ABSTRACT
Increasing evidence has confirmed the existence of cancer stem cells (CSCs) in both hematological malignancies and solid tumors. However, the origin of CSCs is still uncertain, and few agents have been capable of eliminating CSCs till now. The aim of this study was to investigate whether bulk pancreatic cancer cells could convert into CSCs under certain conditions and explore whether metformin and curcumin can kill pancreatic CSCs. Aspc1, Bxpc3 and Panc1 pancreatic cancer cells were cultured in stem cell culture medium (serum-free Dulbecco's modified Eagle medium/Nutrient Mixture F-12 containing basic fibroblast growth factor, epidermal growth factor, B27 and insulin) for 5 days and it was found that all the pancreatic cancer cells aggregated into spheres and expressed pancreatic cancer stem cell surface markers. Then characteristics of Panc1 sphere cells were analyzed and cytotoxicity assays were performed. The results show that Panc1 sphere cells exhibited CSC characteristics and were more resistant to conventional chemotherapy and more sensitive to metformin and curcumin than their parent cells. These findings suggested that bulk pancreatic cancer cells could acquire CSC characteristics under certain conditions, which may support the “yin-yang” model of CSCs (interconversion between bulk cancer cells and CSCs). These results also showed that metformin and curcumin could be candidate drugs for targeting pancreatic CSCs.
Introduction
The cancer stem cell hypothesis proposes that cancer stem cells (CSCs) comprise only a small proportion of tumor, have powerful self-renewal capacity and tumor-initiating ability.Citation1,2 CSCs share similar cell surface markers and self-renewal pathways with normal stem cells, have potent differentiation capacity and are resistant to chemotherapy and radiation.Citation1,2 Conventional anticancer therapies kill the rapidly proliferating bulk cancer cells but spare the relatively quiescent CSCs, resulting in cancer recurrence.
CSCs were first identified in acute myeloid leukemia (AML).Citation3,4 Till now, CSCs have been isolated in most hematological malignancies and solid tumors. Li et al. first isolated pancreatic CSCs from primary pancreatic cancer cells using the cell surface markers CD44, CD24 and ESA in 2007.Citation5 These CD44+CD24+ESA+ pancreatic CSCs represented a small minority (only 0.2-0.8%) of the total tumor cells but were 100 times more tumorigenic than bulk cancer cells. Huang et al. identified pancreatic CSCs expressing CD44 and CD24 in the Panc1 pancreatic cancer cell line, in which only 2.1-3.5% of the cells were CD44+CD24+. Compared with CD44-CD24- Panc1 pancreatic cancer cells, CD44+CD24+ CSCs have a 20-fold higher tumorigenic potential but a lower growth rate in vitro.Citation6 In the Bxpc-3 and Panc03.27 pancreatic cancer cell lines, slowly cycling cells exhibited chemotherapy resistance, an epithelial-to-mesenchymal transition (EMT) fingerprint and increased invasive potential and tumorigenic potential, indicating that the slowly cycling pancreatic cancer cells were CSCs.Citation7 Gavirhi et al. showed that Panc1 cells aggregated to form spheres in serum-free Dulbecco's modified Eagle medium/Nutrient Mixture F-12(DMEM/F-12) containing B27, fungizone, heparin, fibroblast growth factor(FGF) and epidermal growth factor(EGF), and the sphere cells showed increased tumor-initiating ability and aggressiveness, which are CSC characteristics.Citation8
Although the characteristics of CSCs have been studied extensively in recent years, there is still some debate about the CSC hypothesis and the origin of CSCs is still uncertain. Tumor heterogeneity has been explained by 2 different models. One model is the stochastic (or clonal evolution) model, which states that most or all cancer cells have inherent tumorigenic potential.Citation9,10 The other model is the hierarchy model, in which only a small subset of cells, i.e., CSCs, can initiate tumor formation.Citation9,10 Some researchers have proposed a more dynamic model, i.e., the yin-yang model of CSCs, in which cancer cells consist of varying growing or replicating populations (yang) and slow growing or non-dividing populations (yin), and the 2 populations may interconvert.Citation11 Many studies have shown that CSCs can produce CSCs and bulk cancer cells, whereas bulk cancer cells can only produce bulk cancer cells.Citation12-15 These results may support the hierarchy model. Other studies have found that CSCs and bulk cancer cells can each generate the other,Citation16,17 which may support the stochastic (or clonal evolution) model.
Pancreatic cancer is a common malignant neoplasm and has a poor prognosis, with a low overall 5-year survival rate of 6.7%.Citation18 Currently, the available anticancer therapies for pancreatic cancer are still insufficient and the remaining CSCs cause tumor recurrence after conventional chemotherapy and radiotherapy. Recently, some compounds have been shown to inhibit the self-renewal of CSCs in vitro and in vivo. Metformin, a standard drug for the treatment of type 2 diabetes, can selectively kill CD44high/CD24low breast CSCs,Citation19 CD133+ pancreatic CSCs,Citation20 CD44high/CD24high pancreatic CSCs,Citation21 CD133+ colorectal CSCs,Citation22 ovarian CSCsCitation23 and human glioblastoma tumor-initiating cells.Citation24 Curcumin, a food spice derived from turmeric, can suppress the self-renewal of human breast CSCs,Citation25,26 colon CSCs,Citation27 laryngeal carcinoma CSCsCitation28 and rat C6 glioma SP cells.Citation29 In this study, whether bulk pancreatic cancer cells could convert into CSCs under certain conditions and whether metformin and curcumin can kill pancreatic CSCs were investigated.
