2F4 expression was lost from the efferent duct epithelium in the E2f4f/f;E2f5+/−;Vil-cre animals (n = 4/4; ). We then analyzed the expression of FOXJ1, a transcription factor required for multiciliogenesis and a marker of multiciliated cells.Citation28,29 In the efferent duct epithelium of E2f4f/f;E2f5+/−;Vil-cre adult males there was a significant reduction in the number of cells that expressed normal levels of FOXJ1, relative to the controls, although many cells expressed very low levels of FOXJ1 (). Quantification of the percentage of nuclei in the epithelium expressing normal levels of FOXJ1 in mutant versus control littermates (n=4 for each genotype) showed that this difference was statistically significant (8.4+/−4% vs. 27.5+/−6%, p < 0.0001). To investigate whether FOXJ1 expression was initiated normally, we examined expression in the efferent ducts at 1 week of age. At this stage of development, where Vil-cre deleted E2F4 in the efferent ducts, staining of adjacent sections for FOXJ1 showed substantially reduced expression levels in E2f4f/f;E2f5+/−;Vil-cre males relative to the controls (n=4/5; ). These results show that efferent duct epithelial development is disrupted at early stages and, even though some cells in the epithelium of E2f4f/f;E2f5+/−;Vil-cre adults express normal levels of FOXJ1, multiciliogenesis fails to occur.
Figure 2. Efferent ducts of adult E2f4f/f;E2f5+/−;Vil-cre mutants lack multiciliated cells. (A) Cilia project into the lumen from the apical surface of multiciliaited cells in controls (Ctrl) but are absent from mutants (B), sections stained with hematoxylin and eosin. (C) Immunohistochemical staining for acetylated tubulin (brown stain) of cilia shows multiciliated cells in controls but not mutants (D). The staining within the lumen of the mutants corresponds to the flagella of spermatozoa. (E) Immunohistochemical staining for E2F4 shows nuclear staining (brown stain) in nuclei of the efferent duct epithelium in the controls but not mutants (F). (G) Immunohistochemical staining for a multiciliated cell expressed transcription factor, FOXJ1 (brown stain) shows nuclear staining in the controls and reduced expression in the mutants (H). Scale bars in C, D, G and H 20 μm; A, B, E and F 10 μm.
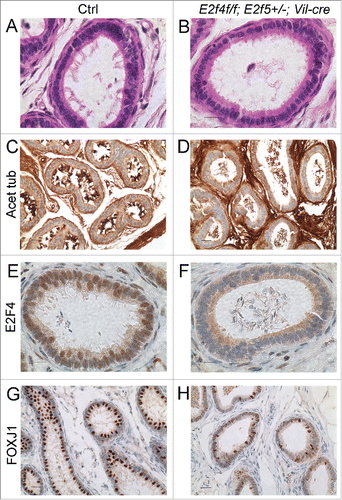
Figure 3. Expression of a multiciliated cell marker, FOXJ1 is reduced early during efferent duct development. Immunohistochemical staining for E2F4 or FOXJ1 (brown stains) in efferent ducts from one week old littermates. (A) Control efferent ducts (Ctrl) showing nuclear expression of E2F4 and (B) the loss of E2F4 from E2f4f/f;E2f5+/−;Vil-cre efferent duct epithelium at this stage. Note that mesenchymal expression of E2F4 is not lost in the mutants. (C) Robust expression of FOXJ1 in the controls in comparison with weak expression in the mutants (D). Scale bars: for each set, left hand panel 20 μm and the right hand panel 10 μm.
