ABSTRACT
Homologous recombination enables the exchange of genetic information between related DNA molecules and is a driving force in evolution. Using single-molecule optical microscopy we have recently shown that members of the Rad51/RecA family of recombinases stabilize paired homologous strand of DNA in precise 3-nt increments. Here we discuss an interesting conceptual implication of these results, which is that the recombinases may actively sense and reorganize their alignment register relative to the bound DNA sequences to ensure optimal base triplet pairing interactions during the early stages of recombination.
Introduction
During homologous recombination (HR), a single–stranded DNA (ssDNA) is paired with the complementary strand of a homologous double-stranded DNA (dsDNA), resulting in displacement of the non–complementary strand. This reaction is referred to as DNA strand exchange.Citation1,2 Strand exchange reactions play essential roles in double–strand DNA break (DSB) repair, the rescue of stalled or collapsed replication forks, chromosomal rearrangements, and horizontal gene transfer.Citation3-5 Strand exchange is also necessary during meiosis, allowing for proper chromosome segregation and the generation of new allelic combinations.Citation6,7
The Rad51/RecA family of DNA recombinases promote homologous recombination in all kingdoms of life.Citation8 These ATP-dependent DNA-binding proteins assemble into long polymeric filaments on the ssDNA overhangs that are generated during the initial stages of HR.Citation1,2 The filaments are called “presynaptic complexes,” and they are responsible for aligning homologous DNA sequences and promoting DNA strand exchange. Interestingly, presynaptic complexes exhibit an extended structure in which the bound DNA is stretched by ˜50% relative to regular B-form DNA. Contrary to initial assumptions, the DNA within these presynaptic complexes is not uniformly extended. Instead, crystal structures of the RecA nucleoprotein complex reveal that the DNA is organized into base triplets, with each triplet maintained in near B-form geometry, while every third phosphodiester bond is stretched to ˜7.8–8.1 Å.Citation9 We refer to this unique nucleic acid structure as RS-DNA (Rad51/RecA-Stretched DNA) to denote its unique characteristics, and to distinguish it from other stretched forms of DNA.
Base triplet pairing during dna recombination
Single-molecule technologies offer an opportunity to probe the molecular mechanisms that underlie DNA recombination in unprecedented detail.Citation10-15 We have developed the single-molecule DNA curtains method to establish a more detailed understanding of the molecular mechanisms that contribute to homologous DNA recombination.Citation16-19 For studies of recombination, we use ssDNA curtains, where long ssDNA molecules are tethered to a lipid bilayer on the surface of a microfluidic sample chamber and then organized into “curtains” using a series of strategically positioned barriers to lipid diffusion; details describing the preparation of DNA curtains can be found in references.Citation16-19 The ssDNA molecules are coated with a GFP-tagged version of replication protein A (RPA), which is a ubiquitous ssDNA-binding protein found in eukaryotes.Citation20 The RPA-ssDNA can then be used as a substrate to assemble presynaptic complexes by addition of the appropriate Rad51/RecA protein along with the required nucleotide cofactor (ATP, AMP-PNP, or ATPγS). These presynaptic complexes are assembled with wild-type recombinases that are not fluorescently tagged, and thus provide a “dark” background against which one can detect the binding of fluorescently tagged partner proteins, fluorescent DNA substrates, or both.
