ABSTRACT
A major goal in haematopoietic stem cell (HSC) research is to define conditions for the expansion of HSCs or multipotent progenitor cells (MPPs). Since human HSCs/MPPs cannot be isolated, NOD/SCID repopulating cell (SRC) assays emerged as the standard for the quantification of very primitive haematopoietic cell. However, in addition to HSCs/MPPs, lympho-myeloid primed progenitors (LMPPs) were recently found to contain SRC activities, challenging this assay as clear HSC/MPP readout. Because our revised model of human haematopoiesis predicts that HSCs/MPPs can be identified as CD133+CD34+ cells containing erythroid potentials, we investigated the potential of human mesenchymal and conventional murine stromal cells to support expansion of HSCs/MPPs. Even though all stromal cells supported expansion of CD133+CD34+ progenitors with long-term myeloid and long-term lymphoid potentials, erythroid potentials were exclusively found within erythro-myeloid CD133lowCD34+ cell fractions. Thus, our data demonstrate that against the prevailing assumption co-cultures on human mesenchymal and murine stromal cells neither promote expansion nor maintenance of HSCs and MPPs.
Introduction
For many years haematopoietic stem cell (HSC) expansion strategies have been addressed by thinking within the framework of the hierarchically organized classical model of haematopoiesis. This model predicts the development of HSCs via multipotent progenitors (MPPs) into lineage restricted daughter cells, either containing the potential to develop all lymphoid cell types, the common lymphoid progenitors (CLPs), or all erythro-myeloid cell types, the common myeloid progenitors (CMPs).Citation1 In 2005 this model was challenged by the discovery of a murine lymphoid-primed multipotent progenitor (LMPPs) containing lymphoid and partial myeloid developmental capacities.Citation2 This led to the formulation of the composite model, mainly proposing that different types of progenitors, the CMP and the LMPP, can create granulocytes.Citation2 Later on, progenitors with LMPP characteristics were also detected in humans.Citation3-5
Dissecting the cell fate of different human progenitors including LMPPs by means of their CD133 and CD45RA expression, we provided evidence for novel lineage relationships within human haematopoiesis (). Contradicting existing models, we demonstrated that neutrophils together with lymphocytes derive exclusively from LMPPs (CD133+CD34+CD45RA+), which also contain myeloid (long-term culture-initiating cell; LTC-IC) and lymphoid (NK cell-initiating cell; NK-IC) long-term potentials.Citation4 In contrast, eosinophils and basophils together with megakaryocytes and erythrocytes derive exclusively from CD133lowCD34+CD45RA− precursors, which largely correspond to CMPs; however, due to the lack of neutrophil potential, we prefer to decipher them as erythro-myeloid progenitors (EMPs) rather than CMPs.Citation4 Furthermore, with the formulation of the composite model, which placed the LMPPs at the same hierarchical level as CLPs and CMPs, the binary character of the classical model was lost. Resolving the origin of the different granulocyte subtypes and demonstrating the asymmetric division of HSCs/MPPs (CD133+CD34+CD45RA−) into LMPPs and EMPs daughter cells, the binary feature of the human haematopoietic model has been restored.Citation4,6
Figure 1. Expansion and functional characterization of CD133+CD34+ cells raised on conventional murine stromal cells (A) Revised model of the haematopoietic tree suggesting an early segregation of lympho-myeloid and erythro-myeloid lineages.Citation4 Progenitor cells exhibiting erythroid differentiation potential are highlighted in red with multipotent progenitors (MPPs) being the only CD133-expressing progenitors with erythroid capabilities. LMPP: lymphoid-primed multipotent progenitor, EMP: erythro-myeloid progenitor, MLP: multi-lymphoid progenitor, GMP: granulocyte-macrophage progenitor, EoBP: eosinophil-basophil progenitor, MEP: megakaryocytic-erythroid progenitor; (B) Experimental strategy; (C) gating strategy for the quantification of CD133+CD34+ and CD133lowCD34+ subpopulations; (D) Fold expansion of absolute CD133+CD34+ cell numbers. (E and F) Fold expansion of HSPCs with LTC-IC, NK-IC and CFC-potential; (G and H) Frequency of erythroid (BFU-E), myeloid (CFU-M, CFU-G and CFU-GM) and erythro-myeloid (CFU-MIX) colonies obtained from CD133+CD34+ and CD133lowCD34+ subpopulations (mean ± SEM; *: p-value < 0.05, **: p-value < 0.01, ***: p-value < 0.001).