Mutation of E2f4 and E2f5 disrupts non-ciliated cell development in the efferent ducts
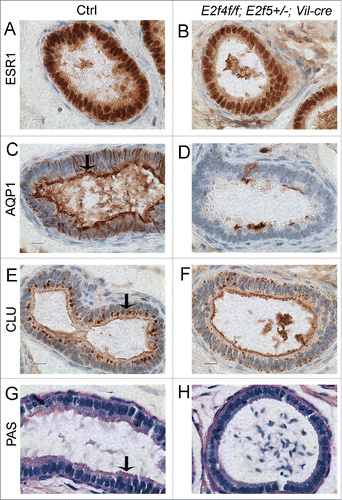
Since the motile cilia are believed to stir the fluid rather than directing its flow from the testis to the epididymis,Citation21 the loss of motile cilia was unlikely to fully explain the dilation of the seminiferous tubules and rete testis in the E2f4f/f;E2f5+/−;Vil-cre males. As E2F4 is expressed in both ciliated and non-ciliated cells of the efferent duct epithelium, we assessed the function of the non-ciliated cells in E2f4f/f;E2f5+/−;Vil-cre mice by analyzing the expression of markers associated with efferent duct absorptive cell function. Initially, we examined the expression of Estrogen receptor 1 (ESR1) which is expressed in both ciliated and non-ciliated cells of the efferent duct epithelium and required for reabsorption of luminal fluid (reviewed in refs.Citation30,31) Our analyses showed the ESR1 was still expressed in mutant males, albeit at a slightly lower level (). We then examined the expression of the water channel protein aquaporin1 (AQP1), involved in water transport across the efferent ducts, and clusterin (also known as SGP-2 or apolipoprotein J), a marker of endocytosis.Citation21,32-34 The majority of clusterin inside efferent duct cells is secreted by the Sertoli cells of the testis, and taken up by the efferent duct epithelium via endocytosis.Citation21 We found that expression of AQP1 () and clusterin () in the efferent ducts was greatly reduced in 5/5 E2f4f/f;E2f5+/−;Vil-cre mice, relative to their littermate controls. This reduction was not uniform but >40 % (for 1/5 mice) or >50 % (for 4/5 mice) of the efferent duct epithelial cells showed complete loss of both AQP1 and clusterin (, Fig. S6). We also observed reduced apical PAS staining in the mutants relative to littermate controls which is additional evidence of a decreased abundance of endocytic vesicle apparatus (n=5/5; ). Thus, we conclude that mutation of E2f4 and E2f5 also compromised the ability of the efferent ducts to reabsorb seminiferous tubule fluid by disrupting the development of non-ciliated cells.
Figure 4. Efferent duct marker analyses indicates abnormal development of the adult E2f4f/f;E2f5+/−;Vil-cre efferent duct epithelium. (A) Immunohistochemical analysis of estrogen receptor 1 (ESR1) expression (brown stain) in control and (B) mutant efferent ducts showing reduced expression in the mutants. (C) Aquaproin 1 (AQP1) expression (brown stain) is predominantly at the apical surface of control efferent ducts (arrow) but poorly expressed in the mutants (D). (E) Clusterin (CLU) a marker for the endocytic apparatus is located predominantly beneath the apical surface in controls (arrow) but poorly expressed in mutant efferent ducts (F). (G) Sections submitted to the PAS reaction show apical staining in the controls but not in the mutants (H) indicating a loss of endocytic apparatus. Scale bars in all panels 10 μm.
Mutation of E2f4 and E2f5 causes abnormal rete testis development
Inefficient reabsorption of seminiferous fluid by the efferent ducts would lead to a back up in flow from the testis causing seminiferous tubule and rete testis dilation. To investigate this further, we analyzed the development of the male reproductive tract at 1 week of age, which is before the production of spermatozoa or seminiferous fluid by the seminiferous tubules. If the testis phenotype was due solely to the failure of seminiferous fluid reabsorption rete testis development should be normal at this age. In contrast, we found that the rete testis was dilated in 5/7 E2f4f/f;E2f5+/−;Vil-cre males, but in 0/12 controls (). In 2/6 animals, we also observed rete testis dilation in double heterozygotes (E2f4+/f;E2f5+/−;Vil-cre) at 1 week of age, but this defect was absent in older animals (n>12), suggesting that it resolved at later stage of development (data not shown). Notably, examination of the Vil-cre transgene expression using the ZsGreen1 Cre recombinase reporter, a β-galactosidase based Cre recombinase reporter, and also IHC for E2F4 indicated that Cre recombinase was not active in the rete testis (Fig. S7 and data not shown). Thus, this rete testis dilation is non-cell autonomous. Based on these observations, we hypothesize that disruption of efferent duct development through E2f4 and E2f5 mutation causes the efferent duct epithelium, testis and/or rete testis to secrete excess fluid early during development and thereby yielding the rete testis dilation at 1 week of age.
Figure 5. Dilation of the rete testis is observed at one week of age in E2f4f/f;E2f5+/−;Vil-cre and E2f5−/− testes and loss of E2F5 reduces the multiciliated cell population. (A) Sagittal sections of testis from one week old control (Ctrl) and (B) E2f4f/f;E2f5+/−;Vil-cre mutant testis showing extensive dilation of the rete testis in the mutant (arrow). (C) Sagittal sections of testis from one week old control (Ctrl) and (D) E2f5−/− mutant testis showing extensive dilation of the rete testis in the mutant (arrow). (E) Sagittal sections of testis from 3 week old control (Ctrl) and (F) E2f5−/− mutant testis showing dilation of the rete testis (arrow) and partial dilation of seminiferous tubules in the mutant. (G) Immunohistochemical staining for FOXJ1, a multiciliated cell marker in one week old control and (H) E2f5−/− mutant efferent ducts showing reduced expression in the mutants. (I) Immunohistochemical staining for FOXJ1 in 3 week old control and (J) E2f5−/− mutant efferent ducts showing reduced expression in the mutants. (K) Immunohistochemical staining for acetylated tubulin staining (brown stain) of cilia shows many multiciliated cells in efferent ducts from 3 week old controls but many fewer multiciliated cells in littermate E2f5−/− mutant efferent ducts. Panels A though F hemotoxylin and eosin stained sections. Scale bars: E and F 400 μm; A, B, C, D 200 μm; I, J, K and L 20 μm; G and H 5μm.