Results
Cell sphere formation
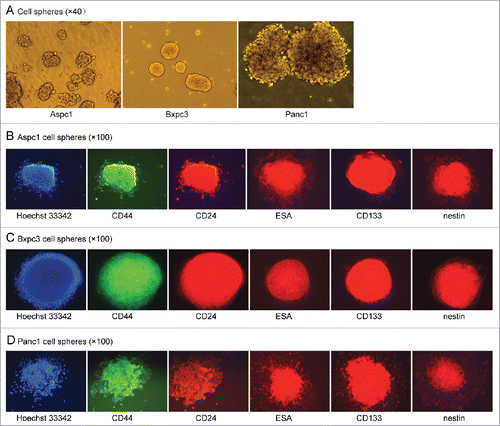
To investigate what percentage of pancreatic cancer cells can form cell spheres, individual-cell suspensions of Aspc1, Bxpc3 and Panc1 pancreatic cancer cells were plated in ultra-low attachment plates in stem cell medium. It was found unexpectedly that all of the Aspc1, Bxpc3 and Panc1 cells aggregated to form spheres within several hours in serum-free DMEM/F-12 containing basic fibroblast growth factor(bFGF), EGF, B27 and insulin (). After having been cultured in the medium for 5 days, the cell spheres were collected by centrifugation, and individual cells were obtained by trypsin digestion. The number of cells in the spheres had not increased.This mean the cells in the spheres are dormant in the culture medium.
Cell immunofluorescence
Cell sphere formation ability is widely accepted as a characteristic of CSCs. So we wonder whether these Aspc1, Bxpc3 and Panc1 sphere cells were CSCs. Firstly we detected the cell surface markers by cell immunofluorescence. Adherent Aspc1, Bxpc3 and Panc1 cells cultured in DMEM containing 10% fetal bovine serum (FBS) only weakly expressed CD44 and did not express CD24, ESA, CD133 or nestin. For sphere cells, we found that all the Aspc1, Bxpc3 and Panc1 shpere cells expressed CD44, CD24, ESA, CD133 and nestin after they had been cultured in serum-free DMEM/F-12 containing bFGF, EGF, B27 and insulin for 5 days. Then we had a dynamic observation and found that all of the sphere cells did not expressed the stem cell markers when they had been cultured in the medium for 24 hours and 48 hours, all the sphere cells began to express the stem cell markers after more than 72 hours, and strongly expressed the markers after more than 96 hours (). These results suggested that the cell surface markers of the sphere cells gradually changed. The different cell markers may mean different cell characteristics.
Figure 1. (A). All of the Aspc1, Bxpc3 and Panc1 cells formed spheres in serum-free DMEM/F-12 containing bFGF, EGF, B27 and insulin. (B, C and D). The sphere cells of Aspc1, Bxpc3 and Panc1 strongly expressed CD44, CD24, ESA, CD133 and nestin after they had been cultured for more than 96 hours in serum-free DMEM/F-12 containing bFGF, EGF, B27 and insulin.
Flow cytometry analysis
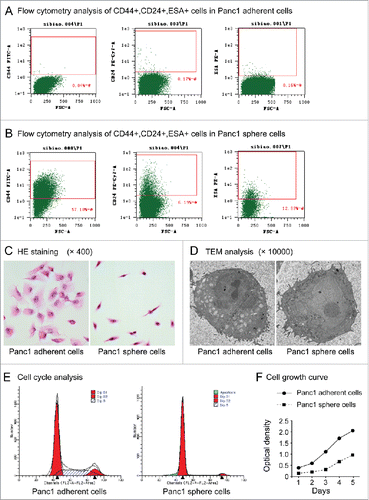
Panc1 cells have been widely used in pancreatic cancer research. Next, characteristics of Panc1 sphere cells (cultured in serum-free DMEM/F-12 containing bFGF, EGF, B27 and insulin for 5 days) and Panc1 adherent cells (cultured in DMEM containing 10% FBS) were analyzed. Flow cytometry analysis revealed that 0.05% ± 0.021% (n=3) of Panc1 adherent cells were CD44+, 0.18%±0.03% (n=3) were CD24+ and 0.08%±0.01% (n=3) were ESA+ (), whereas 62.99% ± 7.931% (n=3) of Panc1 sphere cells were CD44+, 6.58%±0.38% (n=3) were CD24+ and 12.88%±0.39% (n=3) were ESA+ (). The cell immunofluorescence showed all the cells in the spheres expressed CD44, CD24 and ESA, while the flow cytometry analysis showed just a certain percentage of the sphere cells expressed the markers. This result may due to the sensitivities and specificities of the 2 analyses are not the same. Even so, the results of flow cytometry analysis showed that there were more cells expressed pancreatic CSC markers in Panc1 sphere cells than in adherent cells (P < 0.001, n=3).
Figure 2. (A). Flow cytometry analysis of CD44+CD24+ESA+ cells in Panc1 adherent cells. (B). Flow cytometry analysis of CD44+CD24+ESA+ cells in Panc1 sphere cells. (C). Cell morphology. After HE staining, Panc1 adherent cells had a polygonal or triangular shape, whereas Panc1 sphere cells had a circular or fusiform shape with a smaller size and a high nucleus-to-cytoplasm ratio. (D). TEM analysis. Panc1 sphere cells exhibited larger nuclei and fewer cytoplasmic organelles than Panc1 adherent cells. (E). Cell cycle. Cell cycle analysis showed that the number of Panc1 sphere cells in the G0/G1 phase was significantly higher than that of adherent Panc1 cells (91.19 ± 0.66% vs. 60.35 ± 1.37%, P < 0.001, n=3). (F). Cell proliferation. The proliferation rate of Panc1 sphere cells was significantly lower than that of Panc1 adherent cells.
Cell morphology and ultrastructure
HE staining revealed that Panc1 sphere cells had a circular or fusiform shape with a smaller size and a high nucleus-to-cytoplasm ratio compared with Panc1 adherent cells, which had a polygonal or triangular shape (). As determined by transmission electron microscopy (TEM) analysis, Panc1 sphere cells exhibited larger nuclei and fewer cytoplasmic organelles than Panc1 adherent cells (). These results showed that the morphology; structure and ultrastructure of the sphere cells are similar to normal stem cells.