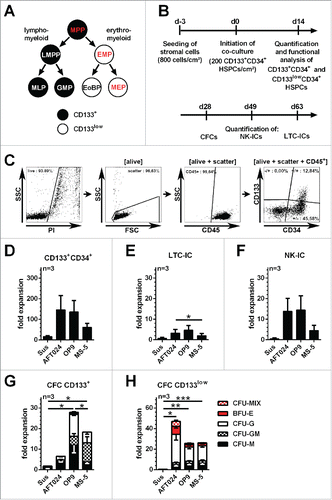
With regard to the revised model, functional in vivo analyses revealed that against the prevailing thinking HSCs/MPPs are not the only primitive progenitors with the capability to reconstitute NOD/SCID mice; LMPPs were also shown to engraft into NOD/SCID mice.Citation4,5 Since long-term human erythropoiesis is not supported Citation7 and analysis of human megakaryocytes as well as discrimination of granulocyte subtypes is commonly neglected in engrafted NOD/SCID mice, proof of erythro-myeloid potentials and thus multipotency of transplanted cells is lacking in many studies reporting expansion protocols of HSCs/MPPs.Citation8 Thus, the interpretation of several such studies has to be challenged.
According to the new lineage relationships, HSCs/MPPs can easily be detected as CD133+CD34+ cells with erythroid potentials in conventional colony-forming cell (CFC) assays.Citation8 In this context, we observed that HSPCs can neither be expanded nor maintained in conventional suspension cultures like IMDM supplemented with 10% FBS and SCF, TPO and FLT3-L (10 ng/ml each).Citation4,6 Since stromal cells have been reported to support maintenance and expansion of primitive haematopoietic cells with LTC-IC, NK-IC and SRC activities,Citation9-12 we tested whether these conditions are sufficient to truly expand HSCs/MPPs. To this end, we tested the haematopoietic support of conventional murine stromal cells and of mesenchymal stromal cells (MSC) raised from different human tissues.
Results
Initially, cold blood (CB) CD133+CD34+ cells were raised for 2 weeks in suspension culture and on AFT024, OP9 or MS5 murine stromal cells (). A massive cell expansion was observed under all conditions. Even though the vast majority of arising cells lost the expression of CD34 and CD133 (), the total number of CD133+/CD133lowCD34+ cells was significantly increased with an average of 16.5±4.7/6.5±2.0-fold in suspension culture and 61/249 to 146/403-fold on the different murine stromal cells (, Table S1, n=3).
Since it has been shown that the phenotype of cultured primitive haematopoietic cells does not necessarily represent their functional properties,Citation13 we purified CD133+CD34+ and CD133lowCD34+ cells by fluorescent cell sorting and introduced them into functional in vitro assays. LTC-IC and NK-IC assays showed that CD133+CD34+ cells harvested from suspension culture contained hardly any long-term potential (, n=3). In contrast, in co-culture with murine stromal cells primitive haematopoietic cells revealing LTC-IC or NK-IC potentials were expanded between 2.0±1.0 to 4.6±2.3-fold or 4.5±2.5 to 14.5±6.9-fold, respectively (, Table S2, n=3). Similarly, total numbers of progenitors containing CFC potential were expanded on murine stromal cells (6.5±2.9 to 27.9±5.3-fold) but not in suspension cultures (, Table S2, n=3). Strikingly, HSCs/MPPs - CD133+ cells showing erythroid potential - were not detected in any of the tested conditions (). Cells containing erythroid potentials were exclusively found within the EMP-lineage restricted CD133lowCD34+ fractions (, Table S2, n=3). Thus, our data imply that under the conditions used in this study HSCs/MPPs can be neither expanded nor maintained in co-cultures with the conventional murine stromal cells AFT024, OP9 and MS5.