Since mutation of E2f5 has been shown to cause excess secretion of cerebrospinal fluid from the choroid plexus,Citation23 we further investigated this rete testes phenotype by additionally analyzing pups that were mutant for E2f5. For this, we examined testis from one week old pups that were E2f4+/f;E2f5−/− or E2f4f/f;E2f5−/− and lacked the Vil-cre transgene. Notably, the rete testis was dilated in 4/5 E2f5 homozygous mutant mice (), whereas only 1/8 of the control littermates (an E2f5+/− animal) showed a minor case of dilation (data not shown). Thus, we conclude that loss of E2f5 alone is able to cause rete testis dilation. We also examined E2f5−/− pups at 3 weeks of age (the oldest they are viable), and observed rete testis dilation in 4/4 E2f4+/f;E2f5−/− and E2f4f/f;E2f5−/− pups, compared with 0/4 control littermates (). The dilated rete testis and dilated seminiferous tubule phenotype was also observed in rare surviving adult E2f5−/− (2/2) and E2f4+/−;E2f5−/− (4/4) mice (data not shown).
Given these results, we next analyzed the development of the efferent duct epithelium to ascertain whether it was also affected by mutation of E2f5. At one week of age the levels of FOXJ1 within the efferent duct epithelium were consistently lower in E2f4f/f;E2f5−/− and E2f4+/f;E2f5−/− pups (without Vil-cre) in comparison with control littermates (n=5/5; ). These data suggested that mutation of E2f5 impacts the multiciliogenesis developmental program within the efferent duct epithelium at this stage. By three weeks of age the levels of FOXJ1 were moderately reduced in the epithelium of E2f4f/f;E2f5−/− or E2f4+/f;E2f5−/− pups relative to control littermates (n= 4/4; ). However, staining for cilia showed a reduction in the abundance, but not a complete loss of multiciliated cells in E2f4f/f;E2f5−/− and E2f4+/f;E2f5−/− pups at 3 weeks of age (n = 4/4; ). Notably, expression of E2F4 was not altered by mutation of E2f5, as judged by immunohistochemistry (data not shown). These data therefore indicate that loss of E2F5 primarily causes the rete testis dilation but it only partially impairs multiciliogenesis. Conversely, loss of E2F4 only impairs development of the multiciliated cells and promotes rete testis dilation when one copy of E2f5 is mutant, implying that these transcription factors function redundantly.
Discussion
This study shows that the development of the murine male reproductive system requires the transcription factors E2F4 and E2F5 and that they have overlapping functions. Loss of E2F4 and E2F5 leads to several phenotypes in the reproductive tract that, at least in E2f4 null, E2f5 heterozygous context, are directly linked to defective efferent duct development. Within the efferent duct epithelium several cell types are affected. First, multiciliated cell development fails when E2F4 is absent in combination with heterozygosity of E2f5. This is illustrated by the absence of cilia and the disrupted expression of FOXJ1, a marker of multiciliated cells, both early during efferent duct development and in adults. This phenotype most likely arises because E2F4 and E2F5 are involved in the activation of the multiciliated cell program.Citation35-37). Analyses of E2f5−/− efferent ducts indicate that at one week of age, the levels of FOXJ1 are lower in comparison with littermate controls indicating that the multiciliogenesis program is disrupted but by 3 weeks of age multiciliated cells can develop albeit with reduced efficiency. At the same time, E2f4 deficiency alone does not disrupt multiciliogenesis in the efferent ducts but the additional deletion of a single E2f5 allele is sufficient to completely abrogate multiciliogenesis. Taken together, these data provide direct support for overlapping roles of E2F4 and E2F5 in the in vivo setting. By extension of this logic, we propose that the failure of multiciliated cell development in the airway epithelium and their normal development in the efferent ducts of E2f4−/− mice Citation18 reflects the fact that E2F5 compensates for the loss of E2F4 in the efferent duct epithelium but not in the airway epithelium. We further predict that loss of E2F4 and E2F5 during the development of other ciliated epithelium, such as that in the fallopian tubes or the ependymal epithelium of the ventricles of the brain, is likely to result in the failure of multiciliated cell development.