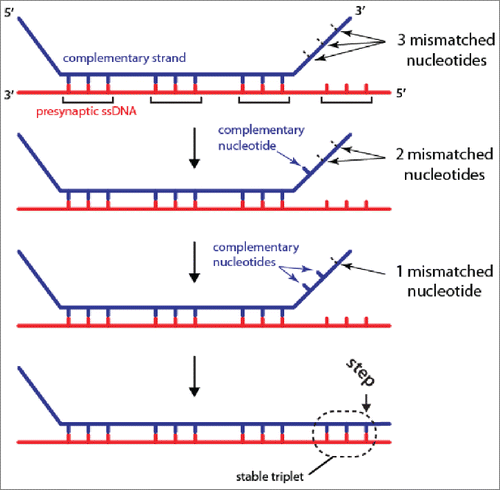
Using fluorescently tagged 70-bp dsDNA substrates we examined how Rad51/RecA presynaptic complexes interacted with duplex DNA provided in trans.Citation19 The dsDNA substrates contained short tracts of microhomology (≤15 -nt) complementary to defined regions of the Rad51/RecA-coated presynaptic ssDNA. Interactions between these dsDNA molecules and the Rad51/RecA presynaptic complexes were monitored by direct visualization with total internal reflection fluorescence microscopy (TIRFM).Citation21 These experiments revealed that substrates bearing ≤7 -nt of microhomology are rapidly sampled and rejected within ∼0.5 seconds. Rad51/RecA presynaptic complexes required at least 8-nt of microhomology to stably capture a dsDNA fragment, in agreement with previous bulk biochemical results,Citation22 leading to a lifetime on the order of ∼10 minutes.Citation19 Importantly, the lifetime measurements are a direct proxy for the stability of the interaction between the presynaptic ssDNA and a given tract of microhomology with the dsDNA. Remarkably, the dsDNA binding stability exhibited a distinctive 3-nt stepping pattern when the length of the microhomology was increased from 8-nt to 15-nt in single nucleotide increments.Citation18,19 That is, the measured stability of the interactions increased in quantized units for each set of 3 homologous nucleotides. Similar 3-nt stepping was observed for human and S. cerevisiae Rad51, human and S. cerevisiae Dmc1 (a meiosis-specific Rad51 paralog), and E. coli RecA, suggesting that it is conserved across the entire Rad51/RecA family. The 3-nt stepping patterns were also readily recapitulated using molecular dynamics or Monte Carlo simulations of a course-grained model for RS-DNA in the absence of any proteins, strongly suggesting that the base triplet architecture of RS-DNA itself was responsible for the observed triplet stepping.Citation18 A crucial implication of these findings is that only fully paired base triplets are capable of forming stable pairing interactions within the context of RS-DNA ().
Figure 1. Base-triplet organization of RS-DNA. Model depicting the base triplet pairing interactions that take place between the presynaptic ssDNA (red) and complementary dsDNA (blue) molecule (the non-complementary dsDNA is omitted for clarity). Stable base triplet interactions are only detected if 3 contiguous nucleotides within the dsDNA are complementary to the presynaptic ssDNA. Additional details are presented in the text, and references.Citation18,19
The problem of protein register
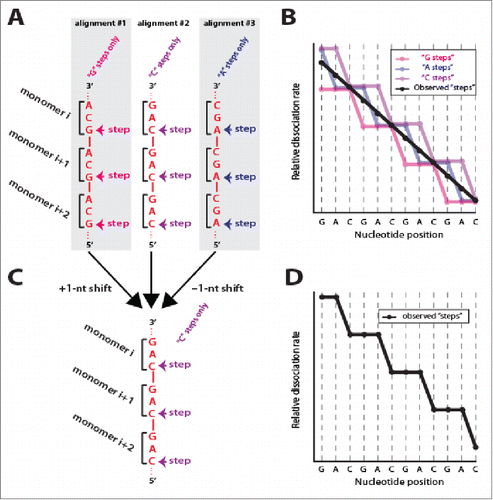
The ability to experimentally detect base triplet stepping behavior highlights an interesting concept related to the early stages of dsDNA interrogation during recombination. Assembly of Rad51/RecA filaments initiates with a rate limiting nucleation step, which is then followed by more rapid extension of the filaments.Citation1,2 If the nucleation occurs at random locations then the Rad51/RecA monomers within any population of presynaptic complexes should exist in 3 different alignment registers with respect to the underlying ssDNA sequence (). There is no reason to believe that one particular register should be preferred over another, and it is reasonable to assume that all 3 would be present within a population of molecules. However, if these 3 distinct alignments existed in roughly equal proportions, then the experimental dsDNA binding data obtained from DNA curtain measurements should reflect a mixture of all 3 possible alignments. As a consequence the triplet patterns should be buried in an average of all data (). But this is not the case, and we observe very clear triplet stepping patterns. Therefore, an interesting implication of the experimentally observed 3-nt stepping is that the protein monomers on different ssDNA molecules must all be aligned in the same register with respect to each other and with respect to the underlying presynaptic ssDNA sequence ().