Cell cycle
Cell cycle analysis showed that the number of Panc1 sphere cells in the G0/G1 phase was significantly higher than for Panc1 adherent cells (91.19 ± 0.66% vs. 60.35 ± 1.37%, P < 0.001, n=3), while the number of Panc1 sphere cells in the S phase was significantly lower than for Panc1 adherent cells (3.98 ± 0.52% vs. 28.86 ± 1.01%, P < 0.001, n=3) (). This result showed that most of the sphere cells are in resting state while the adherent cells are not.
Cell growth curve
Individual cells of both Panc1 sphere cell and adherent cell were all cultured in DMEM containing 10% FBS and cell proliferation was observed. The result showed that when the sphere cells were cultured in medium containing serum they began to proliferate and the growth is significantly slower than that of the adherent cells ().
Cell spontaneous migration
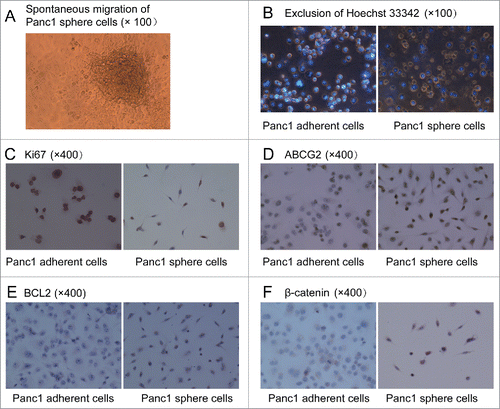
Suspensions of Panc1 cell spheres (Panc1 cell spheres in DMEM/F-12 containing bFGF, EGF, B27 and insulin) were transferred into 96-well plates and serum was added to the medium. 8 hours later, the spheres had adhered to the bottom. 24 hours later, many cells from the edges of the spheres had migrated out of the spheres spontaneously () and then gradually spreaded in the whole bottom of the plate. This result was an accidental discovery in our research and meant that the Panc1 sphere cells had an ability of spontaneous migration like normal stem cells. In Panc1 adherent cells spontaneous migration had never been observed.
Figure 3. (A). Spontaneous migration. After serum was added into the medium, Panc1 cell spheres in DMEM/F-12 containing bFGF, EGF, B27 and insulin adhered to the bottom in 96-well plates and many cells from the edges of the spheres migrated out of the spheres spontaneously and then gradually spreaded in the whole bottom of the plate. (B). Exclusion of Hoechst 33342. After incubation with Hoechst 33342 (2.5 μg/ml), the fluorescent staining of Panc1 sphere cells was significantly weaker than that of Panc1adherent cells. (C). Almost all of the Panc1 adherent cells were Ki67 positive, whereas few Panc1 sphere cells were Ki67 positive. (D, E and F). Expression levels of ABCG2, BCL2 and ß-catenin were much higher in Panc1 sphere cells than in Panc1 adherent cells. ß-catenin was localized to the cell membrane of adherent cells, whereas it was localized to the cytoplasm and nucleus of sphere cells.
Hoechst 33342 efflux
After individual cells were incubated with Hoechst 33342 (2.5 μg/ml) for 30 min at 37°C, the fluorescent staining in Panc1 sphere cells was significantly weaker than in Panc1 adherent cells observed under a fluorescence microscope (). This result suggested the sphere cells can pump out Hoechst 33342 like normal stem cells and CSCs.
mRNA levels of Gli1, Notch1, ß-catenin and Oct4
To investigate the activity of self -renewal pathways and the stem cell gene expression in the cells, we detected the mRNA levels of Gli1, Notch1, ß-catenin, which play important roles in Hedgehog, Notch and Wnt/ß-catenin pathways, and Oct4, one of the most important stem cell gene. The mRNA levels of Gli1, Notch1, ß-catenin and Oct4 in Panc1 sphere cells were 6.9-fold, 2.2-fold, 2.1-fold and 1.8-fold higher, respectively, than in Panc1 adherent cells. These results suggested that the activity of the self -renewal pathways and the Oct4 gene expression in Panc1 sphere cells were higher than in Panc1 adherent cells.
Ki67, ABCG2(ATP-binding cassette superfamily G member 2), BCL2 and ß-catenin expression
Ki67, ABCG2, BCL2 and ß-catenin expression in Panc1 sphere cells and adherent cells were detected by cell immunohistochemistry. After we found that the proliferation rate of Panc1 sphere cells was significantly lower than Panc1 adherent cells, we detect the expression of the proliferation-associated Ki-67 antigen and found that compared with Panc1 adherent cells, fewer Panc1 sphere cells were Ki67-positive (). we also found that the levels of ABCG2 and BCL2 expression were higher in Panc1 sphere cells than in Panc1 adherent cells (). ABCG2, an ATP-binding cassette (ABC) efflux transporter, is one of the putative biomarkers of CSCs. BCL2 protein acts as a key regulator in cell apoptosis pathway and antiapoptosis is one of the characteristics of CSCs. The high expression of ABCG2 and BCL2 in Panc1 sphere cells meant that the cells may be resistant to chemotherapies. ß-catenin plays an important role in Wnt/ß-catenin pathway and the localization of ß-catenin in the nucleus and cytoplasm means the activation of the self-renewal pathway. In realtime PCR, we found the mRNA level of ß-catenin in Panc1 sphere cells was higher than in Panc1 adherent cells. In cell immunohistochemistry, it was shown that ß-catenin was localized to the cell membrane of adherent Panc1 cells, whereas it was localized to the cytoplasm and nucleus of Panc1 sphere cells (). This result also meant that the activity of Wnt/ß-catenin pathway in Panc1 sphere cells was higher than in Panc1 adherent cells.