Within the efferent duct epithelium the development of the non-ciliated absorptive cells is also disrupted in the E2f4f/f;E2f5+/−;Vil-cre males. In this case we see a significantly reduction in the presence of 2 proteins important for absorptive cell function, Aquaporin 1 and Clusterin, within the efferent ducts suggesting that their absorptive capacity is compromised. This was further supported by the disruption of PAS staining that is indicative of endocytic vesicle apparatus. We hypothesize that the inability of the efferent ducts in the E2f4f/f;E2f5+/−;Vil-cre males to appropriately reabsorb seminiferous fluid contributes to the dilation of the rete testes and seminiferous tubules at later stages. However, this cannot fully account for the dilation of the rete testis observed since this is observed in one week old pups, prior to the normal time when the seminiferous tubules produce seminiferous fluid. Since this dilation defect is observed in the E2f4f/f;E2f5+/−;Vil-cre males, in which E2f4 deletion occurs specifically in the efferent ducts, we presume this must involve an efferent duct defect. We speculate that the efferent ducts, instead of absorbing fluid, are secreting excess fluid, which backs-up into the rete testis, causing it to dilate. Alternatively, disruption of efferent duct epithelium could result in abnormal signaling from the efferent ducts to the rete testis that causes the rete testis itself to secrete excess fluid. Given that the mutant rete testis is substantially larger than normal, there is also overgrowth of the rete testis. This could be a response to the excess fluid and/or altered signaling between the efferent duct and rete testes.
In estrogen receptor 1 (Esr1) mutants, which display a similar phenotype in the rete testis and testis,(reviewed in Citation30,31) rete testis dilation was observed as early as postnatal day 10 Citation38 suggesting that it may also occur prior to the stage when the seminiferous tubules are secreting fluid. Interestingly, in the Esr1 mutant mouse it was clearly established that the efferent ducts have an inability to reabsorb seminiferous tubule fluid and even secreted fluid into the lumen,Citation39 which could account for the observed rete testis dilation. We did not detect a substantial reduction in Esr1 expression in E2f4f/f;E2f5+/−;Vil-cre efferent ducts. However, we cannot rule out that the observed modest reduction of ESR1 levels, and/or an unappreciated defect in ESR1 function, contributes the testis dilation defects in our E2f4/E2f5 mutant models. Interestingly, other transgenic mouse models have been shown to develop rete testis dilation through modulation of Esr1 expression (Lgr4) versus through direct effects upon fluid reabsorption that are likely ESR1-independent Na+/H+ exchanger 3 (Slc9a3) and Carbonic Anhydrase II (Car2).Citation40-43
In contrast to our model, Esr1 mutation does not prevent the development of multiciliated cells, but does decrease the number of formed cilia by more than 50% .44 It is unclear whether the multiciliated cell defect in E2f4f/f;E2f5+/−;Vil-cre males contributes to the observed infertility and/or whether the multiciliated cell and rete testes dilation defects influence one another. Analysis of the male reproductive tract in the FoxJ1 mutant, which specifically disrupts multiciliated cell development, could help answer this question, but this has not been reported. It is also possible that the stirring or mixing function of efferent ductule cilia is essential for homogenous reabsorption of fluid by the nonciliated cells, as previously predicted.Citation45-47 Additionally we speculate that the near absence of sperm from the epididymis could be caused by impaired fluid flow, an occlusion or a defect in smooth muscle contraction, which may rely upon signaling from the efferent ducts.
Irrespective of the contribution of the multiciliogenesis defect, we believe that the primary problem is an accumulation of luminal fluid that reflects both impaired absorption and excess secretion. Interestingly, mutation of E2f5 lead to excessive secretory activity of the choroid plexus epithelium causing hydrocephalus Citation23 and transmission electron microscopy and histochemical analysis showed that the nasal respiratory epithelium of E2f4 mutant embryos resembles that of a secretory epithelium, instead of the normal, predominantly multiciliated epithelium.Citation18 Taken together with the results presented here, these data suggest that E2F4 and E2F5 play key roles in repressing a secretory cell program for which the cofactors and genes involved are yet to be established. Furthermore, this secretory phenotype has not been observed following mutation of other E2Fs indicating that it is a specific, overlapping role of E2F4 and E2F5.
Materials and methods
Generation of a conditional, floxed, allele of E2f4
Construction of the E2f4 conditional allele will be published elsewhere, details are available upon request.