Figure 2. Evidence for uniform protein–ssDNA register during strand exchange. (A) Predicted random alignment registers of Rad51/RecA protein monomers (depicted as brackets) with a presynaptic ssDNA with the sequence d(GAC)n. Rad51/RecA can align in any of 3 different registers with respect to any ssDNA, and each register should yield a different “stepping” pattern. (B) Relative dissociation rates reflecting each of the 3 different possible registers. Assuming random protein alignment, then the experimental data should represent the average of these 3 registers (black line). (C) A ±1 –nt shift of the ssDNA relative to the protein monomers channels the proteins into a single alignment. (D) Schematic highlighting that experimental data reflect a single alignment register, rather than the average of 3 different registers.
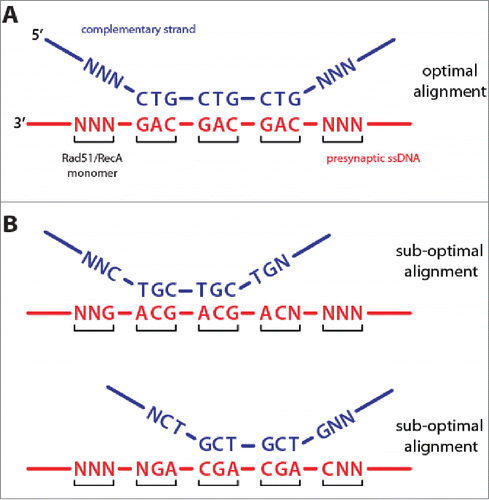
How is it possible that the proteins are all aligned in the same register, and how might this common alignment register contribute to duplex DNA recognition and strand exchange? These observations are readily explained if protein monomers within the presynaptic complex are capable of shifting just ±1–nucleotide with respect to their original binding register (). One-dimensional diffusion of proteins on both ssDNA and dsDNA is now a well-documented phenomenon,Citation23-31 and we have previously observed 1D diffusion of human Rad51 along dsDNA,Citation32 supporting the notion that Rad51/RecA monomers within the presynaptic complex might also be capable of sliding at least short distances along ssDNA. The ability of Rad51/RecA to slide just ±1–nucleotide would ensure optimal alignment of DNA triplet sequences during the early stages of homology recognition and strand exchange. This can be illustrated by considering how a 9-nt tract of microhomology embedded within an otherwise nonhomologous duplex may align with a complementary ssDNA within the context of the presynaptic complex. There are 3 possible recombinase-ssDNA alignment registers, but only one of these alignments allows all 9-nucleotides to pair with the presynaptic ssDNA (). The remaining 2 registers should only allow for 6-nucleotides to pair with the presynaptic ssDNA, while the remaining 3-nucleotides form discontinuous tracts of 1-nt and 2-nt at either end of the microhomology (). These terminal nucleotides should not stably pair with the presynaptic ssDNA because they cannot form complete RS-DNA base triplets. Similar arguments can be made for longer tracts of microhomology and even fully homologous sequences. The ability of Rad51/RecA to shift register with respect to the underlying ssDNA may ensure optimal alignment of complementary sequences during the search for homology and during the very earliest stages of strand invasion, implying that Rad51/RecA can sense and respond to the sequence and alignment of the incoming homologous DNA strand.