Tumor-initiating ability
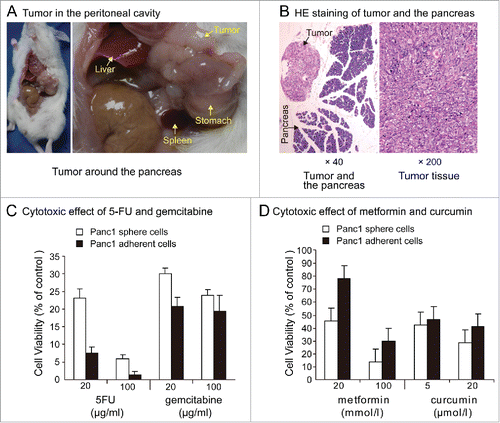
Eight weeks after the intraperitoneal injection of 1×106 Panc1 sphere cells, 2 of the 6 non-obese diabetic/severe combined immunodeficiency disease(NOD/SCID) mice presented with ascites. White tumor nodules around the pancreas appeared in one of the 2 mice with ascites (). Tumor nodules around the pancreas meant that these Panc1 sphere cells had a homing ability like normal stem cell. In the group receiving Panc1 adherent cell intraperitoneal injections, no tumors or ascites appeared in any of the 6 mice.
Figure 4. (A). Tumor in peritoneal cavity. Eight weeks after the intraperitoneal injection of 1000,000 individual Panc1 sphere cells, tumor nodules around the pancreas appeared in one of the 6 NOD/SCID mice. (B). HE staining of tumor and the pancreas. (C). Chemoresistance. Exposure to 5-FU (20,100 μg / ml) or gemcitabine (20,100μg / ml) for 5 days, cell viability was significantly higher in Panc1 sphere cells than in adherent cells. (D). Cytotoxic effects of metformin and curcumin. Cell growth of Panc1 adherent cells and sphere cells can all be inhibited by metformin and curcumin. Exposure to metformin(20, 100 mmol/l)or curcumin(5, 20μmol/l)for 5 days, cell viability was significantly lower in Panc1 sphere cells than in adherent cells.
Chemoresistance
After individual Panc1 adherent cells and sphere cells were plated in 96-well plates and cultured in DMEM containing 10% FBS for 24 hours, 5-fluorouracil(5-FU) and gemcitabine were added into the medium. Results of cytotoxic effects showed that in the presence of 5-FU (20,100 μg/ml) or gemcitabine (20,100μg/ml) for 5 days, cell viability was significantly higher in Panc1 sphere cells than in adherent cells (). This result meant that Panc1 sphere cells were more resistant to chemotherapy drugs than Panc1 adherent cells.
Cytotoxic effects of metformin and curcumin
After individual Panc1 adherent cells and sphere cells were plated in 96-well plates and cultured in DMEM containing 10% FBS for 24 hours, metformin and curcumin were added into the medium. Cell growth of Panc1 adherent cells and sphere cells can all be inhibited by metformin and curcumin. Exposure to metformin(20, 100 mmol/l)or curcumin(5, 20μmol/l)for 5 days, cell viability was significantly lower in Panc1 sphere cells than in adherent cells. The most obvious difference is to low concentration of metformin.The inhibitaion of Panc1 adherent cell growth was mild while cell viability of Panc1 sphere cells was less than 50% in expourse to 20 mmol/l metformin ().
Discussion
In the present study, whether bulk pancreatic cancer cells could convert into CSCs under certain conditions and whether metformin and curcumin could kill pancreatic CSCs were investigated. Aspc1, Bxpc3 and Panc1 pancreatic cancer cells all formed cell spheres when they were cultured in serum-free DMEM/F-12 containing EGF, bFGF, B27 and insulin. Dynamic observation of the cell surface markers in 5 days showed that all the sphere cells expressed the stem cell surface markers CD24, CD44, ESA, CD133 and nestin form the third day after they had been cultured in the medium and the expression level of the stem cell markers increased in the next 2 days. Because Panc1 cells have been widely used in pancreatic cancer research, further studies on Panc1 sphere cells (having been cultured in serum-free DMEM/F-12 containing bFGF, EGF, B27 and insulin for 5 days) and adherent cells were performed in our study. Flow cytometry analysis showed that there were more cells expressed markers CD24, CD44 and ESA in Panc1 sphere cells than in adherent cells.This result was not consistent with the cell immunofluorescence, which showed all the cells in the spheres expressed CD44, CD24 and ESA, may due to the sensitivities and specificities of the 2 analyses are not the same. After having been cultured in the medium for 5 days, the sphere cells had CSC characteristics, such as normal stem cell morphology and structure (small size, high nucleus-to-cytoplasm ratio and few organelles) and increased activity of the Hedgehog, Notch and Wnt/ß-catenin self-renewal pathways (this increased activity was indicated by the upregulation of Gli1, Notch1 and ß-catenin, which play important roles in these signaling pathways and the localization of ß-catenin to the nucleus and cytoplasm). Furthermore, the sphere cells had increased mRNA level of stem cell gene Oct4, increased expression of the anti-apoptotic protein BCL2 and the multi-drug resistance transporter ABCG2, ability to exclude Hoechst 33342 dyeresistance and resistance to 5-FU and gemcitabine. These results also supported that the sphere cells had acquired CSC characteristics even though the cell spheres were not generated by single cells.
Moreover, we found that after the spheres had attached to culture plates in medium containing serum, Panc1 cells in the spheres can migrate to the distant area spontaneously. In vivo, after Panc1 sphere cells were intraperitoneally injected into NOD/SCID mice, metastatic tumor nodules developed around the pancreas. These results demonstrated that the sphere cells were similar to normal stem cells with regard to spontaneous migration and homing. In Panc1 adherent cells, the ability of spontaneous migration and homing ability had never been shown.We cannot explain our finding that the Panc1 sphere cells, despite having so many stem cell characteristics, did not exhibit increased proliferation in vitro. In study of Huang et al. compared with CD44-CD24- Panc1 pancreatic cancer cells, CD44+CD24+ CSCs have a 20-fold higher tumorigenic potential but a lower growth rate in vitro.Citation6 This result is similar to ours.