Animal maintenance and genotyping
For this study mice were maintained on a mixed C57BL/6 × 129Sv background in a conventional facility. All animal procedures followed protocols approved by the MIT Institutional Animal Care and use Committee. Genotyping was performed by PCR using standard procedures and the following primers for the E2f4 conditional allele: F4cC, gccattaagcctcagctctgtctgg and F4cU, gtgcaccctgagatgtttagtctgg resulting in a 200bp product for the wild-type allele and a 293bp product for the conditional, floxed, allele. F4cC and a separate primer, ctggaacttgcaatgtagacaagg were used to detect the locus following recombination by Cre recombinase (244bp product). Genotyping for the E2f5 alleles was performed using the following primers: agacacggagtgggtcagatttggg, tctgtcccctacaagacagacaggc and attcgcagcgcatcgccttctatcg resulting in a 350bp product for the wild-type allele and 520bp product for the mutant allele.
Genotyping for the Vil-Cre transgene was performed as described.Citation48 Genotyping for the lacZ and ZsGreen1 Cre reporter alleles was conducted as described on the Jackson Laboratories' web site (http://www.jax.org/), stock numbers 009427 and 007906 respectively.
In all experiments controls included the following genotypes E2f4+/f; E2f5+/+ or E2f4+/f; E2f5+/− with and without Vil-cre as well as animals of any genotype that lacked Vil-cre. Mutants in this study are defined as E2f4f/f; E2f5+/−;Vil-cre with or without a Cre recombinase reporter allele. Apart from the loss of E2F4 expression we did not detect any phenotype in E2f4f/f; E2f5+/+;Vil-cre animals using the assays described in this paper unless specified otherwise, however, these animals were not used as controls. Male E2f4f/f; E2f5+/−; Vil-cre mice arose from crosses at approximately Mendelian frequency indicating that this genotype did not cause premature lethality.
Histological analysis and immunohistochemistry
Collected tissues were fixed in either modified Davidson's fixative or formalin (3.7% formaldehyde in phosphate buffered saline, PBS) for 24 hours at room temperature or overnight at 4°C, respectively and then dehydrated via an ethanol series prior to embedding in paraffin for sectioning at 5μm. For all procedures slides were re-hydrated through an ethanol series following de-waxing in xylenes and rinsed in water or PBS, as required. For each tissue at least 2 different levels were examined and one section from each level stained with haematoxylin and eosin (H&E) using standard procedures. Periodic acid-Shiff (PAS) staining was performed as described previously.Citation18 Images were captured on a Nikon Eclipse E600 using a SPOT RT digital camera and SPOT Advanced software.
The quantification of seminiferous tubule dilation was performed using ImageJ software. For each animal a minimum of 10 randomly chosen tubules per testis were measured. The total diameter of each tubule was measured perpendicular and midway along the longest axis of the tubule visible in the section and the diameter of the lumen measured at the same position and angle. The percentage of the tubule diameter comprised of by epithelium was calculated from these measurements (tubule diameter – lumen diameter/tubule diameter) and the data subject to an unpaired Student's t-test. At each time point a minimum of 4 pairs of controls and mutant littermates were analyzed.
For analysis of testis at 1 and 3 weeks of age for each pair of testes 4 midline sections were cut 300μm apart. If a dilated rete was observed in these sections then this sample was scored as positive.
Immunohistochemistry was performed essentially as described previously Citation18 using the following antibodies: E2F4,(1:1 LLF4.2 Citation16) acetylated α-tubulin (1:8000, http://www.sigmaaldrich.com/catalog/product/sigma/t6793?lang=enandregion=US), FOXJ1 (1:400, http://www.ebioscience.com/human-mouse-foxj1-antibody-purified-2a5.htm), ERα (1:1000, http://www.scbt.com/datasheet-542-eralpha-mc-20-antibody.html), AQUAPORIN 1 (1:300, http://www.scbt.com/datasheet-20810-aqp1-h-55-antibody.html), CLUSTERIN (1:200, http://www.scbt.com/datasheet-8354-clusterin-h-330-antibody.html). For each marker analyzed a minimum of 4 pairs of control and mutant littermate sections were stained and, unless stated otherwise, all scored with the described phenotype. Following immunohistochemistry slides were counterstained with hematoxylin in a Thermo Gemini stainer and coverslips added using the Thermo Consul cover slipper. The percentage of cells exhibiting normal levels of FOXJ1 expression was calculated by counting a minimum of 350 total efferent duct epithelial nuclei per sample from 4 pairs of independent mutant and control littermate samples. The data is presented as mean +/− one standard deviation and was subject to an unpaired Student's t-test.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
2015CC6884-file002.docx
Download MS Word (62.8 KB)Supplemental_Figures.zip
Download Zip (210.1 MB)Acknowledgments
We wish to thank Dirk de Rooij, Roderick Bronson and members of the Lees lab for their advice, Sylvie Robine for the Vil-cre allele, David Livingston for the E2f5 allele and the Koch Institute Swanson Biotechnology Center for technical support, specifically the Hope Babette Tang Histology facility and the ES Cell and Transgenics facility.