Figure 3. Protein register and base triplet pairing. (A) Optimized alignment register that maximizes base triplet pairing interactions for an example where the complementary dsDNA strand (blue) harbors 9 nucleotides of microhomology that are complementary to the presynaptic ssDNA (red). For clarity, the non-complementary dsDNA strand is not shown. “N” corresponds to any non-complementary nucleotide. (B) Two alternative alignment registers for the same DNA sequences will yield sub-optimal triplet pairing interactions. Details are presented in the main text.
The precise length of the invading presynaptic ssDNA strand is difficult to quantify. However, current estimates suggest that the heteroduplex product of strand exchange can be hundreds or even thousands of bases pairs in length.Citation33,34 So alternative alignment registers that result in 1–2 unpaired nucleotides at either end will have little impact on the overall stability of the final heteroduplex intermediate. Why then might Rad51/RecA shift register with respect to the presynaptic ssDNA during the early stages of homology recognition and strand exchange? One possibility is that this capacity may play a role in the initial interrogation of DNA sequences while searching for homology. This initial search process requires the rapid sampling and rejection of numerous short nonhomologous DNA sequences while looking for short tracts of microhomology. The ability of Rad51/RecA to shift register may help ensure that sub-optimally aligned intermediates are not prematurely rejected during the early stages of recombination.
How might the Rad51/RecA presynaptic complex sense the optimal sequence alignment? In the simplified consideration of protein register, each monomer is depicted as interacting with a single base triplet (). However, close inspection of the RecA-ssDNA crystal structure reveals that each monomer also contacts the flanking nucleotides that lie immediately 5′ and 3′ to the B-form like triplet.Citation9 Although speculative, it is possible that these contacts allow protein monomers within the presynaptic complex to directly sense, and presumably respond to, the pairing status of the adjacent RS-DNA triplets. Additional work will be essential to more fully understand the dynamic processes that take place within the interior of the presynaptic complex.
Closing thoughts
The ability to study recombination intermediates by single-molecule optical microscopy has yielded many new insights into how Rad51/RecA presynaptic complexes interrogate dsDNA and promote strand invasion. However, many important questions remain to be addressed. For instance, why does initial recognition involve 8-nt (as opposed to 6 or 9), and does the length of microhomology required for initial recognition vary with reaction temperature? How do other recombination proteins, such as members of the Rad52 epistasis group of genes,Citation3-5 modulate dsDNA sampling by Rad51, and what is their impact on strand invasion? Finally, how is dsDNA sampling and capture influenced by the presence of chromatin and other dsDNA-binding proteins? These and many other questions can now be addressed by directly visualizing individual recombination reactions in real time.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
I am grateful to Daniel Duzdevich for comments on the manuscript, and members of the Greene laboratory for helpful discussions.
Funding
Work in the Greene laboratory is support by the NIH (GM074739, GM082848) and NSF (MCB1154511).
References