Cell sphere formation in vitro is widely accepted as one of the characteristics of CSCs. In most previous studies, only CSCs can form cell spheres in serum-free medium, whereas non-CSCs can not. In our study, all of the pancreatic cancer cells (including CSCs and bulk cancer cells) aggregated to form spheres when single cells were cultured in serum-free DMEM/F-12 containing EGF, bFGF, B27 and insulin and the cells in the spheres gradually acquired CSC characteristics. In study of Gavirhi et al., Panc1 cells also aggregated to form spheres in serum-free DMEM/F-12 containing B27, fungizone, heparin, EGF and FGF, and the sphere cells showed stem cell functionalities.Citation8 This result is similar to ours. How the pancreatic cancer cells acquired CSC characteristics in the serum-free DMEM/F-12 medium containing EGF, bFGF, B27 and insulin? It may be that the self–renewal pathways of the bulk cancer cells were activated by bFGF or EGF, which are members of cell signaling pathways. It is also possible that the bulk cancer cells de-differentiated spontaneously when they were in serum-free medium (i.e., a condition without differentiating factors) or that CSCs induced the de-differentiation of non-CSCs in the same sphere by cell signaling transduction between the neighboring cells. In general, normal stem cells can differentiate into somatic cells, but not vice versa; however, under certain conditions, somatic cells can de-differentiate into stem cells. Fu et al. showed that normal human skin cells de-differentiated into stem cells in the presence of EGF.Citation30-32 Chaffer et al. also demonstrated that CD44lo basal-like human mammary epithelial cells and the transformed epithelial cells could all spontaneously de-differentiated into CD44hi stem cells and CD44hi CSCs respectively, and the convertion of transformed epithelial cells to CSCs was more efficiently than the untransformed cells.Citation33 The conversion of bulk cancer cells into CSCs may be simpler due to the genomic instability of cancer cells. Gao’s study shows that the enrichment of CSCs may result either from an increased symmetric self-renewal division rate of CSCs or a reprogramming of non-CSCs to a stem cell state.Citation34 Hu et al. and Faurobert et al. also propose the plasticity of tumor cells.Citation35,36 All these mean that non CSCs can convert into CSCs. Our results also indicate that tumor cell characteristics is microenvironment-dependent and bulk cancer cells can convert to CSCs under certain conditions, which are consistent with the yin-yang model of CSCs.Citation11
The study of Klevebring et al. suggests that there may be a dynamic conversion between breast cancer CSCs and non CSCs in vivo for similar frequencies of most somatic mutations are shared between the 2 group of cells.Citation37 Zhou et al.’s study demonstrates that dormant tumor cells in ovarian cancer exhibit CSC characteristics and cisplatin enhances these characteristics.Citation38 Kleffel et al. Propose that CSCs and dormant cells in cancer, which are resistant to chemotherapy and radiotherapy and are responsible for tumor recurrence, may be the one and same.Citation39 All these support that CSCs may be just cancer cells in dormant stage while not a special group of cancer cells. In the present study, Panc1 sphere cells had a lower growth rate in vitro than adherent cells, as well as fewer Ki67-positive cells, indicating that the Panc1 sphere cells were relatively quiescent. Additionally, the cell number in the Panc1 cell spheres had not increased after 5 days of culture, and most of the cells in Panc1 spheres were in the G0/G1 phase, indicating that the Panc1 sphere cells with CSC characteristics might be dormant cancer cells. So, the results of our study also support the view that CSCs might be just cancer cells in a resting state.
Conventional anticancer therapies kill rapidly proliferating bulk cancer cells while sparing the relatively quiescent CSCs. In the present study, Panc1 sphere cells were more resistant to 5-FU and gemcitabine than their corresponding adherent cells. It has been shown that metformin and curcumin can suppress the self-renewal of CSCs in several cancer cells. So whether metformin and curcumin can kill the Panc1 sphere cells was investigated in this study. We found that cell growth of Panc1 adherent cells and sphere cells can all be inhibited by metformin and curcumin. Panc1 sphere cells were more sensitive to the 2 compounds than Panc1 adherent cells. The most obvious difference was in low concentration of metformin. In exposure to 20 mmol/l metformin , the cell growth inhibitaion was mild in Panc1 adherent cells while obvious cytotoxicity was shown in Panc1 sphere cells. For the Panc1 sphere cells have CSC characteristics, these results mean that metformin and curcumin may be candidate drugs for targeting pancreatic CSCs. It has been shown that metformin kills CSCs by activate AMPK and inactivate mTORCitation20,21 and curcumin targets CSCs by down-regulation of STAT3–NFκB signaling.Citation26,27
Further studies on the molecular mechanisms by which metformin and curcumin kill pancreatic CSCs are required.
Conclusion
At present, the origin of CSCs is still uncertain. In most previous studies, CSCs can generate bulk cancer cells and not vice versa. In the present study, Panc1 pancreatic cancer cells aggregated into spheres in serum-free DMEM/F-12 containing bFGF, EGF, B27 and insulin. After having been cultured in the medium for 5 days, the sphere cells exhibited CSC characteristics, indicating that bulk pancreatic cancer cells could convert into CSCs under such condition. This result suggestes that the pancreatic cancer cells are CSCs or bulk cancer cells is determined by their microenvironment. Under some conditions, the pancreatic cancer cells are bulk cancer cells, while under other conditions, they are CSCs. Therefore, only conventional chemotherapy and radiotherapy combined with anti-CSC agents will be able to eradicate all cancer cells. Metformin and curcumin may be candidate drugs for targeting pancreatic CSCs.
Materials and methods
Cell culture
Aspc1, Bxpc3 and Panc1 adherent cells were cultured in DMEM containing 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA), 1% penicillin and streptomycin (Invitrogen, Carlsbad, CA, USA) in a humidified incubator under 5% CO2 at 37°C.