Funding
Funding was provided by grant NIH-P01 CA42063 to J.A.L. J.A.L is a Daniel K. Ludwig Scholar.
References
- Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol 2002; 3:11-20; PMID:11823794; http://dx.doi.org/10.1038/nrm714
- Attwooll C, Lazzerini Denchi E, Helin K. The E2F family: specific functions and overlapping interests. Embo J 2004; 23:4709-16; PMID:15538380; http://dx.doi.org/10.1038/sj.emboj.7600481
- Chen HZ, Tsai SY, Leone G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer 2009; 9:785-97; PMID:19851314; http://dx.doi.org/10.1038/nrc2696
- McClellan KA, Slack RS. Specific in vivo roles for E2Fs in differentiation and development. Cell Cycle 2007; 6:2917-27; PMID:17993781; http://dx.doi.org/10.4161/cc.6.23.4997
- Chong JL, Wenzel PL, Saenz-Robles MT, Nair V, Ferrey A, Hagan JP, Gomez YM, Sharma N, Chen HZ, Ouseph M, et al. E2f1-3 switch from activators in progenitor cells to repressors in differentiating cells. Nature 2009; 462:930-4; PMID:20016602; http://dx.doi.org/10.1038/nature08677
- Blais A, Dynlacht BD. E2F-associated chromatin modifiers and cell cycle control. Curr Opin Cell Biol 2007; 19:658-62; PMID:18023996; http://dx.doi.org/10.1016/j.ceb.2007.10.003
- Sadasivam S, DeCaprio JA. The DREAM complex: master coordinator of cell cycle-dependent gene expression. Nat Rev Cancer 2013; 13:585-95; PMID:23842645; http://dx.doi.org/10.1038/nrc3556
- DeGregori J, Johnson DG. Distinct and Overlapping Roles for E2F Family Members in Transcription, Proliferation and Apoptosis. Curr Mol Med 2006; 6:739-48; PMID:17100600
- Chong JL, Tsai SY, Sharma N, Opavsky R, Price R, Wu L, Fernandez SA, Leone G. E2f3a and E2f3b contribute to the control of cell proliferation and mouse development. Mol Cell Biol 2009; 29:414-24; PMID:19015245http://dx.doi.org/10.1128/MCB.01161-08
- Cloud JE, Rogers C, Reza TL, Ziebold U, Stone JR, Picard MH, Caron AM, Bronson RT, Lees JA. Mutant mouse models reveal the relative roles of E2F1 and E2F3 in vivo. Mol Cell Biol 2002; 22:2663-72; PMID:11909960; http://dx.doi.org/10.1128/MCB.22.8.2663-2672.2002
- Danielian PS, Friesenhahn LB, Faust AM, West JC, Caron AM, Bronson RT, Lees JA. E2f3a and E2f3b make overlapping but different contributions to total E2f3 activity. Oncogene 2008; 27:6561-70; PMID:18663357; http://dx.doi.org/10.1038/onc.2008.253
- Tsai SY, Opavsky R, Sharma N, Wu L, Naidu S, Nolan E, Feria-Arias E, Timmers C, Opavska J, de Bruin A, et al. Mouse development with a single E2F activator. Nature 2008; 454:1137-41; PMID:18594513; http://dx.doi.org/10.1038/nature07066
- Wu L, Timmers C, Maiti B, Saavedra HI, Sang L, Chong GT, Nuckolls F, Giangrande P, Wright FA, Field SJ, et al. The E2F1-3 transcription factors are essential for cellular proliferation. Nature 2001; 414:457-62; PMID:11719808; http://dx.doi.org/10.1038/35106593
- Rempel RE, Saenz-Robles MT, Storms R, Morham S, Ishida S, Engel A, Jakoi L, Melhem MF, Pipas JM, Smith C, et al. Loss of E2F4 activity leads to abnormal development of multiple cellular lineages. Mol Cell 2000; 6:293-306; PMID:10983977; http://dx.doi.org/10.1016/S1097-2765(00)00030-7
- Humbert PO, Rogers C, Ganiatsas S, Landsberg RL, Trimarchi JM, Dandapani S, Brugnara C, Erdman S, Schrenzel M, Bronson RT, et al. E2F4 is essential for normal erythrocyte maturation and neonatal viability. Mol Cell 2000; 6:281-91; PMID:10983976; http://dx.doi.org/10.1016/S1097-2765(00)00029-0
- Moberg K, Starz MA, Lees JA. E2F-4 switches from p130 to p107 and pRB in response to cell cycle reentry. Mol Cell Biol 1996; 16:1436-49; PMID:8657117; http://dx.doi.org/10.1128/MCB.16.4.1436