- Bianco PR, Tracy RB, Kowalczykowski SC. DNA strand exchange proteins: a biochemical and physical comparison. Front Biosci 1998; 3:D570-603; PMID:9632377
- Morrical SW. DNA-Pairing and Annealing Processes in Homologous Recombination and Homology-Directed Repair. Cold Spring Harb Perspect Biol 2015; 7:a016444; PMID:25646379; http://dx.doi.org/10.1101/cshperspect.a016444
- Symington LS, Rothstein R, Lisby M. Mechanisms and regulation of mitotic recombination in Saccharomyces cerevisiae. Genetics 2014; 198:795-835; PMID:25381364; http://dx.doi.org/10.1534/genetics.114.166140
- San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem 2008; 77:229-57; PMID:18275380; http://dx.doi.org/10.1146/annurev.biochem.77.061306.125255
- Heyer WD, Ehmsen KT, Liu J. Regulation of homologous recombination in eukaryotes. Annu Rev Genet 2010; 44:113-39; PMID:20690856; http://dx.doi.org/10.1146/annurev-genet-051710-150955
- Neale MJ, Keeney S. Clarifying the mechanics of DNA strand exchange in meiotic recombination. Nature 2006; 442:153-8; PMID:16838012; http://dx.doi.org/10.1038/nature04885
- Brown MS, Bishop DK. DNA Strand Exchange and RecA Homologs in Meiosis. Cold Spring Harb Perspect Biol 2014; 7:a016659; PMID:25475089
- Lin Z, Kong H, Nei M, Ma H. Origins and evolution of the recA/RAD51 gene family: evidence for ancient gene duplication and endosymbiotic gene transfer. Proc Natl Acad Sci U S A 2006; 103:10328-33; PMID:16798872; http://dx.doi.org/10.1073/pnas.0604232103
- Chen Z, Yang H, Pavletich NP. Mechanism of homologous recombination from the RecA-ssDNA/dsDNA structures. Nature 2008; 453:489-4; PMID:18497818; http://dx.doi.org/10.1038/nature06971
- Sanchez H, Reuter M, Yokokawa M, Takeyasu K, Wyman C. Taking it one step at a time in homologous recombination repair. DNA Repair (Amst) 2014; 20:110-8; PMID:24636751; http://dx.doi.org/10.1016/j.dnarep.2014.02.012
- Forget AL, Kowalczykowski SC. Single-molecule imaging brings Rad51 nucleoprotein filaments into focus. Trends Cell Biol 2010; 20:269-76; PMID:20299221; http://dx.doi.org/10.1016/j.tcb.2010.02.004
- Hilario J, Kowalczykowski SC. Visualizing protein-DNA interactions at the single-molecule level. Curr Opin Chem Biol 2010; 14:15-22; PMID:19945909; http://dx.doi.org/10.1016/j.cbpa.2009.10.035
- Candelli A, Modesti M, Peterman EJ, Wuite, G.J. Single-molecule views on homologous recombination. Q Rev Biophys 2013; 46:323-48; PMID:24016421; http://dx.doi.org/; http://dx.doi.org/10.1017/S0033583513000073
- Spies M. There and back again: new single-molecule insights in the motion of DNA repair proteins. Curr Opin Struct Biol 2013; 23:154-60; PMID:23260129; http://dx.doi.org/10.1016/j.sbi.2012.11.008
- Carrasco C, Dillingham MS, Moreno-Herrero F. Single molecule approaches to monitor the recognition and resection of double-stranded DNA breaks during homologous recombination. DNA Repair (Amst) 2014; 20:119-29; PMID:24569169; http://dx.doi.org/10.1016/j.dnarep.2014.02.002
- Gibb B, Silverstein TD, Finkelstein IJ, Greene EC. Single-stranded DNA curtains for real-time single-molecule visualization of protein-nucleic acid interactions. Anal Chem 2012; 84:7607-12; PMID:22950646; http://dx.doi.org/10.1021/ac302117z
- Greene EC, Wind S, Fazio T, Gorman J, Visnapuu ML. DNA curtains for high-throughput single-molecule optical imaging. Methods Enzymol 2010; 472:293-315; PMID:20580969; http://dx.doi.org/10.1016/S0076-6879(10)72006-1
- Lee JY, Terakawa T, Qi Z, Steinfeld JB, Redding S, Kwon Y, Gaines WA, Zhao W, Sung P, Greene EC. DNA RECOMBINATION. Base triplet stepping by the Rad51/RecA family of recombinases. Science 2015; 349:977-81; PMID:26315438; http://dx.doi.org/10.1126/science.aab2666