Cell sphere formation assay
Individual-cell suspensions of Aspc1, Bxpc3 and Panc1 cells were plated (100,000 cells/well) in 6-well ultra-low attachment plates in serum-free Dulbecco's modified Eagle medium/Nutrient Mixture F-12 (DMEM/F-12) (Invitrogen, Carlsbad, CA, USA) containing 20 μg/l basic fibroblast growth factor (bFGF), 20 μg/l epidermal growth factor (EGF), B27 (1:50) (Invitrogen, Carlsbad, CA, USA), 4 μg/l insulin (China Novo Nordisk, Beijing, China), 1% penicillin and streptomycin (Invitrogen, Carlsbad, CA, USA) in a humidified incubator under 5% CO2 at 37°C. In the following analysis, sphere cells were cultured in the medium for 5 days except for cell immunofluorescence.
Cell immunofluorescence assay
Aspc1, Bxpc3 and Panc1 adherent cells and sphere cells (sphere cells had been cultured in serum-free DMEM/F-12 containing bFGF, EGF, B27 and insulin for 24 hours, 48 hours, 72 hours,96 hours or 120 hours ) were plated onto coverslips and cultured in DMEM containing 10% FBS for 12 hours, then washed with PBS, fixed with 95% ethanolacetone, blocked in PBS containing 1% bovine serum albumin (BSA) (Invitrogen, Carlsbad, CA, USA) and incubated with the primary antibodies (1:100 dilution) overnight at 4°C. The cells on the coverslips were then washed with PBS and incubated with a fluorescent secondary antibody (1:800 dilution) at room temperature in the dark for 1 hour. After being washed with PBS, the coverslips were examined under a fluorescence microscope(IX71, Olympus, Japan). The antibodies included the following: primary antibodies to CD44, CD24, ESA, CD133 and nestin (Abcam, Cambridge, MA, USA) and secondary antibodies labeled with FITC, fluorescein DyLight 549 and DyLight 649 (Jackson Immunoresearch, West Grove, PA).
Flow cytometry analysis
Individual Panc1 adherent cells and sphere cells were resuspended in PBS and stained with the directly conjugated monoclonal antibodies FITC anti-human CD44 antibody (1:50) (Biolegend, San Diego, CA,USA) ,PE/Cy7 anti-human CD24 antibody (1:50) (Biolegend, San Diego, CA,USA) or PE anti-human ESA antibody (1:50) (Biolegend, San Diego, CA,USA) for 20 min at 4°C. Then the cells were washed with PBS for three times. Flow cytometry analysis was performed on a BD FACS-Aria II device (BD Biosciences, San Jose, CA, USA).
Cell morphology and transmission electron microscopy (TEM) analysis
Cell morphology of Panc1 adherent cells and sphere cells was visualized using hematoxylin and eosin (HE) staining. Cells were fixed in 2.5% glutaraldehyde and postfixed in 2% osmium tetroxide. The samples were dehydrated in a graded ethanol series and embedded in Epon.Ultrathin sections were contrasted with uranyl acetate and lead citrate, and then examined by a Hitachi-800 transmission electron microscope (Hitachi High-Technologies Co, Japan).
Cell cycle assay
Individual-cell suspensions of Panc1 adherent cells and sphere cells were harvested and washed with PBS, fixed in cold (4°C) 70% ethanol, incubated with 50 μg/ml RNase (Invitrogen, Carlsbad, CA, USA) and 60 μg/ml propidium iodide (Sigma-Aldrich Co. St. Louis, MO, USA) and analyzed by flow cytometry to evaluate the cell cycle. The final data were averaged from 3 independent experiments.
Cell growth curve assay
Individual cell suspensions of Panc1 adherent cells and sphere cells were plated in 96-well plates (5,000 individual cells/well) and cultured in DMEM containing 10% FBS. Cellular proliferation was assayed for 5 consecutive days. The Cell Counting Kit-8 (CCK-8)(Dojindo, Kumamoto, Japan) solution was used according to the manufacturer’s instructions. Briefly, 100 μl of fresh medium containing 10 μl of the CCK-8 solution was added to each well and incubated at 37°C for 1 h. The optical density was measured at 450 nm by a microplate reader. Cell growth curves were generated from the average optical density values of the 3 independent experiments.
Cell spontaneous migration assay
The suspensions of Panc1 cell spheres in DMEM/F-12 containing bFGF, EGF, B27 and insulin were transferred into 96-well plates and serum was added to the medium. 8 hours and 24 hours later, the sphere cells were observed with a light microscopy to detect spontaneous migration.
Hoechst 33342 efflux assay
Individual Panc1 adherent cells and sphere cells were re-suspended in PBS and incubated with Hoechst 33342 (Sigma-Aldrich Co. St. Louis, MO, USA) at a final concentration of 2.5 μg/ml for 30 min at 37°C. After three washes in PBS, the cell suspensions were plated onto coverslips and observed under a fluorescence microscope.
Real-time quantitative reverse transcription PCR assay
Total RNA of Panc1 adherent cells and sphere cells was extracted using RNAiso reagent according to the manufacturer's protocol (Takara Bio, Otsu, Shiga Japan). ß-Actin amplification was used as an internal control for the normalization of gene expression. After the RNA was reverse transcribed to cDNA, real-time polymerase chain reaction (PCR) amplification with the primers listed in was performed using the following cycling conditions: 50°C for 2 min, 95°C for 10 min and 40 cycles of denaturation at 95°C for 15 s and of annealing and extension at 60°C for 1 min, 95°C for 15 s, 60°C for 15 s and 95°C for 15 s. PCRs were performed on Applied Biosystems 7900HT Real-Time PCR System (Applied Biosystems,Foster City, CA, USA).
Table 1. Primers of Gli1, Notch1, β-catenin, Oct4 and β-actin.