- Gaubatz S, Lindeman GJ, Ishida S, Jakoi L, Nevins JR, Livingston DM, Rempel RE. E2F4 and E2F5 play an essential role in pocket protein-mediated G1 control. Molecular cell 2000; 6:729-35; PMID:11030352; http://dx.doi.org/10.1016/S1097-2765(00)00071-X
- Danielian PS, Bender Kim CF, Caron AM, Vasile E, Bronson RT, Lees JA. E2f4 is required for normal development of the airway epithelium. Dev Biol 2007; 305:564-76; PMID:17383628; http://dx.doi.org/10.1016/j.ydbio.2007.02.037
- Deschenes C, Alvarez L, Lizotte ME, Vezina A, Rivard N. The nucleocytoplasmic shuttling of E2F4 is involved in the regulation of human intestinal epithelial cell proliferation and differentiation. J Cell Physiol 2004; 199:262-73; PMID:15040009; http://dx.doi.org/10.1002/jcp.10455
- Garneau H, Paquin MC, Carrier JC, Rivard N. E2F4 expression is required for cell cycle progression of normal intestinal crypt cells and colorectal cancer cells. J Cell Physiol 2009; 221:350-8; PMID:19562678; http://dx.doi.org/10.1002/jcp.21859
- Hess RA. The Efferent Ductules: Structure and Function. In: Hinton BRaB, ed. The Epididymis: From Molecules to Clinical Practice: Springer, 2002:49-80
- el Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 2004; 39:186-93; PMID:15282745; http://dx.doi.org/10.1002/gene.20042
- Lindeman GJ, Dagnino L, Gaubatz S, Xu Y, Bronson RT, Warren HB, Livingston DM. A specific, nonproliferative role for E2F-5 in choroid plexus function revealed by gene targeting. Genes Dev 1998; 12:1092-8; PMID:9553039; http://dx.doi.org/10.1101/gad.12.8.1092
- Horvat B, Osborn M, Damjanov I. Expression of villin in the mouse oviduct and the seminiferous ducts. Histochemistry 1990; 93:661-3; PMID:2329063; http://dx.doi.org/10.1007/BF00272210
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 2010; 13:133-40; PMID:20023653; http://dx.doi.org/10.1038/nn.2467
- Abe K, Takano H. Changes in distribution and staining reactivity of PAS-positive material in the mouse epididymal duct after efferent duct ligation. Arch Histol Cytol 1988; 51:433-41; PMID:2464973; http://dx.doi.org/10.1679/aohc.51.433
- O'Hara L, Welsh M, Saunders PT, Smith LB. Androgen receptor expression in the caput epididymal epithelium is essential for development of the initial segment and epididymal spermatozoa transit. Endocrinology 2011; 152:718-29; PMID:21177831; http://dx.doi.org/10.1210/en.2010-0928
- Chen J, Knowles HJ, Hebert JL, Hackett BP. Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left-right asymmetry. J Clin Invest 1998; 102:1077-82; PMID:9739041; http://dx.doi.org/10.1172/JCI4786
- Brody SL, Yan XH, Wuerffel MK, Song SK, Shapiro SD. Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am J Respir Cell Mol Biol 2000; 23:45-51; PMID:10873152; http://dx.doi.org/10.1165/ajrcmb.23.1.4070
- Hess RA, Fernandes SA, Gomes GR, Oliveira CA, Lazari MF, Porto CS. Estrogen and its receptors in efferent ductules and epididymis. J Androl 2011; 32:600-13; PMID:21441425; http://dx.doi.org/10.2164/jandrol.110.012872
- Joseph A, Shur BD, Hess RA. Estrogen, efferent ductules, and the epididymis. Biol Reprod 2011; 84:207-17; PMID:20926801; http://dx.doi.org/10.1095/biolreprod.110.087353
- Ruz R, Gregory M, Smith CE, Cyr DG, Lubahn DB, Hess RA, Hermo L. Expression of aquaporins in the efferent ductules, sperm counts, and sperm motility in estrogen receptor-α deficient mice fed lab chow vs. casein. Mol Reprod Dev 2006; 73:226-37; PMID:16261609; http://dx.doi.org/10.1002/mrd.20390