Due to potential xenogeneic barriers in some signaling pathways, which might be required for the maintenance of HSCs/MPPs,Citation14-16 we also tested the haematopoietic support of primary MSCs. MSCs were raised from the aortagonad-mesonephros (AGM),Citation17 placental tissue, umbilical cord artery and vein as well as from bone marrow (BM) fat and mononuclear cells (MNCs). Like bona fide MSCs,Citation18,19 all obtained stromal cells expressed the cell surface antigens CD44, CD73, CD90, CD105 and CD146, were negative for CD14, CD31, CD34 and CD45 (, Fig. S1, Table S3) and revealed osteogenic, adipogenic and chondrogenic potentials (, Fig. S2). To test for their haematopoietic support, MSCs were co-cultured with CD133+CD34+ cells in accordance with previous experiments. Suspension cultures and co-cultures with AFT024 stromal cells served as controls.
Figure 2. Phenotypical/functional characterization of primary human MSCs and expansion of phenotypical and functional primitive haematopoietic cells in co-culture with primary human MSCs (A) Plots: Representative analyze of the cell surface marker expression on MSC BM MNC 1 (black histograms) in comparison to isotype-controls (white histogram). Numbers indicate the mean fluorescence intensity (MFI) of the specific staining. Pictures: Representative morphology of MSC BM Fat 6 and proof of tri-lineage differentiation potential into Osteocytes, Adipocytes and Chondrocytes. (Scale-bar: 50µm) Fold-expansion of (B) primitive haematopoietic cells revealing (C) LTC-IC, (D) NK-IC- and (E and F) CFC potentials in co-culture with human MSCs derived from different tissues, AFT024 stromal cells and in suspension culture. Primitive haematopoietic cells revealing CFC potentials were subdivided regarding their arising colony types (BFU-E, CFU-M, CFU-G and CFU-GM and CFU-MIX) (mean ± SEM; */#: p-value < 0.05, tested against *Sus or #AFT024).
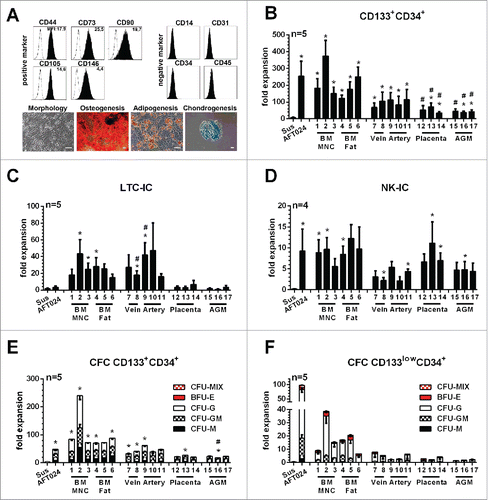
Again, we observed a massive expansion of CD45+ and CD34+ cells under all conditions. Strikingly, CD133+CD34+ cells were expanded on all MSCs in higher amounts than in suspension cultures (6.4±2.8-fold), highest on MSCs derived from BM samples (122±18 to 373±73-fold) followed by AFT024 (253±89-fold), MSCs derived from umbilical cord (67±26 to 113±62-fold), placenta (34±6 to 71±22-fold) and AGM (37±7 to 44±15-fold, , Table S4). MSCs derived from BM samples (15±4 to 43±17-fold) and umbilical cord (16±3 to 47±25-fold) supported LTC-IC expansion much better than AFT024 (3.3±1.4-fold) or MSCs derived from placenta (2.9±1.3 to 6.7±4.1-fold) and AGM (0.9±0.4 to 3.3±1.2-fold, , Table S5). Comparable expansion rates of primitive haematopoietic cells with NK-IC capabilities were observed on MSCs from adult tissue (5.6±1.7 to 12.3±2.1-fold) and the placenta (6.7±1.9 to 11.1±5.0-fold) as on AFT024 (9.2±4.7-fold). Less NK-IC expansion was seen on umbilical cord (2.1±0.6 to 5.4±1.2-fold) and AGM (4.4±1.9 to 4.8±1.9-fold) MSCs than on AFT024 cells (, Table S5). Primitive haematopoietic cells containing CFC potentials were also expanded on all MSCs, obtaining almost a 2-fold higher CFC expansion on BM-derived MSCs than on AFT024 cells (, Table S5). Strikingly, independent of the given stromal cells used, none of the expanded CD133+CD34+ populations revealed erythroid potential. Again, primitive haematopoietic cells with erythroid differentiation potential were exclusively found in the EMP-restricted CD133lowCD34+ cell fraction (). Thus, our data demonstrate that even though BM-derived MSCs support expansion of primitive haematopoietic cells with LTC-IC, NK-IC and CFC potential more effectively than the murine stromal cells, they are not sufficient to support expansion or even maintenance of HSCs/MPPs under these conditions.