Cell immunohistochemistry
Individual Panc1 adherent cells and sphere cells were cultured in DMEM containing 10% FBS and grown on coverslips for 12 hours, washed with PBS, fixed with 95% ethanolacetone, blocked in PBS containing 1% BSA and incubated with the primary antibodies (1:100 dilution) overnight at 4°C. After incubation with a biotinylated secondary antibody at room temperature for 1 hour, the horseradish peroxidase-labeled avidin-biotin complex (ABC-HRP) method was performed with the Elite ABC kits (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer's instructions. The primary antibodies included the following: mouse anti-human Ki67, mouse anti-human BCL2, mouse anti-human ß-catenin (DOKA, Denmark) and mouse anti-human ABCG2 (R&D Systems, Minneapolis, MN, USA).
Tumorigenicity assay
1×106 individual Panc1 cells and sphere cells were injected intraperitoneally into 6 5-week-old male NOD/SCID mice respectively. Eight weeks later, 6 animals in each group were euthanized, and tumor formation in abdominal cavity was observed.
Histopathologic examination
Samples of tumor and pancreas were fixed in formalin and embedded in paraffin. After sections of the tissue having been stained with HE, microscopic examination was performed.
Chemoresistance and cytotoxic effects of metformin and curcumin
Individual Panc1 adherent cells and sphere cells were plated in 96-well plates (5,000 cells/well, in triplicate) in 200 μl of DMEM containing 10% FBS. The cells were cultured for 24 hours and then incubated with 5-FU (20 or 100 μg/ml) (Alexis, Lausanne, CH), gemcitabine (20 or 100 μg/ml) (Alexis, Lausanne, CH), metformin (20 or 100 mmol/l) ((Sigma-Aldrich Co. St. Louis, MO, USA) or curcumin (5 or 20 μmol/l) (Sigma-Aldrich Co. St. Louis, MO, USA). After incubation for 5 days, the viability of the cells was assayed using CCK-8 according to the manufacturer’s instructions, and final values were averaged from 3 independent experiments.
Statistical analysis
For the analysis, t-tests were used, and P ≤ 0.05 was considered statistically significant. Statistical analysis was performed using the SPSS version 13.0.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Ethics statement
The animal experiments were approved by the Experimental Animal Center of Second Military Medical University (Shanghai, China) and by the Second Military Medical University Animal Ethics Committee.
Funding
This work was funded by a grant from the National Natural Science Foundation of China (No. 30600730).
References
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature 2011; 414:105-11; http://dx.doi.org/10.1038/35102167
- Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol.2007; 18: 460-66; PMID:18023337; http://dx.doi.org/10.1016/j.copbio.2007.10.007
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994; 367:645-8; PMID:7509044; http://dx.doi.org/10.1038/367645a0
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997; 3:730-7; PMID:9212098; http://dx.doi.org/10.1038/nm0797-730
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res 2007; 67:1030-7; PMID:17283135; http://dx.doi.org/10.1158/0008-5472.CAN-06-2030
- Huang P, Wang CY, Gou SM, Wu HS, Liu T, Xiong JX. Isolation and biological analysis of tumor stem cells from pancreatic adenocarcinoma. World J Gastroenterol 2008;14:3903-7; PMID:18609717; http://dx.doi.org/10.3748/wjg.14.3903
- Dembinski JL, Krauss S. Characterization and functional analysis of a slow cycling stem cell-like subpopulation in pancreas adenocarcinoma. Clin Exp Metastasis 2009; 26:611-3; PMID:19421880; http://dx.doi.org/10.1007/s10585-009-9260-0
- Gaviraghi M, Tunici P, Valensin S, Rossi M, Giordano C, Magnoni L, Dandrea M, Montagna L, Ritelli R, Scarpa A, et al. Pancreatic cancer spheres are more than just aggregates of stem marker-positive cells. Biosci Rep 2011; 31:45-5; PMID:20426768; http://dx.doi.org/10.1042/BSR20100018
- Rowan K. Are cancer stem cells real? After four decades, debate still simmers. J Natl Cancer Inst 2009; 101:546-7; PMID:19351923; http://dx.doi.org/10.1093/jnci/djp083
- Catalano V, Gaggianesi M, Spina V, Iovino F, Dieli F, Stassi G, Todaro M. Colorectal cancer stem cells and cell death. Cancers 2011; 3:1929-46; PMID:24212789; http://dx.doi.org/10.3390/cancers3021929
- Zhou J, Zhang Y. Cancer stem cells: Models, mechanisms and implications for improved treatment. Cell Cycle 2008; 7:1360-70; PMID:18418062; http://dx.doi.org/10.4161/cc.7.10.5953
- Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci USA 2004; 101:781-6
- Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK. A distinct "side population" of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA 2004; 101:14228-33; PMID:15381773; http://dx.doi.org/10.1073/pnas.0400067101
- Chiba T, Kita K, Zheng YW, Yokosuka O, Saisho H, Iwama A, Nakauchi H, Taniguchi H. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology 2006; 44:240-51; PMID:16799977; http://dx.doi.org/10.1002/hep.21227
- Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, Maclaughlin DT, Donahoe PK. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci USA 2006; 103:11154-9; PMID:16849428; http://dx.doi.org/10.1073/pnas.0603672103