- Qi Z, Redding S, Lee JY, Gibb B, Kwon Y, Niu H, Gaines WA, Sung P, Greene EC. DNA sequence alignment by microhomology sampling during homologous recombination. Cell 2015; 160:856-69; PMID:25684365; http://dx.doi.org/10.1016/j.cell.2015.01.029
- Wold MS. Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu Rev Biochem 1997; 66:61-92; PMID:9242902; http://dx.doi.org/10.1146/annurev.biochem.66.1.61
- Axelrod D. Total internal reflection fluorescence microscopy. Methods Cell Biol 1989; 30:245-70; PMID:2648112; http://dx.doi.org/10.1016/S0091-679X(08)60982-6
- Hsieh P, Camerini-Otero CS, Camerini-Otero RD. The synapsis event in the homologous pairing of DNAs: RecA recognizes and pairs less than one helical repeat of DNA. Proc Natl Acad Sci U S A 1992; 89:6492-6; PMID:1631148; http://dx.doi.org/10.1073/pnas.89.14.6492
- Blainey PC, van Oijen AM, Banerjee A, Verdine GL, Xie XS. A base-excision DNA-repair protein finds intrahelical lesion bases by fast sliding in contact with DNA. Proc Natl Acad Sci U S A 2006; 103:5752-7; PMID:16585517; http://dx.doi.org/10.1073/pnas.0509723103
- Elf J, Li GW, Xie XS. Probing transcription factor dynamics at the single-molecule level in a living cell. Science 2007; 316:1191-4; PMID:17525339; http://dx.doi.org/10.1126/science.1141967
- Lee KS, Marciel AB, Kozlov AG, Schroeder CM, Lohman TM, Ha T. Ultrafast redistribution of E. coli SSB along long single-stranded DNA via intersegment transfer. J Mol Biol 2014; 426:2413-21; PMID:24792418; http://dx.doi.org/10.1016/j.jmb.2014.04.023
- Leith JS, Tafvizi A, Huang F, Uspal WE, Doyle PS, Fersht AR, Mirny LA, van Oijen AM. Sequence-dependent sliding kinetics of p53. Proc Natl Acad Sci U S A 2012; 109:16552-7; PMID:23012405; http://dx.doi.org/10.1073/pnas.1120452109
- Nguyen B, Sokoloski J, Galletto R, Elson EL, Wold MS, Lohman TM. Diffusion of human replication protein A along single-stranded DNA. J Mol Biol 2014; 426:3246-61; PMID:25058683; http://dx.doi.org/10.1016/j.jmb.2014.07.014
- Schwarz FW, Tóth J, van Aelst K, Cui G, Clausing S, Szczelkun MD, Seidel R. The helicase-like domains of type III restriction enzymes trigger long-range diffusion along DNA. Science 2013; 340:353-6; PMID:23599494; http://dx.doi.org/10.1126/science.1231122
- Tafvizi A, Mirny LA, van Oijen AM. Dancing on DNA: kinetic aspects of search processes on DNA. Chemphyschem 2011; 12:1481-9; PMID:21560221; http://dx.doi.org/10.1002/cphc.201100112
- Zhou R, Kozlov AG, Roy R, Zhang J, Korolev S, Lohman TM, Ha T. SSB functions as a sliding platform that migrates on DNA via reptation. Cell 2011; 146:222-32; PMID:21784244; http://dx.doi.org/10.1016/j.cell.2011.06.036
- Ha T, Kozlov AG, Lohman TM. Single-molecule views of protein movement on single-stranded DNA. Annu Rev Biophys 2012; 41:295-319; PMID:22404684; http://dx.doi.org/10.1146/annurev-biophys-042910-155351
- Graneli A, Yeykal CC, Robertson RB, Greene EC. Long-distance lateral diffusion of human Rad51 on double-stranded DNA. Proc Natl Acad Sci U S A 2006; 103:1221-6; PMID:16432240; http://dx.doi.org/10.1073/pnas.0508366103
- Chung WH, Zhu Z, Papusha A, Malkova A, Ira G. Defective resection at DNA double-strand breaks leads to de novo telomere formation and enhances gene targeting. PLoS Genet 2010; 6:e1000948; PMID:20485519; http://dx.doi.org/10.1371/journal.pgen.1000948
- Jinks-Robertson S, Michelitch M, Ramcharan S. Substrate length requirements for efficient mitotic recombination in Saccharomyces cerevisiae. Mol Cell Biol 1993; 13:3937-50; PMID:8321201; http://dx.doi.org/10.1128/MCB.13.7.3937