Discussion
Here, we systematically compared the haematopoietic support of the murine stromal cell lines AFT024, OP9 and MS5 and of human MSCs derived from different tissues, for the first time. Against the prevailing hypothesis we show that within 2-weeks of culture neither the murine cell lines nor the primary human MSCs promoted expansion or even maintenance of human CB-derived HSCs/MPPs. Instead, arising progeny was highly enriched for lympho-myeloid progenitor populations with long-term myeloid (LTC-IC) and lymphoid (NK-IC) potentials but lacking erythro-myeloid capabilities. In summary, our data demonstrate that all stromal cells tested do support expansion of lympho-myeloid progenitors but not of HSCs/MPPs.
At first glance, these data may appear contradictory to results of previous studies, which have demonstrated that both murine stromal cells, as well as primary human MSCs, support expansion of primitive haematopoietic cells containing the capability to engraft immune deficient mice.Citation12,20-23 However, as stated above, in addition to HSCs/MPPs, SRC activities were also found within LMPP fractions lacking erythro-myeloid differentiation potentials.Citation4,5 Furthermore, by comparing engraftment potentials of retrovirally marked baboon primitive haematopoietic cells in NOD/SCID mice and baboon, we previously provided evidence that cells other than true long term repopulating HSCs/MPPs are read out in the NOD/SCID xenotransplantation model.Citation24 Thus, documented expansion of SRC activity is not sufficient to claim expansion of multipotent HSCs/MPPs, unless the presence of human erythro-myeloid lineage-potentials has specifically been confirmed.Citation8,25 Due to the report that long-term human erythropoiesis is not supported in NOD/SCID mice,Citation7 to our best knowledge only two studies searched for the presence of human erythroid cells in the peripheral blood of successfully engrafted NOD/SCID mice.Citation26,27 Even though both studies detected human erythrocytes in the peripheral blood, their origin was not analyzed. It remains elusive, whether they derived from the same progenitors as the SRCs and thus from HSCs/MPPs or from co-transplanted, lineage-restricted progenitors with erythroid potentials. As a consequence, previous studies defining their read-outs of multipotency based on the classical model of human haematopoiesis should be reconsidered critically.
Using this new read out for multipotent HSCs/MPPs we show that under the conditions used, none of the stromal cells were sufficient to support expansion or even maintenance of HSCs/MPPs. This finding is in line with the observation that in murine HSC niches, both MSCs and endothelial cells are required for the HSC maintenance.Citation28-30 It will be interesting to learn whether MSCs together with endothelial cells or other cell types may allow for the in vitro expansion of HSCs/MPPs. Altogether, these data support our previous conclusion that testing for the erythroid potential of CD133+CD34+ HSPCs provides a simple but meaningful assay to test for the presence of HSCs/MPPs.Citation8
Experimental procedures
Cell sources
Human placenta, umbilical cord, CB and BM samples were obtained from unrelated donors after informed consent according to the Declaration of Helsinki. Mononuclear cells (MNCs) from CB and BM were isolated by Ficoll (Biocoll Separating Solution, Biochrom AG, Berlin, Germany) density gradient centrifugation as described previously.Citation31
Haematopoietic cells
CD34+ cells were enriched from CB MNCs by magnetic cell separation using the MidiMacs technique according to the manufacturer's instructions (Miltenyi Biotec, Bergisch Gladbach, Germany).