- Platet N, Mayol JF, Berger F, Hérodin F, Wion D. Fluctuation of the SP/non-SP phenotype in the C6 glioma cell line. FEBS Lett 2007; 581:1435-40; PMID:17362939; http://dx.doi.org/10.1016/j.febslet.2007.02.071
- Mitsutake N, Iwao A, Nagai K, Namba H, Ohtsuru A, Saenko V, Yamashita S. Characterization of side population in thyroid cancer cell lines: cancer stem-like cells are enriched partly but not exclusively. Endocrinology 2007; 148:1797-803; PMID:17234707; http://dx.doi.org/10.1210/en.2006-1553
- Sun H, Ma H, Hong G, Sun H, Wang J.Survival improvement in patients with pancreatic cancer by decade: a period analysis of the SEER database, 1981-2010. Sci Rep 2014; 4:6747; PMID:25339498; http://dx.doi.org/10.1038/srep06747
- Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res 2009; 69:7507-11; PMID:19752085; http://dx.doi.org/10.1158/0008-5472.CAN-09-2994
- Gou S, Cui P, Li X, Shi P, Liu T, Wang C.Low concentrations of metformin selectively inhibit CD133?cell proliferation in pancreatic cancer and have anticancer action. PLoS One 2013; 8:e63969; PMID:23667692; http://dx.doi.org/10.1371/journal.pone.0063969
- Lee H, Park HJ, Park CS, Oh ET, Choi BH, Williams B, Lee CK, Song CW. Response of breast cancer cells and cancer stem cells to metformin and hyperthermia alone or combined. PLoS One 2014; 9(2):e87979; PMID:24505341; http://dx.doi.org/10.1371/journal.pone.0087979
- Zhang Y, Guan M, Zheng Z, Zhang Q, Gao F, Xue Y. Effects of metformin on CD133+ colorectal cancer cells in diabetic patients. PLoS One 2013; 8:e81264; PMID:24278407; http://dx.doi.org/10.1371/journal.pone.0081264
- Shank JJ, Yang K, Ghannam J, Cabrera L, Johnston CJ, Reynolds RK, Buckanovich RJ. Metformin targets ovarian cancer stem cells in vitro and in vivo. Gynecol Oncol 2012; 127:390-7; PMID:22864111; http://dx.doi.org/10.1016/j.ygyno.2012.07.115
- Würth R, Pattarozzi A, Gatti M, Bajetto A, Corsaro A, Parodi A, Sirito R, Massollo M, Marini C, Zona G, et al. Metformin selectively affects human glioblastoma tumor-initiating cell viability: A role for metformin-induced inhibition of Akt. Cell Cycle 2013; 12:145-56
- Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, Liu S, Dontu G, Wicha MS. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat 2010; 122:777-85; PMID:19898931; http://dx.doi.org/10.1007/s10549-009-0612-x
- Chung SS, Vadgama JV. Curcumin and Epigallocatechin Gallate Inhibit the Cancer Stem Cell Phenotype via Downregulation of STAT3-NFκB Signaling. Anticancer Res. 2015; 35:39-46; PMID:25550533
- Lin L, Liu Y, Li H, Li PK, Fuchs J, Shibata H, et al.Targeting colon cancer stem cells using a new curcumin analogue, GO-Y030. Br J Cancer 2011; 105:212-20; http://dx.doi.org/10.1038/bjc.2011.200
- Zhang H1, Yu T, Wen L, Wang H, Fei D, Jin C. Curcumin enhances the effectiveness of cisplatin by suppressing CD133+ cancer stem cells in laryngealcarcinoma treatment. Exp Ther Med 2013; 6:1317-21; PMID:24223665
- Fong D, Yeh A, Naftalovich R, Choi TH, Chan MM. Curcumin inhibits the side population (SP) phenotype of the rat C6 glioma cell line: towards targeting of cancer stem cells with phytochemicals. Cancer Lett 2010; 293:65-72; PMID:20089354; http://dx.doi.org/10.1016/j.canlet.2009.12.018
- Li H, Fu X, Zhang L, Sun T, Wang J: In vivo dedifferentiation of human epidermal cells. Cell Biol Int.2007; 31:1436-41; PMID:17689109; http://dx.doi.org/10.1016/j.cellbi.2007.05.016
- Fu X, Sun X, Li X, Sheng Z. Dedifferentiation of epidermal cells to stem cells in vivo. Lancet 2011; 358:1067-8; http://dx.doi.org/10.1016/S0140-6736(01)06202-X
- Zhang C, Fu X, Chen P, Bao X, Li F, Sun X, Lei Y, Cai S, Sun T, Sheng Z. Dedifferentiation derived cells exhibit phenotypic and functional characteristics of epidermal stem cells. J Cell Mol Med.2010; 14:1135-45; PMID:19426155
- Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, Brooks M, Reinhardt F, Su Y, Polyak K, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci USA 2011; 108:7950-55; PMID:21498687; http://dx.doi.org/10.1073/pnas.1102454108
- Gao X, McDonald JT, Naidu M, Hahnfeldt P, Hlatky L. A proposed quantitative index for assessing the potential contribution of reprogramming to cancer stem cell kinetics. Stem Cells Int 2014; 2014:249309; PMID:24955094
- Hu Y, Yu X, Liu S, Liu S. Cancer stem cells: a shifting subpopulation of cells with stemness. Med Hypotheses 2013; 80:649-55; PMID:23484674; http://dx.doi.org/10.1016/j.mehy.2013.01.009
- Faurobert E, Bouin AP, Albiges-Rizo C. Microenvironment, tumor cell plasticity, and cancer. Curr Opin Oncol 2015; 27:64-70; http://dx.doi.org/10.1097/CCO.0000000000000154
- Klevebring D, Rosin G, Ma R, Lindberg J, Czene K, Kere J, Fredriksson I, Bergh J, Hartman J. Sequencing of breast cancer stem cell populations indicates a dynamic conversion between differentiation states in vivo. Breast Cancer Res 2014; 16:R72; PMID:24998755; http://dx.doi.org/10.1186/bcr3687
- Zhou N, Wu X, Yang B, Yang X, Zhang D, Qing G. Stem cell characteristics of dormant cells and cisplatininduced effects on the stemness of epithelial ovarian cancer cells. Mol Med Rep 2014; 10:2495-504; PMID:25119644
- Kleffel S, Schatton T. Tumor dormancy and cancer stem cells: two sides of the same coin. Adv Exp Med Biol 2013; 734:145-79; PMID:23143979; http://dx.doi.org/10.1007/978-1-4614-1445-2_8