- Oliveira CA, Carnes K, Franca LR, Hermo L, Hess RA. Aquaporin-1 and -9 are differentially regulated by oestrogen in the efferent ductule epithelium and initial segment of the epididymis. Biol Cell 2005; 97:385-95; PMID:15850448; http://dx.doi.org/10.1042/BC20040078
- Oliveira CA, Victor-Costa AB, Hess RA. Cellular and regional distributions of ubiquitin-proteasome and endocytotic pathway components in the epithelium of rat efferent ductules and initial segment of the epididymis. J Androl 2009; 30:590-601; PMID:19269934; http://dx.doi.org/10.2164/jandrol.108.007310
- Stubbs JL, Vladar EK, Axelrod JD, Kintner C. Multicilin promotes centriole assembly and ciliogenesis during multiciliate cell differentiation. Nat Cell Biol 2012; 14:140-7; PMID:22231168; http://dx.doi.org/10.1038/ncb2406
- Ma L, Quigley I, Omran H, Kintner C. Multicilin drives centriole biogenesis via E2f proteins. Genes Dev 2014; 28:1461-71; PMID:24934224; http://dx.doi.org/10.1101/gad.243832.114
- Brooks ER, Wallingford JB. Multiciliated Cells. Curr Biol 2014; 24:R973-R82; PMID:25291643; http://dx.doi.org/10.1016/j.cub.2014.08.047
- Lee KH, Park JH, Bunick D, Lubahn DB, Bahr JM. Morphological comparison of the testis and efferent ductules between wild-type and estrogen receptor α knockout mice during postnatal development. J Anat 2009; 214:916-25; PMID:19538635; http://dx.doi.org/10.1111/j.1469-7580.2009.01080.x
- Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, Lubahn DB. A role for oestrogens in the male reproductive system. Nature 1997; 390:509-12; PMID:9393999; http://dx.doi.org/10.1038/37352
- Hoshii T, Takeo T, Nakagata N, Takeya M, Araki K, Yamamura K. LGR4 regulates the postnatal development and integrity of male reproductive tracts in mice. Biol Reprod 2007; 76:303-13; PMID:17079737; http://dx.doi.org/10.1095/biolreprod.106.054619
- Zhou Q, Clarke L, Nie R, Carnes K, Lai LW, Lien YH, Verkman A, Lubahn D, Fisher JS, Katzenellenbogen BS, et al. Estrogen action and male fertility: roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proc Natl Acad Sci U S A 2001; 98:14132-7; PMID:11698654; http://dx.doi.org/10.1073/pnas.241245898
- Mendive F, Laurent P, Van Schoore G, Skarnes W, Pochet R, Vassart G. Defective postnatal development of the male reproductive tract in LGR4 knockout mice. Dev Biol 2006; 290:421-34; PMID:16406039; http://dx.doi.org/10.1016/j.ydbio.2005.11.043
- Davies B, Baumann C, Kirchhoff C, Ivell R, Nubbemeyer R, Habenicht UF, Theuring F, Gottwald U. Targeted deletion of the epididymal receptor HE6 results in fluid dysregulation and male infertility. Mol Cell Biol 2004; 24:8642-8; PMID:15367682; http://dx.doi.org/10.1128/MCB.24.19.8642-8648.2004
- Hess RA, Bunick D, Lubahn DB, Zhou Q, Bouma J. Morphologic changes in efferent ductules and epididymis in estrogen receptor-α knockout mice. J Androl 2000; 21:107-21; PMID:10670526
- Hess RA. Estrogen in the adult male reproductive tract: a review. Reprod Biol Endocrinol 2003; 1:52; PMID:12904263; http://dx.doi.org/10.1186/1477-7827-1-52
- Hess RA. Disruption of estrogen receptor signaling and similar pathways in the efferent ductules and initial segment of the epididymis. Spermatogenesis 2014; 4:e979103; PMID:26413389; http://dx.doi.org/10.4161/21565562.2014.979103
- Hess RA. Small tubules, surprising discoveries: from efferent ductules in the turkey to the discovery that estrogen receptor α is essential for fertility in the male. Anim Reprod 2015; 12:7-23
- Kucherlapati MH, Nguyen AA, Bronson RT, Kucherlapati RS. Inactivation of conditional Rb by Villin-Cre leads to aggressive tumors outside the gastrointestinal tract. Cancer Res 2006; 66:3576-83; PMID:16585182; http://dx.doi.org/10.1158/0008-5472.CAN-05-2699