Mesenchymal stromal cells (MSCs)
Primary human MSCs were raised and cultured from MNCs from different tissues. MNCs were obtained either from BM aspirates or from fetal placental vessels, which were purged with PBS through the umbilical vein, followed by perfusion with DMEM containing 0.125 % Trypsin and 0.1 g/l Collagenase. Umbilical cord-MSCs were either raised from umbilical arteries or veins which were purged twice with PBS, etched from the cord, cut into rings, and stuck to the bottom of a tissue culture 6-well. Obtained MSCs were cultured as described previously.Citation32
Co-culture experiments
Sort-purified CD133+CD34+ cells (200/cm2) were seeded on murine stromal cells (AFT024, OP9 or MS5) or primary repetition of definition, placental tissue, umbilical cord (artery and vein) and adult bone marrow repetition of definition-derived MSCs and co-cultured for 14 days (). Co-culture was performed in IMDM (Lonza, Basel, Switzerland) supplemented with 20% FBS (Biochrom, Berlin, Germany), 100 U/ml penicillin, 100 U/ml streptomycin (Life Technologies, Karlsruhe, Germany) and SCF, TPO and FLT3-L (Peprotech, Rocky Hill, USA) each at 10 ng/ml final concentration with 50% of exchange of culture medium on day7.
Flow cytometric analysis and sorting
For flow-cytometric analysis haematopoietic cells and MSCs were stained with different combinations of monoclonal fluorochrome-conjugated antibodies (see Table S5) for at least 20 min at 4°C. Propidium-Iodide (PI) was used for dead cell exclusion. Appropriate isotype-matched, control monoclonal antibodies were used to determine the level of background staining in all experiments. Flow cytometric analyses were performed on a FC500 flow cytometer equipped with the CXP 2.2 software (Beckman Coulter) (). Cells were sorted using a FACSAria I cell sorter. The sort-purity was routinely assessed by recovery of sorted cells and was >99.5 %.
Haematopoietic cell assays
For CFC assays, 400 sort-purified CD133+CD34+ or CD133lowCD34+ cells were transferred into 1 ml MethoCult H4434 (StemCell Technologies, Vancouver, Canada). Haematopoietic colonies were scored after 14 days discriminating colony forming unit- (CFU-) macrophage (M), granulocyte (G), granulocyte-macrophage (GM) and burst forming unit-erythrocyte (BFU-E). Colonies consisting of erythroid and myeloid cells were scored as CFU-MIX. For LTC-IC and NK-IC assays 6000 CD133+CD34+were sorted and analyzed in limiting dilutions as described previously.Citation4
MSC assays
The adipogenic, osteogenic, and chondrogenic differentiation capability of BM-, artery-, vein-, AGM- and placenta-derived MSC was analyzed as described previously.Citation32,33
Statistical analysis
Statistical analysis was performed using GraphPad Prism Version 5. All data are given as mean ± standard error of the mean (SEM). Significance analyses was performed with the paired Student t test (*: p < 0.05; **: p < 0.01; ***: p <0.001).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
B. G. and S. R. conceived the study, designed the experiments and wrote the manuscript. S. R. and A. G. performed the experiments, all authors contributed to analysis and interpretation of the data and writing of the manuscript.
Supplementary Files
Download MS Word (2.5 MB)Acknowledgments
Bone marrow samples were kindly provided by the Stem Cell Department of Red Cross Blood Service West and UCB samples by Angela Köninger and Rainer Kimmig from the Department of Gynecology at the University Hospital Essen.
References
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature 2001; 414:105-11; PMID:11689955; http://dx.doi.org/10.1038/35102167
- Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell 2005; 121:295-306; PMID:15851035; http://dx.doi.org/10.1016/j.cell.2005.02.013
- Goardon N, Marchi E, Atzberger A, Quek L, Schuh A, Soneji S, Woll P, Mead A, Alford KA, Rout R, et al. Coexistence of LMPP-like and GMP-like leukemia stem cells in acute myeloid leukemia. Cancer Cell 2011; 19:138-52; PMID:21251617; http://dx.doi.org/10.1016/j.ccr.2010.12.012
- Görgens A, Radtke S, Möllmann M, Cross M, Dürig J, Horn PA, Giebel B. Revision of the human hematopoietic tree: granulocyte subtypes derive from distinct hematopoietic lineages. Cell Reports 2013; 3:1539-52; PMID:23707063; http://dx.doi.org/10.1016/j.celrep.2013.04.025
- Kohn LA, Hao QL, Sasidharan R, Parekh C, Ge S, Zhu Y, Mikkola HK, Crooks GM. Lymphoid priming in human bone marrow begins before expression of CD10 with upregulation of L-selectin. Nat Immunol 2012; 13:963-71; PMID:22941246; http://dx.doi.org/10.1038/ni.2405
- Görgens A, Ludwig AK, Möllmann M, Krawczyk A, Dürig J, Hanenberg H, Horn PA, Giebel B. Multipotent hematopoietic progenitors divide asymmetrically to create progenitors of the Lympho-Myeloid and Erythro-Myeloid Lineages. Stem Cell Reports 2014; 3(6):1058-72; PMID:25448068
- Mazurier F, Doedens M, Gan OI, Dick JE. Rapid myeloerythroid repopulation after intrafemoral transplantation of NOD-SCID mice reveals a new class of human stem cells. Nat Med 2003; 9:959-63; PMID:12796774; http://dx.doi.org/10.1038/nm886
- Görgens A, Radtke S, Horn PA, Giebel B. New relationships of human hematopoietic lineages facilitate detection of multipotent hematopoietic stem and progenitor cells. Cell Cycle 2013; 12:3478-82; PMID:24189527; http://dx.doi.org/10.4161/cc.26900
- Kelly SS, Sola CB, de Lima M, Shpall E. Ex vivo expansion of cord blood. Bone Marrow Transplant 2009; 44:673-81; PMID:19802023; http://dx.doi.org/10.1038/bmt.2009.284
- Chung YS, Choi B, Kwon CH, Joh JW, Kim SJ. AFT024 cell line in co-culture system using high pore density insert (HPDI) maintains hematopoietic stem/progenitor cells (HSCs/HPCs) as more primitive state through histone modification. Transplantation Proc 2010; 42:4611-8; PMID:21168747; http://dx.doi.org/10.1016/j.transproceed.2010.09.175
- Feugier P, Li N, Jo DY, Shieh JH, MacKenzie KL, Lesesve JF, Latger-Cannard V, Bensoussan D, Crystal RG, Rafii S, et al. Osteopetrotic mouse stroma with thrombopoietin, c-kit ligand, and flk-2 ligand supports long-term mobilized CD34+ haematopoiesis in vitro. Stem Cells Dev 2005; 14:505-16; PMID:16305336; http://dx.doi.org/10.1089/scd.2005.14.505
- Khoury M, Drake A, Chen Q, Dong D, Leskov I, Fragoso MF, Li Y, Iliopoulou BP, Hwang W, Lodish HF, et al. Mesenchymal stem cells secreting angiopoietin-like-5 support efficient expansion of human hematopoietic stem cells without compromising their repopulating potential. Stem Cells Dev 2011; 20:1371-81; PMID:21142526; http://dx.doi.org/10.1089/scd.2010.0456
- Dorrell C, Gan OI, Pereira DS, Hawley RG, Dick JE. Expansion of human cord blood CD34(+)CD38(−) cells in ex vivo culture during retroviral transduction without a corresponding increase in SCID repopulating cell (SRC) frequency: dissociation of SRC phenotype and function. Blood 2000; 95:102-10; PMID:10607692
- Chen Q, Khoury M, Chen J. Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc Natl Acad Sci U S A 2009; 106:21783-8; PMID:19966223; http://dx.doi.org/10.1073/pnas.0912274106
- Kalberer CP, Siegler U, Wodnar-Filipowicz A. Human NK cell development in NOD/SCID mice receiving grafts of cord blood CD34+ cells. Blood 2003; 102:127-35; PMID:12637322; http://dx.doi.org/10.1182/blood-2002-07-2024
- Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood 1986; 67:257-67; PMID:3002522
- Wang XY, Lan Y, He WY, Zhang L, Yao HY, Hou CM, Tong Y, Liu YL, Yang G, Liu XD, et al. Identification of mesenchymal stem cells in aorta-gonad-mesonephros and yolk sac of human embryos. Blood 2008; 111:2436-43; PMID:18045971
- Wegmeyer H, Broske AM, Leddin M, Kuentzer K, Nisslbeck AK, Hupfeld J, Wiechmann K, Kuhlen J, von Schwerin C, Stein C, et al. Mesenchymal Stromal Cell Characteristics Vary Depending on Their Origin. Stem Cells Dev 2013; 22(19):2606-18; PMID:23676112
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284:143-7; PMID:10102814; http://dx.doi.org/10.1126/science.284.5411.143
- Ando K, Yahata T, Sato T, Miyatake H, Matsuzawa H, Oki M, Miyoshi H, Tsuji T, Kato S, Hotta T. Direct evidence for ex vivo expansion of human hematopoietic stem cells. Blood 2006; 107:3371-7; PMID:16391011; http://dx.doi.org/10.1182/blood-2005-08-3108
- Horn PA, Kiem HP. Expansion of SCID repopulating cells does not prove expansion of hematopoietic stem cells. Blood 2006; 108:771; author reply -2; PMID:16822905; http://dx.doi.org/10.1182/blood-2006-02-002618
- Fan X, Gay FP, Ong SY, Ang JM, Chu PP, Bari S, Lim TK, Hwang WY. Mesenchymal stromal cell supported umbilical cord blood ex vivo expansion enhances regulatory T cells and reduces graft versus host disease. Cytotherapy 2013; 15:610-9; PMID:23419678; http://dx.doi.org/10.1016/j.jcyt.2012.12.007
- Pinho S, Lacombe J, Hanoun M, Mizoguchi T, Bruns I, Kunisaki Y, Frenette PS. PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med 2013; 210:1351-67; PMID:23776077; http://dx.doi.org/10.1084/jem.20122252
- Horn PA, Thomasson BM, Wood BL, Andrews RG, Morris JC, Kiem HP. Distinct hematopoietic stem/progenitor cell populations are responsible for repopulating NOD/SCID mice compared with nonhuman primates. Blood 2003; 102:4329-35; PMID:12816869; http://dx.doi.org/10.1182/blood-2003-01-0082
- Horn PA, Blasczyk R. Severe combined immunodeficiency-repopulating cell assay may overestimate long-term repopulation ability. Stem Cells 2007; 25:3271-2; PMID:17761755; http://dx.doi.org/10.1634/stemcells.2007-0477
- Drake AC, Khoury M, Leskov I, Iliopoulou BP, Fragoso M, Lodish H, Chen J. Human CD34+ CD133+ hematopoietic stem cells cultured with growth factors including Angptl5 efficiently engraft adult NOD-SCID Il2rγ-/- (NSG) mice. PLOS One 2011; PMID: 21559522; http://dx.doi.org/10.1371/journal.pone.0018382
- Isern J, Martín-Antonio B, Martín AM, López JA, Sánchez-Aguilera A, Martín-Pérez D, Marín P, Van Pel M, Vázquez J, Scheding S, et al. Cell Rep 2013; 3(5): 1714-24; PMID: 23623496; http://dx.doi.org/10.1016/j.celrep.2013.03.041
- Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 2013; 495:227-30; PMID:23434756; http://dx.doi.org/10.1038/nature11926
- Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 2013; 495:231-5; PMID:23434755; http://dx.doi.org/10.1038/nature11885
- Raynaud CM, Butler JM, Halabi NM, Ahmad FS, Ahmed B, Rafii S, Rafii A. Endothelial cells provide a niche for placental hematopoietic stem/progenitor cell expansion through broad transcriptomic modification. Stem Cell Res 2013; 11:1074-90; PMID:23978474; http://dx.doi.org/10.1016/j.scr.2013.07.010
- Giebel B, Corbeil D, Beckmann J, Hohn J, Freund D, Giesen K, Fischer J, Kogler G, Wernet P. Segregation of lipid raft markers including CD133 in polarized human hematopoietic stem and progenitor cells. Blood 2004; 104:2332-8; PMID:15231568; http://dx.doi.org/10.1182/blood-2004-02-0511
- Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 2014; PMID:24445866
- Hemeda H, Jakob M, Ludwig AK, Giebel B, Lang S, Brandau S. Interferon-gamma and tumor necrosis factor-α differentially affect cytokine expression and migration properties of mesenchymal stem cells. Stem Cells Dev 2010; 19:693-706; PMID:20067407; http://dx.doi.org/10.1089/scd.2009.0365
